INTRODUCTION
F ungal keratitis (FK), mainly induced by Fusarium and Aspergillus infection, is one of the main causes of blindness[1]. Corneal trauma, excessive usage of contact lens and corticosteroids abuse increase the incidence of FK[2].The treatment of FK remains challenging due to the lack of understanding of mechanism. Innate immunity serves as a key point to the defense of FK and has an essential role in finding new treatments[3].
The different immune responses between C57BL/6 and BALB/c mice have been revealed in numerous disease models,such as bacterial keratitis[4] and retinal diseases[5]. In bacterial keratitis models, BALB/c mice featuring self-limiting infection are classified as resistant murine, whereas C57BL/6 mice exhibit a susceptible character to bacterial infection. Different manifestations are also presented in fungal infected corneas of BALB/c and C57BL/6 mice[6].
Macroautophagy (called “autophagy” here) can mediate the degradation of long-lived proteins and injured organelles by autophagosomes and then fuse them to lysosomes to maintain the intracellular homeostasis[7]. Starvation, stress, hypoxia,tumor and infection can markedly induce the production of autophagy[8]. Previous studies indicate that Beclin-1 plays a crucial role in startup phase of autophagy[9] and LC3 is the only reliable marker on autophagic membrane[10]. In addition,the p62 protein [sequestosome 1 (SQSTM1)] is the typical protein that autophagy degraded[11] and lysosome-associated membrane proteins 1 (LAMP-1) can reflect the activation of autolysosome[12]. An increasing number of studies have shown that bacterial and fungal infection can markedly up-regulate the production of autophagy through many signaling pathways in human macrophages[13-14] and autophagy plays an essential role in maintaining immune homeostasis[15]. Bacteria increase the production of autophagy in human corneal limbal epithelial cells, and autophagy has a great anti-inflammatory effect on infection[16]. However, despite growing studies for anormal production of autophagy in numerous diseases, the expression of autophagy in Aspergillus fumigatus (A. fumigatus) keratitis remains unclear. In this paper, we found that autophagy was related to the process of FK caused by A. fumigatus and we described the disparate expression of autophagy between C57BL/6 and BALB/c mice corneas.
MATERIALS AND METHODS
Ethical Approval The study was under the approval from the Institutional Research Ethics Committee at the Affiliated Hospital of Qingdao University. The experimental animals were treated abided by the Statement for the Use of Animals in Ophthalmic and Vision Research made by the ARVO.
Aspergillus fumigatus Culture A. fumigatus (No.3.0772)was purchased from China General Microbiological Culture Collection Center and cultured in Sabouraud medium. After being shaken in 200 mL Erlenmeyer flasks in Sabouraud liquid medium for 3d, A. fumigatus were separated into 20-40 μm fractions. After being washed with sterile phosphate buffer saline (PBS) 3 times, the liquid supernatant was discarded. The fungal fractions were diluted to 1×108 colony-forming units(CFU)/mL with DMEM (Gibco, San Diego, CA, USA).
Murine Model of Fungal Keratitis C57BL/6 mice (SPF,female, 8wk) and BALB/c mice (SPF, female, 8wk) were bought from Changzhou Cavens Laboratory Animal Co., Ltd. (Jiangsu,China). Totally 8% chloral hydrate was used to anesthetize mice by intraperitoneal injection, and then the central corneal epithelium was scraped within 2-mm diameters. The experimental ocular surface (left eyes) was topically covered with a 5-μL aliquot of A. fumigatus, and the right eyes were chosen as normal control eyes. Soft contact lenses were put into the eyes and then the eyelids were sutured. Mice corneas were obtained at 1, 3 and 5d post infection (p.i.) for Western blot; eyeballs were removed at 1, 3 and 5d p.i. for immunofluorescent staining and hematoxylin-eosin (H&E) staining.
Western Blot Analysis Ultrasound was used to ground corneas and RIPA lysis buffer (Solarbio) plus 1 mmol/L PMSF(Solarbio) was used to lyse corneas for 2h. Corneas were centrifuged for 10min and then collected the supernatant. The concentration of protein was tested and SDS sample buffer was added to the protein. After boiling for 10min, the protein was separated on 12% acrylamide SDS-PAGE and transferred to PVDF membrane (Solarbio). The membranes were blocked with blocking buffer (Beyotime, Jiangsu, China) at 37℃ for 2h and incubated with polyclonal antibodies to GAPDH (1:1000;Elabscience, Wuhan, China), LC3 (1:1000; Cell Signaling Technology, Massachusetts, USA), SQSTM1/p62 (1:1000;Cell Signaling Technology), Beclin-1 (1:1000; Cell Signaling Technology), LAMP-1 (1:1000; Cell Signaling Technology)at 4℃ overnight. After washed with PBST (PBS containing 0.05% Tween 20; Bio-Rad, Hercules, CA, USA), membranes were incubated with secondary antibodies (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 37℃ for 1h, and then detected with BeyoECL Plus (Beyotime, Shanghai, China).
