INTRODUCTION
F ungal keratitis (FK) is recognized as a serious disease which can cause ocular pain, stromal destruction, corneal thinning, perforation and blindness especially in developing countries[1-2]. Aspergillus is one of the main pathogenic fungi.Especially, Aspergillus fumigatus (A. fumigatus) can induce a strong host immune inflammatory reaction of FK[3]. Currently,owing to the lack of efficacious antifungal drugs, increasing numbers of researchers are focusing on the mechanisms of uncontrolled overactive host innate immune inflammatory responses in FK[4]. Inflammation degree in the cornea is determined by the body immunocyte status and secretion of pro- and anti-inflammatory cytokines[5-6].Calcitonin gene-related peptide (CGRP) is a 37 amino acid neuropeptide, which is widely distributed in the nervous systems[7-8]. Therefore, CGRP plays prominent immunoregulatory roles of macrophages[9], neutrophils[10], dendritic cells[11] and other immune cells in inflammation[12]. Results have shown that CGRP plays anti-inflammatory role in immune reactions.CGRP can inhibit the lipopolysaccharide (LPS)-induced release of interleukin-1β (IL-1β) and tumor necrosis factor-α(TNF-α)[13]. CGRP also reduces neutrophil recruitment and opsonophagocytic killing of Streptococcus pyogenes[10].Recent researches have shown that CGRP was obviously increased in different inflammatory diseases[9,14-16]. Studies have demonstrated CGRP treatment can up-regulate proinflammatory factors and down-regulate anti-inflammatory factors to reduce inflammation reactions[17-19]. Maruyama et al[20] found that CGRP inhibited β-glucan-induced inflammatory reactions by direct inhibition of nuclear factor-κB (NF-κB) in Candida osteomyelitis. In addition, Kashem et al[21] found that exogenous addition of CGRP can rescue the susceptibility of C.albicans cutaneous infection which was increased by ablation of sensory neurons. A previous study that described the nerve architecture and distribution of sensory neuropeptides in mouse cornea indicated that CGRP is one of the most abundant in this tissue[22]. However, the role of CGRP has never been tested in experimental keratitis induced by A. fumigatus. Our study investigated the release of CGRP in C57BL/6 corneas and in cultured primary macrophages in response to A. fumigatus exposure. In addition, we investigated the effect of CGRP on inflammatory cytokine secretion to determine the role of CGRP in inhibition of inflammation.
MATERIALS AND METHODS
Ethical Approval This study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of the Affiliated Hospital of Qingdao University. The treatments of mice were based on the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Mice and Reagents Adult (8-week) female C57BL/6 mice(specific pathogen-free; SPF) were purchased from Jinan Pengyue Experimental Animal Co., Ltd. (Jinan, Shandong Province, China). All the mice were weighed between 20-30 g and carefully housed in the laboratory conditions. Sabouroud culture was obtained from Babio biotech (Jinan, Shandong Province, China). Thioglycollate medium came from Hope Bio-Technology Co., Ltd. (Qingdao, Shandong Province,China). Sodium thioglycollate was from Xiya Chemical Industry Co., Ltd. (Shanghai, China). Fetal bovine serum (FBS)and Dulbecco’s modified Eagle’s medium (DMEM) were derived from Gibco (San Diego, California, USA). RNAiso Plus, reverse-transcription polymerase chain reaction (RTPCR) kit and TB Green™ Premix Ex Taq™ II were obtained from TaKaRa (Dalian, Liaoning Province, China). BCA Protein Assay Kit, ammonium persulfate, phosphate buffer saline (PBS), phenylmethylsulfonyl fluoride (PMSF) and radio immunoprecipitation assay (RIPA) lysis buffer were obtained from Solarbio (Beijing, China). Polyvinylidene difluoride(PVDF) membrane, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), sample loaded buffer,confining liquid and enhanced chemiluminescence (ECL)kit were derived from Beyotime Biotechnology (Shanghai,China). GADPH antibody, IL-1β and TNF-α ELISA kits were form Elabscience (Wuhan, China). CGRP was from GenScript Biotech Corp. (New Jersey, USA) and CGRP8-37 was from Abcam (Cambridge, UK). CGRP monoclonal antibody and TNF-α antibody were purchased from Cell Signaling Technology (CST, Danvers, MA, USA). Optimum cutting temperature (OCT) compound was from SAKURA Tissue-Tek(Torrance, CA, USA).
Preparation of Aspergillus fumigatus Standard strain A.fumigatus were purchased from China General Microbiological Culture Collection Center, species number 3.0772 (Beijing,China). A. fumigatus was inoculated in Sabouroud medium at 37℃ for 2-3d, collected with sterile PBS, sterilized by 75%alcohol, and then diluted with DMEM to yield an inoculum with 1×108 colony-forming units (CFU)/mL with a hemacytometer.A. fumigatus conidia was also harvested in sterile PBS, reached the final concentrations of 2.5×107 CFU/mL.
Animal Models of Fungal Keratitis The abrasion of all C57BL/6 mice corneal epithelium was caused by a 30-gauge needle during isoflurane inhalation anesthesia. Then 2 μL A. fumigatus conidia suspension (2.5×107 CFU/mL) was injected into corneal stroma of mice. And the control group was subjected to intra-stromal injection with 2 μL PBS. All corneas were evaluated under the slit lamp and the disease scores (1, 3 and 5d) were recorded according to an established scale. In a word, FK were categorized by turbidity level, ulcer area and morphology into mild (score 0-5), moderate (score 6-9) and severe (score 10-12) infection[23]. Mice corneas (n=6/group/time), enucleation followed by cornea excision with a scalpel and microscissors, were prepared for RT-PCR and Western blot on 1, 3 and 5d post infection (p.i.), respectively. Then whole eyes (n=3/group/time) were also harvested to obtain frozen tissue sections for immunohistofluorescence (IHF) staining.