Immunofluorescent Staining The eyeballs of mice were removed, and frozen rapidly in optimal cutting temperature compound (O.C.T.; SAKURA Tissue-Tek, Torrance, CA, USA)by liquid nitrogen. The 10 μm slices were fixed in acetone for 5min and washed with PBS. The slices were blocked with 10% blocking serum (Solarbio) at 37℃ for 30min and then incubated with rabbit anti-mouse LC3 antibody (1:200;Cell Signaling Technology) at 4℃ overnight. After washed with PBS, the slices were stained with FITC-conjugated goat anti-rabbit antibody (1:100; Bioss, Beijing, China) for 1h and with DAPI for 10min. The slices were photographed by fluorescence and confocal fluorescence microscope.
Hematoxylin-eosin Staining The lens were removed from the eyeballs of mice and the eyeballs were filleted under cryostat.The slices were stained according to routine methods[17] and photographed under a light microscopy.
Statistical Analysis Kruskal-Wallis was used to test the significance of the clinical score, and two-way ANOVA was used for Western blot and immunofluorescent staining. Data in this study was shown as mean±SD and considered to be significant at P≤0.05. The images in this study were processed by Adobe Photoshop CS6 for aesthetics with the authenticity assured. All experiments were repeated once to ensure the credibility.
RESULTS
Higher Keratitis Severity in C57BL/6 Mice than in BALB/c Mice after A. fumigatus Infection To determine the different susceptibility to A. fumigatus between two types of mice,disease severity was observed with slit lamp. Results showed that disease severity was gradually aggravated in two types of mice at 1 and 3d p.i., and alleviated at 5d p.i. Results revealed that corneal edema and opacity in C57BL/6 mice (Figure 1A) were more severe than that in BALB/c mice (Figure 1D;P<0.01) at 1d p.i. Disease response was increased in corneas of C57BL/6 mice (Figure 1B) compared with BALB/c mice(Figure 1E; P<0.05) at 3d p.i. Corneal edema and opacity in C57BL/6 mice (Figure 1C) were more severe than that in BALB/c mice (Figure 1F; P<0.01) at 5d p.i. Clinical scores was shown in Figure 1G.
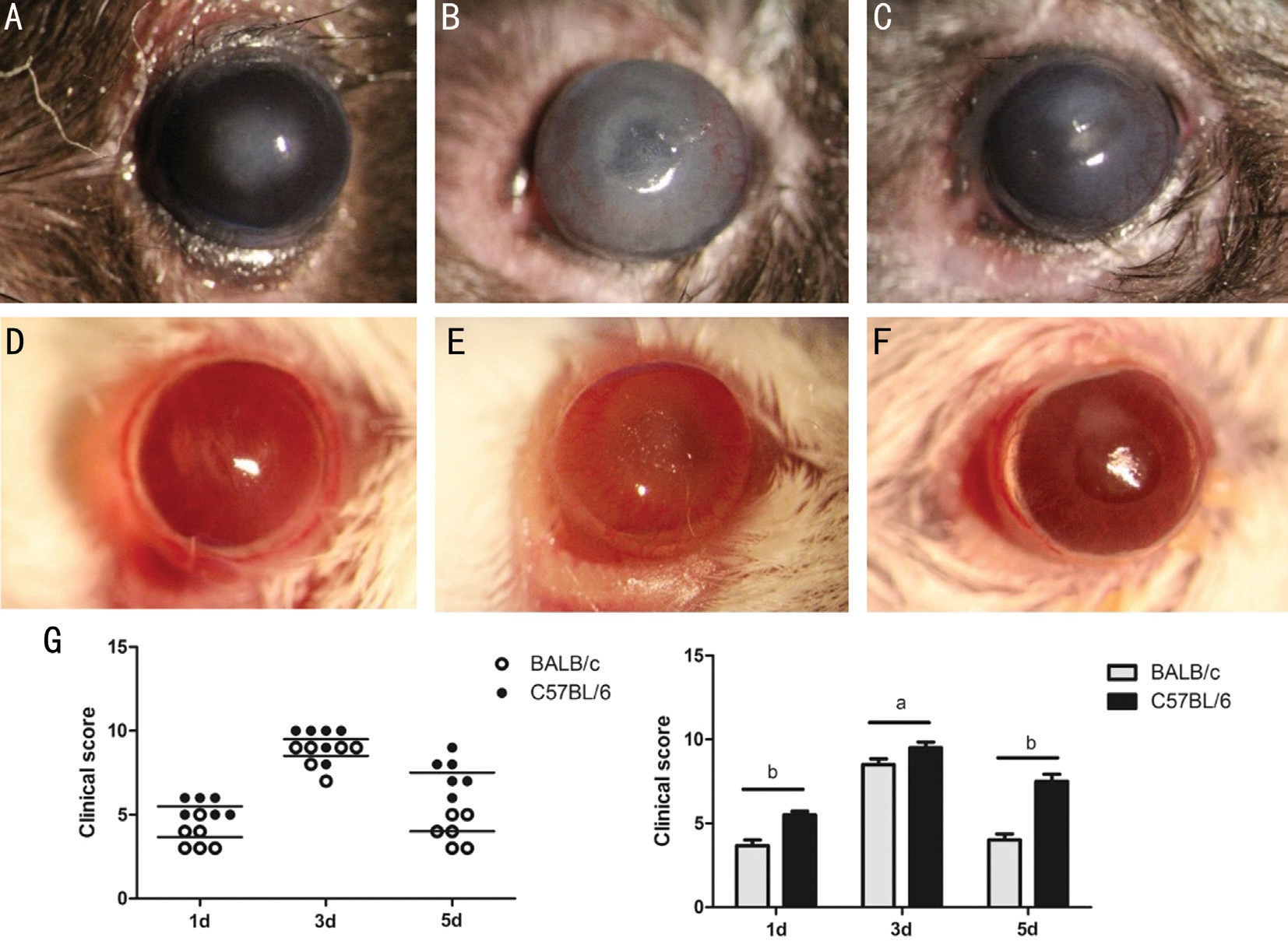
Figure 1 Corneal infection by A. fumigatus in C57BL/6 and BALB/c mice A-C: Infected corneas of C57BL/6 mice at 1, 3 and 5d p.i.; D-F:Infected corneas of BALB/c mice at 1, 3 and 5d p.i.; G: Clinical scores were shown as mean±SD. Each symbol represented an individual mouse.
aP<0.05; bP<0.01.
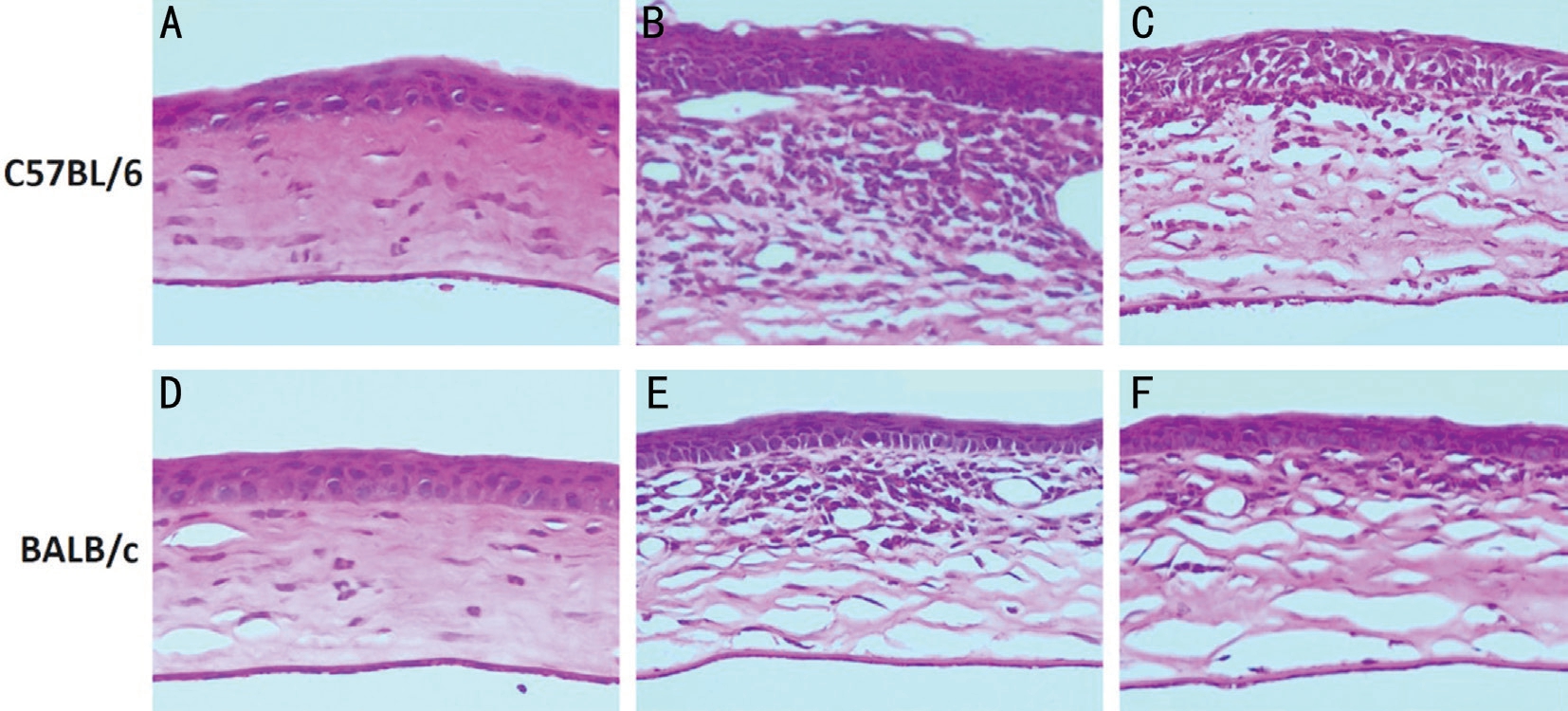
Figure 2 Pathological changes in corneas of C57BL/6 and BALB/c mice after A. fumigatus infection A-C: Pathological changes in corneas of C57BL/6 mice at 1, 3 and 5d p.i.; D-F: Pathological changes in corneas of BALB/c mice at 1d, 3d and 5d p.i. Magnification (A-F): ×200.