Primary Mice Peritoneal Macrophage Isolation Primary mice peritoneal macrophages were extracted in accordance with our previous reports[24]. In brief, female C57BL/6 mice(8-week) were given 1 mL of 3% thioglycollate medium by intraperitoneal injection. Mice were euthanized in 7d by cervical dislocation to harvest macrophages by peritoneal lavage with pre-cooled DMEM. Peritoneal macrophages were collected by centrifugation at 1200 rpm for 10min at 4℃.
Cell Culture and Stimulation Macrophages were cultured in DMEM and 10% FBS growth medium. Cellular suspension(1×106 cells/mL) was plated in 12-well-plates for mRNA detection and 6-well cell culture plates for protein expression,then cultured at 37℃ humidified 5% CO2 incubator. After 2h,the culture medium was completely changed, the non-adherent cells were removed.Macrophages were infected with A. fumigatus hyphae (final concentration of 5×106 CFU/mL). An approximated proportion is equivalent to 5 CFU/macrophage. After hatching for 0, 4,8 and 12h, macrophages were harvested to test the levels of CGRP mRNA and protein by real-time PCR and Western blot. Immunocytofluorescence (ICF) staining was also used to detect the CGRP protein level of control groups and A.fumigatus-infected groups (8h incubation).Macrophages were treated with A. fumigatus suspension,after different concentrations of CGRP (1, 10 and 100 nmol/L)and CGRP8-37 (0.1 and 1 μmol/L) pretreatment for 30min respectively. All groups were cultured at 37℃ for 12 or 24h to detect the secretion of cytokines.
Real-time Polymerase Chain Reaction Total RNA in samples was separated by RNAiso plus reagent and quantified by spectrophotometry. The cDNA was synthesized by reverse transcription of total RNA (2 μg) which was used in the following real-time polymerase chain reaction (PCR; 20 μL reaction volumes) with TB Green™ Premix Ex Taq™ II using specific primers: 95℃ for 30s, followed by 40 cycles of 95℃for 5s and 60℃ for 30s and a final stage of 95℃ for 15s, 60℃for 30s, and 95℃ for 15s. DNA contamination was assessed by melting curve. Different sets of target gene products were quantified by the 2ΔΔCT method[25]. Table 1 lists are primer sequences in this study.
Western Blot Corneas of C57BL/6 and macrophages were lysed in RIPA lysis buffer containing 1 mmol/L PMSF for 2h. The lysates were centrifuged (12 000 rpm, 15min, 4℃) to collect supernatant. Then BCA Protein Assay Kit was used to measure protein concentration. After adding the SDS loading buffer, the supernatant was heated at 109.4℃ for 10 min in a metal bath. The total protein was separated from SDS-PAGE and transferred to PVDF membranes. The membranes were blocked by confining liquid and incubated with a monoclonal antibody to mouse GAPDH, and a monoclonal antibody to mouse CGRP at 4℃. After several PBST scrubbing, the PVDF membranes were incubated with the corresponding peroxidaseconjugated secondary anti-rabbit antibody. Signals were visualized by using ECL kit.
Immunohistofluorescence Staining IHF staining was tested as reported before[4]. Briefly, the eyeballs of C57BL/6 mice were removed. They were embedded in OCT, and then frozen in liquid nitrogen timely. Tissue specimens were cut into 3-μm thick tissue sections, placed on glass slides, stored at 37℃for 6h, then fixed in acetone for 5min. Nonspecific binding was blocked with donkey serum diluted 1:100 with PBS at room temperature for 30min. Sections were incubated with monoclonal rabbit anti-mouse CGRP antibodies (CST,Danvers, MA, USA) diluted 1:400 overnight at 4℃, and followed by FITC-conjugated donkey anti-rabbit secondary antibody (Elabscience, Wuhan, China; 1:200, 1.5h, no light,room temperature). Isotype IgG was used as negative control.Cell nuclei were stained with DAPI. Slice digital images were taken with a fluorescence microscope (Zeiss Axiovert microscope, at 40× magnification).