Larger Number of Inflammatory Cells and Higher Severity of Ulcer in C57BL/6 Mice Corneas vs BALB/c Mice Corneas H&E Staining was used to observe different pathological changes of corneas between C57BL/6 and BALB/c mice. Results showed that pathological changes of corneas were gradually aggravated in corneas of C57BL/6 and BALB/c mice at 1 and 3d p.i., and alleviated at 5d p.i. The number of inflammatory cells was larger and severity of ulcer was higher in corneas of C57BL/6 mice (Figure 2A) versus BALB/c mice(Figure 2D) at 1d p.i. C57BL/6 mice corneas (Figure 2B)showed worsened pathological changes than BALB/c mice corneas (Figure 2E) at 3d p.i. Pathological changes of corneas were more aggravated in C57BL/6 mice (Figure 2C) than in BALB/c mice (Figure 2F) at 5d p.i., which were consistent with the result of disease severity.
Higher Expression of Autophagy-related Proteins in Corneas of C57BL/6 Mice vs BALB/c Mice after Stimulation with A. fumigatus To determine the expression of autophagy in corneas of C57BL/6 and BALB/c mice after A. fumigatus infection, protein levels of Beclin-1, LC3, LAMP-1 and p62 were detected in normal and infected corneas of mice at 1, 3 and 5d by Western blot. Results showed that the expression of LAMP-1, Beclin-1 and LC3 was gradually elevated in corneas of mice after A. fumigatus infection at 1, 3 and 5d,whereas autophagic adaptor protein p62 was down-regulated.Higher expression of LAMP-1 (Figure 3B; P<0.05; P<0.01;P<0.01), Beclin-1 (Figure 3C; P<0.01; P<0.001; P<0.001),and LC3 (Figure 3E; P<0.001; P<0.001; P<0.001) was shown in C57BL/6 mice corneas than in BALB/c mice corneas at 1,3 and 5d p.i., while the expression of p62 (Figure 3D; P<0.05;P<0.05; P<0.05) was lower in C57BL/6 mice. These results showing that the level of autophagy in corneas of C57BL/6 mice was higher than that in BALB/c mice corneas after A. fumigatus infection.
Higher Accumulation of LC3 Protein in Corneas of C57BL/6 Mice vs BALB/c Mice after A. fumigatus Infection To further determine the expression of autophagy in corneas of BALB/c and C57BL/6 mice p.i., the fluorescence of LC3 was observed by fluorescence microscope and confocal microscope after A. fumigatus infection. Results demonstrated that the fluorescence of LC3 was gradually elevated in C57BL/6 and BALB/c mice corneas at 1, 3 and 5d p.i. The integrated option density (IOD) of LC3 was significantly increased in corneas of C57BL/6 mice (Figure 4B) compared with BALB/c mice(Figure 4F; P<0.05) at 1d p.i. The fluorescence of LC3 in C57BL/6 mice corneas (Figure 4C) was stronger than that in BALB/c mice corneas (Figure 4G; P<0.01) at 3d p.i. The IOD of LC3 was increased in corneas of C57BL/6 mice (Figure 4D)compared with BALB/c mice (Figure 4H; P<0.01) at 5d p.i.,which was in line with the expression of LC3 proteins tested by Western blot. Enlarged images clearly showed that the number of LC3 pots was larger in the cytoplasm of C57BL/6 mice corneas (Figure 4I) versus BALB/c (Figure 4J) mice corneas at 3d p.i. Quantitation of IOD was shown in Figure 4K.