Table 1 Nucleotide sequence of mouse primers for real-time PCR amplification
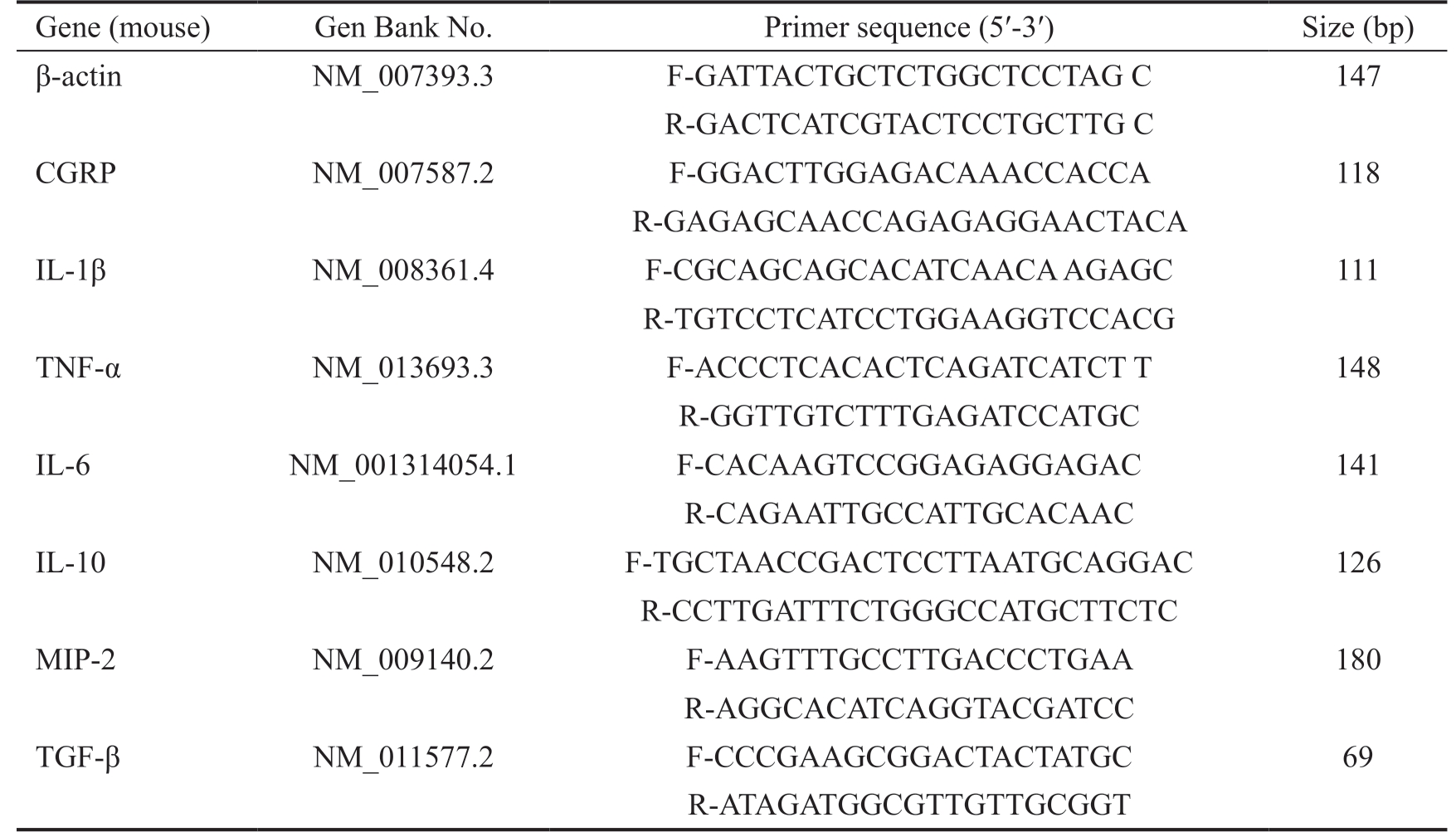
PCR: Polymerase chain reaction; F: Forward; R: Reverse.
Gene (mouse) Gen Bank No. Primer sequence (5′-3′) Size (bp)β-actin NM_007393.3 F-GATTACTGCTCTGGCTCCTAG C 147 R-GACTCATCGTACTCCTGCTTG C CGRP NM_007587.2 F-GGACTTGGAGACAAACCACCA 118 R-GAGAGCAACCAGAGAGGAACTACA IL-1β NM_008361.4 F-CGCAGCAGCACATCAACA AGAGC 111 R-TGTCCTCATCCTGGAAGGTCCACG TNF-α NM_013693.3 F-ACCCTCACACTCAGATCATCT T 148 R-GGTTGTCTTTGAGATCCATGC IL-6 NM_001314054.1 F-CACAAGTCCGGAGAGGAGAC 141 R-CAGAATTGCCATTGCACAAC IL-10 NM_010548.2 F-TGCTAACCGACTCCTTAATGCAGGAC 126 R-CCTTGATTTCTGGGCCATGCTTCTC MIP-2 NM_009140.2 F-AAGTTTGCCTTGACCCTGAA 180 R-AGGCACATCAGGTACGATCC TGF-β NM_011577.2 F-CCCGAAGCGGACTACTATGC 69 R-ATAGATGGCGTTGTTGCGGT
Immunocytofluorescence Staining Macrophages were seeded on four-chamber slides (Thermo Fisher Scientifc,Massachusetts, USA) with or without A. fumigatus hyphae treatment for 8h to detect CGRP localization by using ICF assays. Briefly, after washing with PBS three times,macrophages were fixed with pre-cooled 4% formaldehyde for 15min. And then 0.5% Triton X-100 was used for permeabilization (20min, room temperature). After blocking with normal donkey serum for 30min, the cells were incubated at a 1:400 dilution of rabbit anti-mouse CGRP (CST, Danvers,MA, USA) overnight at 4℃. Donkey anti-rabbit antibody was used as secondary antibody, followed by washing with PBST for three times. Nuclei were stained with 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) for 5min. Fluorescence was visualized using Zeiss Axiovert microscope at 40×magnification.
Enzyme-linked Immunosorbent Assay Macrophages culture medium was collected and centrifuged at 1000× g for 10min to separate the supernatant. According to manufacturer’s instructions (Elabscience), ELISA kit for mouse IL-1β and TNF-α was used to quantify the concentration of IL-1β and TNF-α protein from different treatment groups. The absorbance was tested at 450 nm (Molecular Devices, Sunnyvale, CA,USA).
Statistical Analyses All numerical data were presented as means±standard errors of the mean (SEM). All experiments were performed at least three times. One-way analysis of variance (ANOVA) test was used to make comparisons among three or more groups, and unpaired two-tailed Student’s t-test also was used to identify between multiple groups. P value less than 0.05 was defined to be statistically significant.
RESULTS
CGRP Expression in Mouse Corneas in Response to Aspergillus fumigatus To observe the progression of A. fumigatus keratitis, C57BL/6 mouse corneas were inoculated with A. fumigatus conidia suspension. Corneal edema even ulceration was significantly at 1d after A. fumigatus infection. Thereafter, severity aggravated gradually and the clinical score reached the highest around 3d, after which the cornea began to alleviate or recover (Figure 1A-1B). Relative mRNA and protein expression of CGRP in uninfected control and A. fumigatus-infected mice corneas were tested by Western blot, IHF and RT-PCR. Results indicated that CGRP mRNA and protein levels were significantly increased in A. fumigatus groups and peaked at 3d p.i., when compared with control(Figure 1C-1D). To confirm these data, CGRP protein was also tested by IHF. CGRP was localized mainly to epithelium and stroma of A. fumigatus-infected mice corneas. While, lower amount of CGRP staining was detected in the control group(Figure 1E).