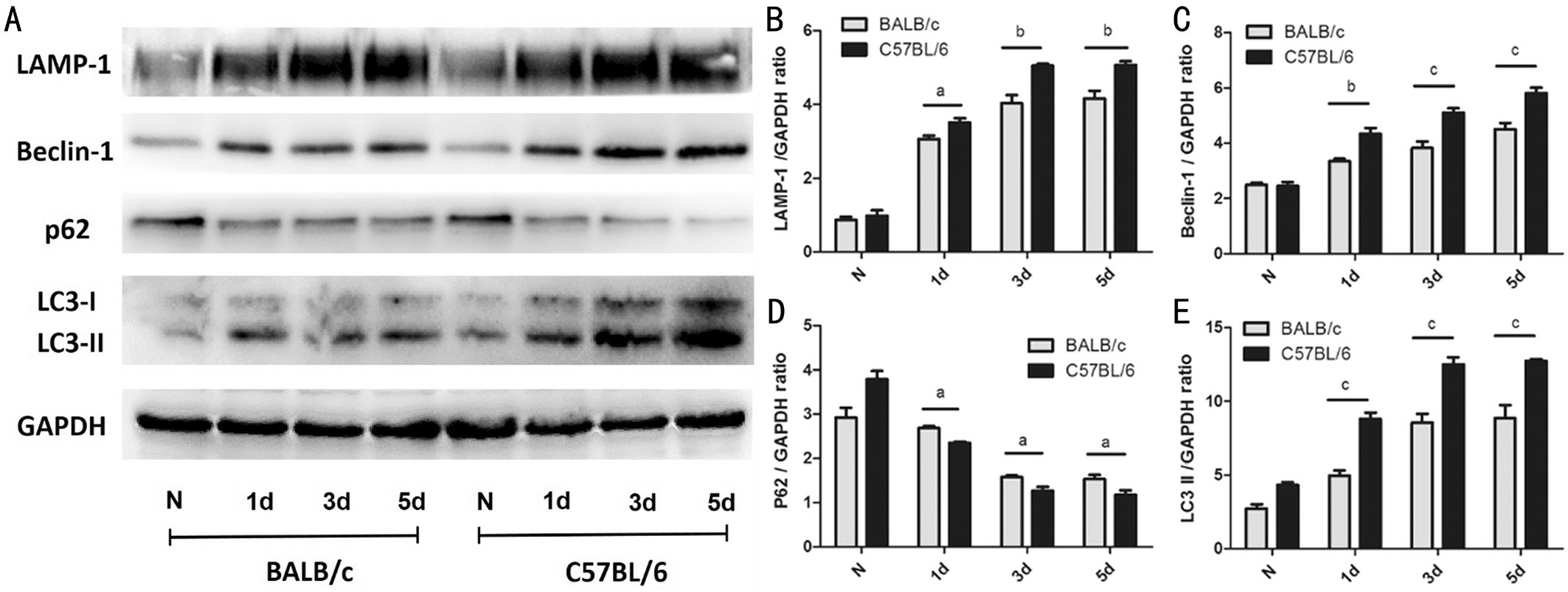
Figure 3 Expression of autophagy-related proteins in corneas of C57BL/6 mice after A. fumigatus infection A: The protein levels of Beclin-1, LC3, LAMP-1 and p62 were detected in normal and infected corneas of mice at 1, 3 and 5d by Western blot; B: The protein level of LAMP-1 in corneas of BALB/c and C57BL/6 mice after 1, 3 and 5d infection; C: The protein level of Beclin-1 in corneas of BALB/c and C57BL/6 mice after 1, 3 and 5d infection; D: The protein level of p62 in normal or infectious corneas of BALB/c and C57BL/6 mice at 1, 3 and 5d p.i.; E: The protein level of LC3 in corneas of BALB/c and C57BL/6 mice after 1, 3 and 5d infection. Quantitation of protein was shown as mean±SD. aP<0.05, bP<0.01, cP<0.001.