CGRP Expression in Primary Macrophages with the Challenge of Aspergillus fumigatus In vitro, we investigated whether CGRP were expressed in macrophages which were interacted with A. fumigatus. RT-PCR, Western blot and ICF staining were used to detect the mRNA and protein levels of CGRP. Data showed that CGRP were upregulated significantly at 4, 8 and 12h in A. fumigatus-infected group than in control group, and peaked at 8h (Figure 2A-2B). To determine these data,CGRP protein was tested by ICF. Comparison in ICF between the two groups at 8h (Figure 2C, green) revealed that A. fumigatusexposed macrophages had increased CGRP staining.
Effect of Exogenous CGRP on Cytokines Production in Macrophages Interacted with Aspergillus fumigatus To assess the role of CGRP in A. fumigatus induced inflammatory responses, macrophages were pretreated with different concentrations of exogenous CGRP (a CGRP agonist; 1, 10 and 100 nmol/L) for 30min. The mRNA expression of IL-1β, TNF-α,IL-6, MIP-2, IL-10 and TGF-β in macrophages was tested by RT-PCR. We also used ELISA to examine IL-1β and TNF-α
protein levels in macrophages supernatant after stimulation with A. fumigatus hyphae for 24h. We found that exogenous CGRP pretreatment (10 and 100 nmol/L) significantly reduced IL-1β mRNA level induced by A. fumigatus hyphae (Figure 3A; P<0.01, P<0.001). And 100 nmol/L CGRP inhibited A. fumigatus hyphae-induced mRNA secretion of TNF-α (Figure 3B; P<0.05) and IL-6 (Figure 3C; P<0.05) at 12h. In contrast,results indicated that a series of concentrations of CGRP (1,10 and 100 nmol/L) significantly enhanced the expression of IL-10 mRNA level after stimulation with A. fumigatus hyphae(Figure 3F; P<0.01, P<0.01, P<0.01). However, data showed that exogenous CGRP (1, 10 and 100 nmol/L) didn’t alter MIP-2 and TGF-β mRNA levels. It indicates that CGRP has no effect on the expression of MIP-2 and TGF-β. IL-1β (Figure 3G; P<0.001) and TNF-α (Figure 3H; P<0.001) protein were reduced followed by CGRP (100 nmol/L) pretreatment when compared with A. fumigatus hyphae-exposed cells after 24h.
Effect of CGRP8-37 on Inflammatory Mediators in Macrophages after Stimulation with A. fumigatus To further investigate the effect of CGRP in vitro, macrophages were pretreated with various concentrations of CGRP8-37 (a CGRP antagonist, 0.1 and 1 μmol/L) for 30min, then followed by A.fumigatus hyphae stimulation. Data indicated that CGRP8-37(0.1 μmol/L) significantly increased the expression of IL-1β mRNA (Figure 4A; P<0.01) after stimulation with A. fumigatus hyphae. We also found that the mRNA levels of TNF-α (Figure 4B; P<0.05, P<0.05) and IL-6 (Figure 4C;P<0.001, P<0.05) were increased with CGRP8-37 (0.1 and 1 μmol/L) pretreatment, as compared with A. fumigatus hyphae-exposed group at 12h, but MIP-2 (Figure 4D), TGF-β(Figure 4E) and IL-10 (Figure 4F) mRNA levels showed no difference after pretreatment, detected by RT-PCR. Results showed that CGRP8-37 (0.1 μmol/L) pretreatment increased the protein levels of IL-1β (Figure 4G, P<0.001) and TNF-α(Figure 4H, P<0.001) induced by A. fumigatus hyphae at 24h,tested by ELISA.
DISCUSSION
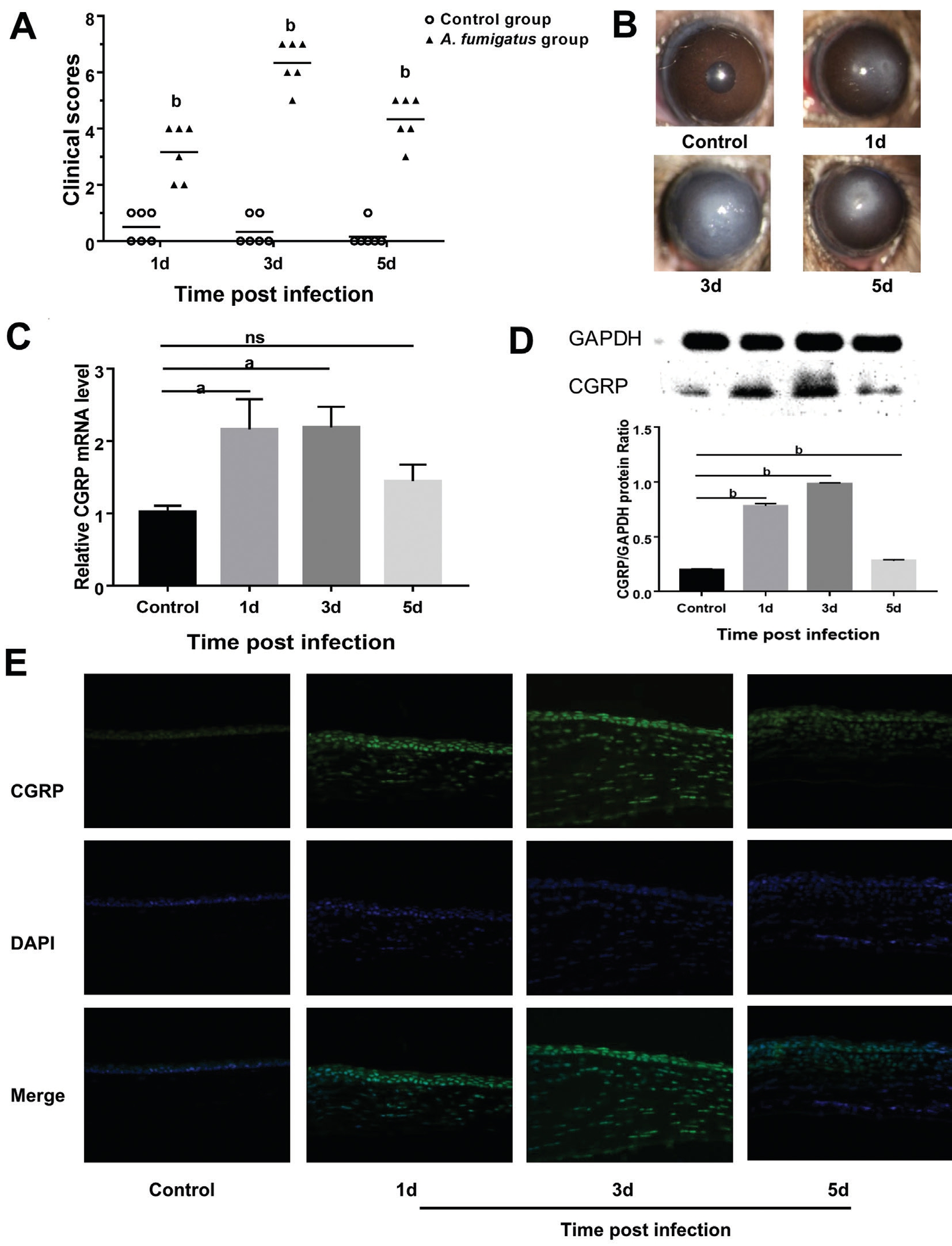