DISCUSSION
Animal models are essential to illuminate the pathogenesis of human disease. Therefore, it is crucial to choose suitable experimental murine to study the pathogenesis of different diseases. Some people with corneal trauma can defense against fungal infection, while some people without any obvious cause exhibit a susceptible character to FK. Several studies have reported that C57BL/6 and BALB/c mice have different susceptibility to numerous infectious disease[6,18-20]. Therefore,we used two types of mice to model the population that is susceptible or resistant to FK, in order to explore the different immune processes in corneas after fungal infection. Results in this study revealed that disease severity was gradually increased in C57BL/6 and BALB/c mice at 1, 3d p.i., and decreased at 5d p.i. Corneal edema and opacity in C57BL/6 mice were more severe than that in BALB/c mice at 1, 3 and 5d p.i. To confirm these results, H&E staining was used to observe pathological changes of corneas after A. fumigatus infection. Results showed that pathological changes of corneas were gradually aggravated in corneas of C57BL/6 and BALB/c mice at 1 and 3d p.i., and alleviated at 5d p.i. The number of inflammatory cells was larger and severity of ulcer was higher in C57BL/6 mice than in BALB/c mice at 1, 3, and 5d after stimulation with A. fumigatus, which was consistent with the result of disease severity. These data consistent with a previous study demonstrating that BALB/c mice are classified as resistant murine to FK, whereas C57BL/6 mice exhibit a susceptible character[6].Published reports have shown that bacterial or fungal infection can markedly up-regulated the production of autophagy in human macrophages[14]. Our results showed that the expression of autophagy-related proteins LC3, Beclin-1 and LAMP-1 was up-regulated, and the expression of p62 was decreased in corneas of C57BL/6 and BALB/c mice at 1, 3 and 5d p.i., which suggested that autophagy may play a potential role in corneas after A. fumigatus infection. Additionally,an increasing number of studies have shown that autophagy can result in decreased susceptibility to infection, clear intracellular pathogens and down-regulate the production of pro-inflammatory cytokines[21-22]. However, autophagy,as a key regulator to maintain immune homeostasis, can be significantly induced by pro-inflammatory proteins such as Dectin-1, ROS and HMGB-1, which expression is higher in C57BL/6 mice than in BALB/c mice after infection[13,23]. And previous studies demonstrated that autophagy is positively correlated with inflammation and pathology in inflammatory bowel disease[7,24]. Thus, we next compared the expression of autophagy in corneas of C57BL/6 mice and BALB/c mice to explore the disparate expression between two types of mice.Results showed that higher expression of LAMP-1, Beclin-1,and LC3 was shown in C57BL/6 mice corneas than in BALB/c mice corneas at 1, 3 and 5d p.i., while the expression of p62 was lower in C57BL/6 mice. These results demonstrated that the expression of autophagy was higher in C57BL/6 mice than in BALB/c mice after A. fumigatus infection. Consistent with the results of Western blot, the fluorescence of LC3 was stronger in C57BL/6 mice than in BALB/c mice after A. fumigatus infection and the number of LC3 pots was larger in the