Figure 1 Expression of CGRP in A. fumigatus-infected C57BL/6 cornea A: Clinical scores indicated statistically significant differences at 1,3 and 5d p.i. between control group and A. fumigatus-infected group; B: Representative eyes from A. fumigatus-treated mice were photographed with a slit lamp at 1 (score 4), 3 (score 7), 5d (score 5); C, D: mRNA and protein levels of CGRP were remarkably greater in the A. fumigatus-infected cornea than in control corneas; E: Greater staining intensity for CGRP was seen in A. fumigatus-infected group compared with control mice. Green:CGRP staining; Blue: Nuclear staining (DAPI). IHF images scale bar: 50 μmol/L. Values represent as means±SEM. aP<0.05; bP<0.001.
Neuropeptides play important roles in fungal infections[20,26].The neuropeptide CGRP has an anti-inflammatory effect and regulates the expression of cytokines[18]. Its protective role has been demonstrated in infectious diseases caused by fungi and bacteria[13,19]. Using the C57BL/6 mouse A. fumigatus keratitis model and primary macrophages, we verified the hypothesis that CGRP plays a role in antifungal innate immune defense. In this study, we demonstrated that C57BL/6 cornea and macrophages release CGRP in response to A. fumigatus.Furthermore, in the mouse model of FK, CGRP expression was generally associated with the severity of keratitis. These findings were consistent with those previous studies showing that CGRP was upregulated in murine lungs and murine macrophages after LPS stimulation[9]. This suggests that CGRP may be an endogenous protective neuropeptide in the pathogenesis of FK.Under the circumstance of fungal infection, massive macrophages aggregate in the limbus or central cornea. These macrophages play a vital role in the innate immune system after infection of A. fumigatus[6]. Neuropeptides play anti-inflammatory roles in many acute and chronic diseases[14,26]. Several researches[18-19]have clearly shown that CGRP inhibited the release of proinflammatory factors. And increased endogenous CGRP can mitigate inflammatory reaction by increasing cAMP levels in the pulmonary inflammatory diseases[15]. Reinshagen et al[27]showed that subcutaneous injection of CGRP8-37 significantly increased the severity of colitis in the rat. Likewise, it has been demonstrated in two ways that CGRP acted as an antiinflammatory mediator in antifungal innate immune defense.We found that CGRP inhibited the A. fumigatus-induced IL-1β, TNF-α, IL-6 secretion and promoted IL-10 secretion in macrophages. Second, CGRP8-37 significantly magnified the A. fumigatus-induced IL-1β, TNF-α, IL-6 secretion.This study is consistent with previous research result that intraperitoneal injection of exogenous CGRP obviously diminishes the histological injury and inflammatory factors levels in acute lung injury induced by LPS[28]. In addition,a similar observation has been reported that dendritic cells with CGRP pretreatment might attenuate allergic airway inflammation in vivo, and increase IL-10 levels[29]. Collectively,our data provided evidence that CGRP might be a protective factor against A. fumigatus by regulating the pro- and antiinflammatory mediators.
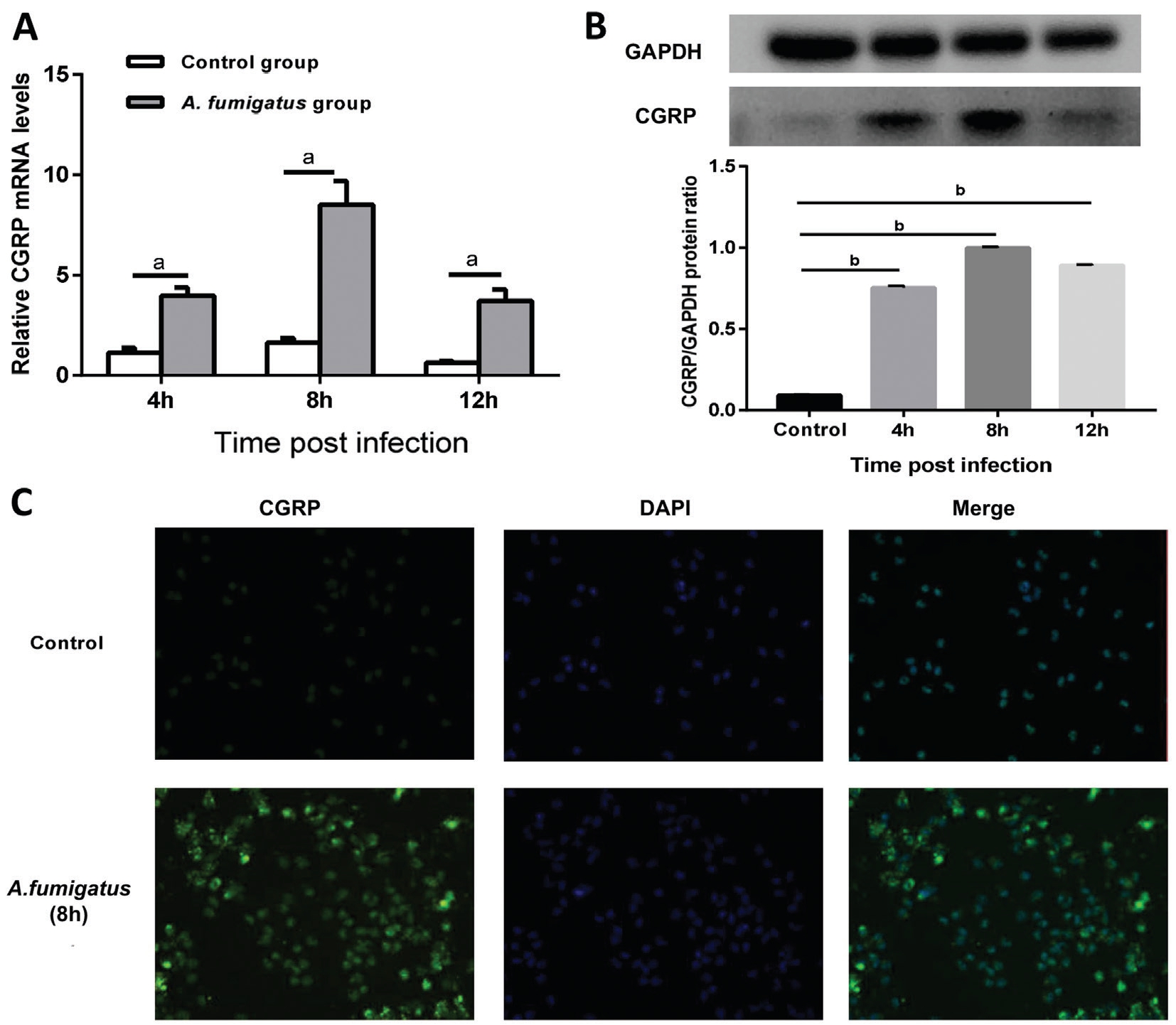
Figure 2 CGRP expression in primary macrophages with infection of A. fumigatus A, B: The levels of CGRP mRNA and protein in macrophages exposed to A. fumigatus were analyzed by RT-PCR and Western blot; C: ICF images showed CGRP protein in primary macrophages without or with exposure to A. fumigatus. Green: CGRP staining; Blue: Nuclear staining (DAPI). ICF images scale bar: 50 μmol/L.Values represent as means±SEM. aP<0.05; bP<0.001.
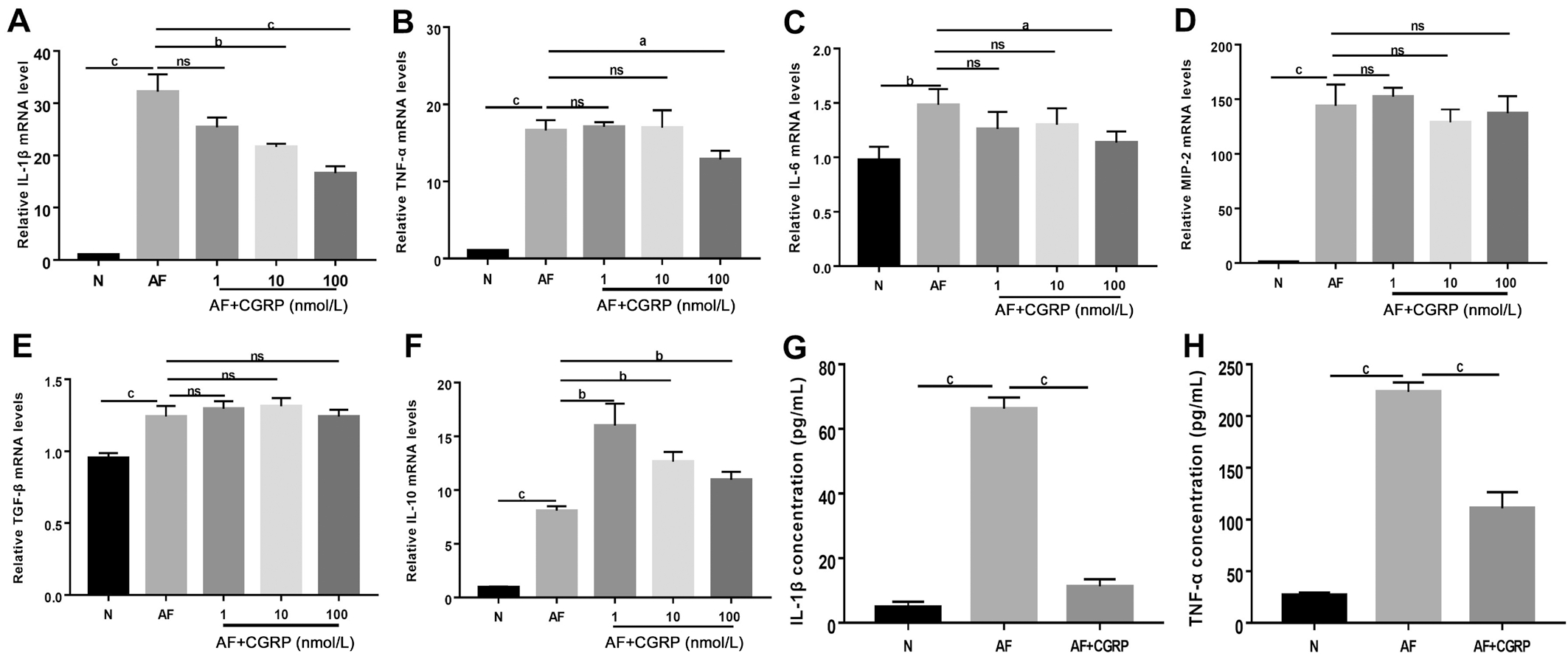
Figure 3 Exogenous CGRP attenuated inflammatory reaction in macrophages challenged with A. fumigatus A-C: The expression of IL-1β, TNF-α and IL-6 mRNA were reduced in different concentrations of CGRP after 12h stimulation with A. fumigatus hyphae; D, E: MIP-2 and TGF-β mRNA levels did not change significantly after various concentrations of CGRP pretreatment; F: Exogenous CGRP significantly increased A. fumigatus hyphae-induced secretion of IL-10 mRNA at 12h; G, H: IL-1β and TNF-α protein levels were significantly reduced at 24h after pretreatment of 100 nmol/L CGRP as compared with A. fumigatus hyphae-exposed control group. aP<0.05, bP<0.01, cP<0.001.