cytoplasm of C57BL/6 mice corneas versus BALB/c mice corneas at 3d p.i. These results suggested that autophagy may be positively correlated with keratitis severity and pathological changes in A. fumigatus keratitis. Our results are consistent with previous studies that the production of autophagy is relative to pathological changes in myopathy[25].Our results are also in line with studies that autophagy is rapidly increased and mitigates the renal injury by regulating inflammation factors after LPS infection in acute kidney injury[26]. We speculate that stronger autophagy response may account for the greater susceptibility of C57BL/6 mice to A. fumigatus keratitis. It is also a possibility that increased levels of inflammation and pathology in C57BL/6 mice may result in more production of autophagy to dispose large amounts of intracellular pathogens and injured organelles to maintain the intracellular and immune homeostasis. However,our findings do not directly prove whether autophagy is the main cause for the observed difference between two types of mice with FK and further studies need be down to definite the role and specific mechanism of autophagy in FK.In summary, our study demonstrates that the expression of autophagy in corneas of C57BL/6 mice is higher than that in BALB/c mice after A. fumigatus infection. Autophagy may be positively correlated with keratitis severity and pathological changes, and may play a potential role in corneas after A. fumigatus infection.
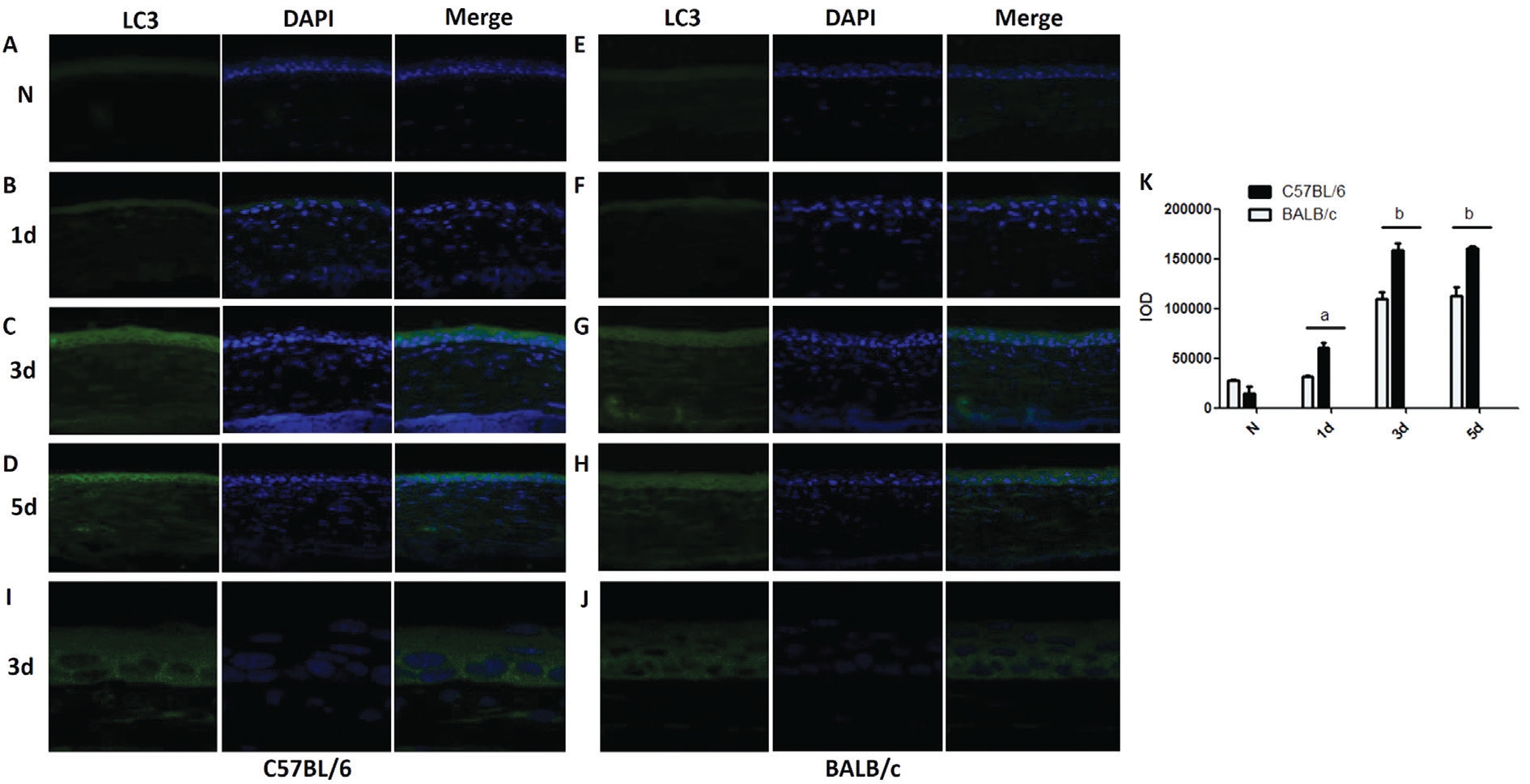
Figure 4 Accumulation of LC3 protein in mice corneas after A. fumigatus infection A-D: The fluorescence of LC3 (green) in normal and infected corneas of C57B/L6/c mice at 1, 3 and 5d p.i.; E-H: The fluorescence of LC3 (green) in normal and infected corneas of BALB/c mice at 1, 3 and 5d p.i.; I: Enlarged images of LC3 pots staining in infected corneas of C57BL/6 mice after 3d stimulation; J: Enlarged images of LC3 pots staining in infected corneas of BALB/c mice after 3d stimulation; K: Quantitation of IOD was shown as mean±SD. aP<0.05; bP<0.01.Magnification (A-H): ×400; Magnification (I-J): ×630.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81470609; No.81500695;No.81700800; No.81870632; No.81800800); Natural Science Foundation of Shandong Province (No.ZR2017BH025;No.ZR2017MH008; No.ZR2013HQ007).
Conflicts of Interest: Li CY, None; Li C, None; Li H, None;Zhao GQ, None; Lin J, None; Wang Q, None; Peng XD,None; Xu Q, None; Zhu GQ, None; Jiang JQ, None.
1 Jiang N, Zhao GQ, Lin J, Hu LT, Che CY, Li C, Wang Q, Xu Q, Peng XD. Indoleamine 2, 3-dioxygenase is involved in the inflammation response of corneal epithelial cells to aspergillus fumigatus infections.PLoS One 2015;10(9):e0137423.
2 Niu YW, Zhao GQ, Li C, Lin J, Jiang N, Che CY, Zhang J, Xu Q. Aspergillus fumigatus increased PAR-2 expression and elevated proinflammatory cytokines expression through the pathway of PAR-2/ERK1/2 in cornea. Invest Ophthalmol Vis Sci 2018;59(1):166-175.
3 Maharana PK, Sharma N, Nagpal R, Jhanji V, Das S, Vajpayee RB.Recent advances in diagnosis and management of mycotic keratitis.Indian J Ophthalmol 2016;64(5):346-357.
4 Li C, McClellan SA, Barrett R, Hazlett LD. Interleukin 17 regulates Mer tyrosine kinase-positive cells in Pseudomonas aeruginosa keratitis.Invest Ophthalmol Vis Sci 2014;55(10):6886-6900.