Figure 4 Effect of CGRP8-37 on cytokines in macrophages induced by A. fumigatus hyphae A-C: CGRP8-37 significantly increased relative mRNA levels of IL-1β, TNF-α and IL-6 compared with A. fumigatus hyphae-exposed control group at 12h; D-F: CGRP8-37 (0.1 and 1 μmol/L)did not change MIP-2, TGF-β and IL-10 mRNA levels, compared with A. fumigatus hyphae-exposed control group; G-H: CGRP8-37 (0.1 μmol/L) upregulated the expression of IL-1β and TNF-α protein induced by A. fumigates hyphae at 24h. aP<0.05, bP<0.01, cP<0.001.
However, we did not detect any differences in MIP-2/TGF-β levels in macrophages which were pretreated with exogenous CGRP or CGRP8-37 induced by A. fumigatus hyphae. While previous reports showed that levels of MIP-2/TGF-β were changed after pretreatment of CGRP or CGRP8-37. Wang et al[30] found that CGRP partially increased MIP-2 levels from the peritoneal macrophages, but did not change from alveolar macrophages in LPS-induced murine sepsis. Yoon and Kim[31] have reported that exogenous CGRP induced the secretion of TGF-β in tubulointerstitial fibrosis. However there is no research on the stimulated mouse macrophages with the infection of A. fumigatus. Discrepancies between our findings and previous might be caused by the difference of strains or disease types. Thus, the levels of MIP-2/TGF-β might be unaffected by CGRP after stimulation with A. fumigatus.Further researches are needed to clarify the importance of CGRP in fungal infections.In summary, we first discovered that CGRP may function as a protector of FK. In the context of fungal infection, strategies to enhance CGRP secretion might be beneficial to prevent the A. fumigatus-associated inflammation. Thus, CGRP, as an anti-inflammatory factor, might have the potential to be a new target in FK therapy. However, these conclusions were based on the responses of cell experiments. The specific mechanism of CGRP in cornea is still enigmatic, continued studies are needed to confirm further the role of CGRP. The study provided experimental basis for clinical application of neuro-immune regulation in FK. Furthermore, it emphasized the necessity of understanding and exploring the complex neuroimmunity mechanisms that contribute to treatment of FK.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81470609; No.81700800;No.81870632; No.81800800); the Youth National Natural Science Foundation of China (No.81500695); Natural Science Foundation of Shandong Province (No.ZR2013HQ007;No.ZR2017MH008; No.ZR2017BH025).
Conflicts of Interest: Yin M, None; Li C, None; Peng XD,None; Zhao GQ, None; Wu Y, None; Zheng HR, None;Wang Q, None; Xu Q, None; Jiang N, None.
1 Nielsen SE, Nielsen E, Julian HO, Lindegaard J, Højgaard K, Ivarsen A, Hjortdal J, Heegaard S. Incidence and clinical characteristics of fungal keratitis in a Danish population from 2000 to 2013. Acta Ophthalmol 2015;93(1):54-58.
2 Garg P, Roy A, Roy S. Update on fungal keratitis. Curr Opin Ophthalmol 2016;27(4):333-339.
3 Heinekamp T, Schmidt H, Lapp K, Pähtz V, Shopova I, Köster-Eiserfunke N, Krüger T, Kniemeyer O, Brakhage AA. Interference of Aspergillus fumigatus with the immune response. Semin Immunopathol 2015;37(2):141-152.
4 Niu YW, Zhao GQ, Li C, Lin J, Jiang N, Che CY, Zhang J, Xu Q. Aspergillus fumigatus increased PAR-2 expression and elevated proinflammatory cytokines expression through the pathway of PAR-2/ERK1/2 in cornea. Invest Ophthalmol Vis Sci 2018;59(1):166-175.
5 Xu Q, Zhao GQ, Lin J, Wang Q, Hu LT, Jiang Z. Role of Dectin-1 in the innate immune response of rat corneal epithelial cells to Aspergillus fumigatus. BMC Ophthalmol 2015;15:126.
6 Lin J, He K, Zhao GQ, Li C, Hu LT, Zhu GQ, Niu YW, Hao GP. Mincle inhibits neutrophils and macrophages apoptosis in A. fumigatus keratitis.Int Immunopharmacol 2017;52:101-109.
7 Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 1983;304(5922):129-135.
8 Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R.Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience 2003;120(3):677-694.
9 Duan JX, Zhou Y, Zhou AY, Guan XX, Liu T, Yang HH, Xie H, Chen P.Calcitonin gene-related peptide exerts anti-inflammatory property through regulating murine macrophages polarization in vitro. Mol Immunol 2017;91:105-113.
10 Pinho-Ribeiro FA, Baddal B, Haarsma R, O’Seaghdha M, Yang NJ,Blake KJ, Portley M, Verri WA, Dale JB, Wessels MR, Chiu IM. Blocking neuronal signaling to immune cells treats streptococcal invasive infection.Cell 2018;173(5):1083-1097.e22.
11 Assas BM, Wakid MH, Zakai HA, Miyan JA, Pennock JL. Transient receptor potential vanilloid 1 expression and function in splenic dendritic cells: a potential role in immune homeostasis. Immunology 2016;147(3):292-304.
12 Asahina A, Moro O, Hosoi J, Lerner EA, Xu S, Takashima A,Granstein RD. Specific induction of cAMP in Langerhans cells by calcitonin gene-related peptide: relevance to functional effects. Proc Natl Acad Sci USA 1995;92(18):8323-8327.
13 Zhou Y, Zhang H, Zhang G, He Y, Zhang P, Sun Z, Gao Y, Tan Y.Calcitonin gene-related peptide reduces Porphyromonas gingivalis LPS-induced TNF-α release and apoptosis in osteoblasts. Mol Med Rep 2018;17(2):3246-3254.
14 Soultanova A, Mikulski Z, Pfeil U, Grau V, Kummer W. Calcitonin peptide family members are differentially regulated by LPS and inhibit functions of rat alveolar NR8383 macrophages. PLoS One 2016;11(10):e0163483.
15 Li W, Wang T, Ma C, Xiong T, Zhu Y, Wang X. Calcitonin generelated peptide inhibits interleukin-1beta-induced endogenous monocyte chemoattractant protein-1 secretion in type II alveolar epithelial cells. Am J Physiol Cell Physiol 2006;291(3):C456-C465.
16 Farmer MA, Taylor AM, Bailey AL, Tuttle AH, MacIntyre LC,Milagrosa ZE, Crissman HP, Bennett GJ, Ribeiro-da-Silva A, Binik YM,Mogil JS. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Sci Transl Med 2011;3(101):101ra91.
17 Augustyniak D, Roszkowiak J, Wiśniewska I, Skała J, Gorczyca D,Drulis-Kawa Z. Neuropeptides SP and CGRP diminish the moraxella Catarrhalis outer membrane vesicle- (OMV-) triggered inflammatory response of human A549 epithelial cells and neutrophils. Mediators Inflamm 2018;2018:4847205.
18 Jablonski H, Kauther MD, Bachmann HS, Jäger M, Wedemeyer C. Calcitonin gene-related peptide modulates the production of proinflammatory cytokines associated with periprosthetic osteolysis by THP-1 macrophage-like cells. Neuroimmunomodulation 2015;22(3):152-165.
19 Gomes RN, Castro-Faria-Neto HC, Bozza PT, Soares MB, Shoemaker CB, David JR, Bozza MT. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock 2005;24(6):590-594.
20 Maruyama K, Takayama Y, Kondo T, et al. Nociceptors boost the resolution of fungal osteoinflammation via the TRP channel-CGRP-jdp2 axis. Cell Rep 2017;19(13):2730-2742.
21 Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity.Immunity 2015;43(3):515-526.
22 He JC, Bazan HE. Neuroanatomy and neurochemistry of mouse cornea. Invest Ophthalmol Vis Sci 2016;57(2):664-674.
23 Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci 2003;44(1):210-216.
24 He K, Yue LH, Zhao GQ, Li C, Lin J, Jiang N, Wang Q, Xu Q, Peng XD, Hu LT, Zhang J. The role of LOX-1 on innate immunity against Aspergillus keratitis in mice. Int J Ophthalmol 2016;9(9):1245-1250.
25 Peng X, Zhao G, Lin J, Qu J, Zhang Y, Li C. Phospholipase Cγ2 is critical for Ca2+ flux and cytokine production in anti-fungal innate immunity of human corneal epithelial cells. BMC Ophthalmol 2018;18:170.
26 Li C, Liu YY, Zhao GQ, Lin J, Che CY, Jiang N, Li N, Zhang J, He K,Peng XD. Role of vasoactive intestinal peptide in Aspergillus fumigatusinfected cornea. Int J Ophthalmol 2018;11(2):183-188.
27 Reinshagen M, Flämig G, Ernst S, Geerling I, Wong H, Walsh JH, Eysselein VE, Adler G. Calcitonin gene-related peptide mediates the protective effect of sensory nerves in a model of colonic injury. J Pharmacol Exp Ther 1998;286(2):657-661.
28 Yang W, Xv M, Yang WC, Wang N, Zhang XZ, Li WZ. Exogenous α-calcitonin gene-related peptide attenuates lipopolysaccharide-induced acute lung injury in rats. Mol Med Rep 2015;12(2):2181-2188.
29 Rochlitzer S, Veres TZ, Kühne K, Prenzler F, Pilzner C, Knothe S,Winkler C, Lauenstein HD, Willart M, Hammad H, Müller M, Krug N, Lambrecht BN, Braun A. The neuropeptide calcitonin gene-related peptide affects allergic airway inflammation by modulating dendritic cell function. Clin Exp Allergy 2011;41(11):1609-1621.
30 Wang X, Ebong SJ, Call DR, Newcomb DE, Bolgos GR, Remick DG.Calcitonin gene-related peptide partially reverses decreased production of chemokines KC and MIP-2 following murine sepsis. Inflammation 2002;26(4):167-174.
31 Yoon SP, Kim J. Exogenous CGRP upregulates profibrogenic growth factors through PKC/JNK signaling pathway in kidney proximal tubular cells. Cell Biol Toxicol 2018;34(4):251-262.