5 Shi HS, Ebrahim AS, Berger EA. A contrast in pathogenic responses between C57BL/6J and BALB/cJ mice using a model of retinal injury. Am J Pathol 2018;188(12):2717-2728.
6 Zou YL, Zhang HB, Li HX, Chen H, Song WG, Wang YQ. Straindependent production of interleukin-17/interferon-γ and matrix remodeling-associated genes in experimental Candida albicans keratitis.Mol Vis 2012;18:1215-1225.
7 Samie M, Lim J, Verschueren E, Baughman JM, Peng I, Wong A, Kwon Y, Senbabaoglu Y, Hackney JA, Keir M, Mckenzie B, Kirkpatrick DS, van Lookeren Campagne M, Murthy A. Selective autophagy of the adaptor TRIF regulates innate inflammatory signaling. Nat Immunol 2018;19(3):246-254.
8 Feng YC, He D, Yao ZY, Klionsky DJ. The machinery of macroautophagy. Cell Res 2014;24(1):24-41.
9 Yang ZF, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 2010;22(2):124-131.
10 Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008;4(2):151-175.
11 Ponpuak M, Davis AS, Roberts EA, Delgado MA, Dinkins C,Zhao ZJ, Virgin HW 4th, Kyei GB, Johansen T, Vergne I, Deretic V.Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity 2010;32(3):329-341.
12 Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition).Autophagy 2016;12(1):1-222.
13 Öhman T, Teirilä L, Lahesmaa-Korpinen AM, Cypryk W, Veckman V, Saijo S, Wolff H, Hautaniemi S, Nyman TA, Matikainen S. Dectin-1 pathway activates robust autophagy-dependent unconventional protein secretion in human macrophages. J Immunol 2014;192(12):5952-5962.
14 Kyrmizi I, Gresnigt MS, Akoumianaki T, Samonis G, Sidiropoulos P,Boumpas D, Netea MG, van de Veerdonk FL, Kontoyiannis DP, Chamilos G. Corticosteroids block autophagy protein recruitment in Aspergillus fumigatus phagosomes via targeting dectin-1/Syk kinase signaling. J Immunol 2013;191(3):1287-1299.
15 Kanayama M, Inoue M, Danzaki K, Hammer G, He YW, Shinohara ML. Autophagy enhances NFκB activity in specific tissue macrophages by sequestering A20 to boost antifungal immunity. Nat Commun 2015;6:5779.
16 Brothers KM, Kowalski RP, Tian SH, Kinchington PR, Shanks RMQ.Bacteria induce autophagy in a human ocular surface cell line. Exp Eye Res 2018;168:12-18.
17 Jeong JH, Yang DS, Koo JH, Hwang DJ, Cho JY, Kang EB. Effect of resistance exercise on muscle metabolism and autophagy in sIBM. Med Sci Sports Exerc 2017;49(8):1562-1571.
18 De Vooght V, Vanoirbeek JA, Luyts K, Haenen S, Nemery B, Hoet PH. Choice of mouse strain influences the outcome in a mouse model of chemical-induced asthma. PLoS One 2010;5(9):e12581.
19 Jovicic N, Jeftic I, Jovanovic I, Radosavljevic G, Arsenijevic N, Lukic ML, Pejnovic N. Differential immunometabolic phenotype in Th1 and Th2 dominant mouse strains in response to high-fat feeding. PLoS One 2015;10(7):e0134089.
20 Schulte S, Sukhova GK, Libby P. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am J Pathol 2008;172(6):1500-1508.
21 Yang ZF, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol 2010;12(9):814-822.
22 Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008;451(7182):1069-1075.
23 Tang DL, Kang R, Livesey KM, Zeh HJrd, Lotze MT. High mobility group box 1 (HMGB1) activates an autophagic response to oxidative stress. Antioxid Redox Signal 2011;15(8):2185-2195.
24 Boada-Romero E, Serramito-Gómez I, Sacristán MP, Boone DL,Xavier RJ, Pimentel-Muiños FX. The T300A Crohn’s disease risk polymorphism impairs function of the WD40 domain of ATG16L1. Nat Commun 2016;7:11821.
25 McClung JM, McCord TJ, Ryan TE, Schmidt CA, Green TD,Southerland KW, Reinardy JL, Mueller SB, Venkatraman TN, Lascola CD, Keum S, Marchuk DA, Spangenburg EE, Dokun A, Annex BH,Kontos CD. BAG3 (bcl-2-associated athanogene-3) coding variant in mice determines susceptibility to ischemic limb muscle myopathy by directing autophagy. Circulation 2017;136(3):281-296.
26 Li T, Liu Y, Zhao J, Miao S, Xu Y, Liu K, Liu M, Wang G, Xiao X.Aggravation of acute kidney injury by mPGES-2 down regulation is associated with autophagy inhibition and enhanced apoptosis. Sci Rep 2017;7(1):10247.