INTRODUCTION
A s a common blindness-causing disease, the inflammatory response of fungal keratitis (FK) to the pathogen is the major factor resulting in damage to the cornea and leading to loss of visual acuity. In the developing countries and regions mainly depending on agriculture, the incidence rate is significantly higher than that in the developed countries and it mainly damage the young agricultural labors, so finding methods to control this disease is very significant. In many developing countries like China, the main pathogens include Fusarium and Aspergillus[1]. Till now, the basic knowledge of FK remains unclear in many aspects, so exploring the molecular mechanism in FK occurrence and development to find effective targets for treatment is of great importance.Impact of the inflammatory reaction plays an important part in this disease. Macrophages could activate the immune system as the first-line defense cells, including the innate and adaptive immune. Macrophage migration inhibitory factor (MIF), a key regulator of the host immune and inflammation response can inhibit the macrophages migrating randomly and promote their infiltration in the site of inflammation. By enhancing the macrophages’ ability to adhesion and phagocytosis, the invading microorganisms are easy to be cleared. MIF can also regulate the immune response through release of cytokines.Studies have shown that MIF-targeted immunosuppression can play important roles in infectious diseases. In an experimental model of pneumococcal pneumonia of mouse, MIF could reduce survival rate with severe lung pathology, more inflammatory cellular infiltration and greater bacterial load[2]through its interaction with CD74[3]. In the case of Dengue virus (DENV) infection, compared with wild-type mice,MIF-/- mice showed less clinical symptoms and lower viral loads[4]. And studies also proved that MIF could significantly reduce consequences from acute Pseudomonas aeruginosa(P. aeruginosa) keratitis as an immunosuppressive agent[5].To fungal infection, studies found that MIF play an important role in host resistance against Aspergillus fumigatus (A.fumigatus) infection by controlling downstream inflammatory cytokine production, myeloperoxidase (MPO) and Th1/Th17 immunity[6-7]. However, the role of MIF in FK has not yet been investigated. In this article, we examined the expression of MIF in three different models of FK, and then blocked MIF by its specific inhibitor [4-Iodo-6-phenylpyrimidine (4-IPP)]to indentify its impact on the clinical outcome and the release of Th1 cytokine tumor necrosis factor-α (TNF-α) and Th2 cytokine interleukin-6 (IL-6), to preliminary explore the role of MIF in the pathogenesis of A. fumigatus keratitis.
SUBJECTS AND METHODS
Ethical Approval This study were given approval by the Ethics Committee of the Affiliated Hospital of Qingdao University and complied with Declaration of Helsinki in 1995(as revised in Edinburgh 2000). All samples were collected under the patients’ consent. All animals were treated in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Human Samples The formalin-fixed, paraffin-embedded cornea tissues collected from 10 FK patients who underwent penetrating keratoplasty and 6 ocular trauma patients who underwent evisceration were prepared for immunohistochemistry.
Cell Cultivation Telomease-immortalized human corneal epithelial cells (THCEs; provided by Eye Institute and Affiliated Xiamen Eye Center, China) were cultured and stimulated by aliquot of A. fumigatus according to methods used by our team[8].
Cultivation of Aspergillus Fumigatus A. fumigatus strain(CCTCC 93024, China General Microbiological Culture Collection Center, Beijing, China) was cultured in Sabouroud dextrose agar at 30℃ for 5d. Strains of A. fumigatus were inoculated to 200 mL Erlenmeyer flasks containing prepared Sabouraud liquid medium (8 g glucose, 2 g mycopeptone,200 mL dH2O). Flasks were shaking cultured at 37℃ and 120 rpm for 72 to 96h, and then the hypha was collected and disrupted into 20-40 μm pieces (1×108 CFU/mL)[9].
Fungal Keratitis Animal Model Wistar rats, female,weighing 200-400 g were provided by Changzhou Cavens Laboratory Animal Co., LTD. (Jiangsu, China). All animals were checked by slit-lamp and excluded those with corneal disease. One and the same physician anesthetized mice,removed the central corneal epithelium (3-mm diameter) of the left eye, and then applied a 5 μL aliquot of A. fumigatus to the ocular surface and covered the ocular surface with a soft contact lens and sutured the eyelids. In the control groups,after the corneal epithelium was removed, we did not give the hyphae suspension and then cover with soft contacts lens and suture the eyelids. In order to implore the suppression effect of 4-IPP (Sigma-Aldrich, Saint Louis, USA) on the produce of MIF, the left eyes (n=6/group/time) received subconjunctival injection with 5 μL 4-IPP of 5 mol/L one day before infection,and after one day, an additional 50 μL 4-IPP solution of 2.5 mol/L were injected intraperitoneally; controls were similarly injected with Dimethyl sulfoxide (DMSO). The corneas were harvested for real-time polymerase chain reaction(PCR), immunohistochemistry staining and Western blot. The clinical scores were calculated according to different degrees of edema, morphology and area of ulceration.
Immunohistochemistry Paraffin-embedded cornea tissues were cut into serial slices of 3 μm thickness. We also collected cultured THCEs stimulated by fungi or not for the experiment.Immunohistochemical staining was done by Polink-2 plus Polymer HRP Detection System and DAB staining method according to the directions. Rabbit anti-MIF antibody (bs-10242R; 1:100) and other kits were purchased from Beijing ZSGB-BIO Co., Ltd. Cells with brown yellow granules in cytoplasm and cell membrane was counted as positive.
Real-time Polymerase Chain Reaction TaqMan realtime PCR method was used to measure the expression of mRNA according to the manufacturer’s amplification.All the kits were provided by Takara BIO INC., Dalian,China. Primers were synthesized by Shanghai Biological Engineering Technology Engineering Service Co.Nucleotide sequences (5’-3’sequences) of the primers:human β-actin, F-TGGCACCCAGCACAATGAA and R-CTAAGTCATAGTCCGCCTAGAAGCA; human MIF(NM_002415), F-GAACAACTCCACCTTCGCCTA and R-CCGTTTATTTCTCCCCACCA; human IL-6,F-AAGCCAGAGCTGTGCAGATGAGTA and R-TGTCCTGCAGCCACTGGTTC; human TNFα, F-TGCTTGTTCCTCAGCCTCTT and R-CAGAGGGCTGATTAGAGAGAGGT; rat β-actin, F-GGAGATTACTGCCCTGGCTCCTA and R-GACTCATCGTACTCCTGCTTGCTG;MIF, F-CGGGTCTACATCAACTATTACGACA and R-CAGCGGTGCAGGTAAGTGAG; rat IL-6,F-CACAAGTCCGGAGAGGAGAC and R-CAGAATTGCCATTGCACAAC; rat TNFα,F-ACCCTCACACTCAGATCATCTT and R-GGTTGTCTTTGAGATCCATGC.
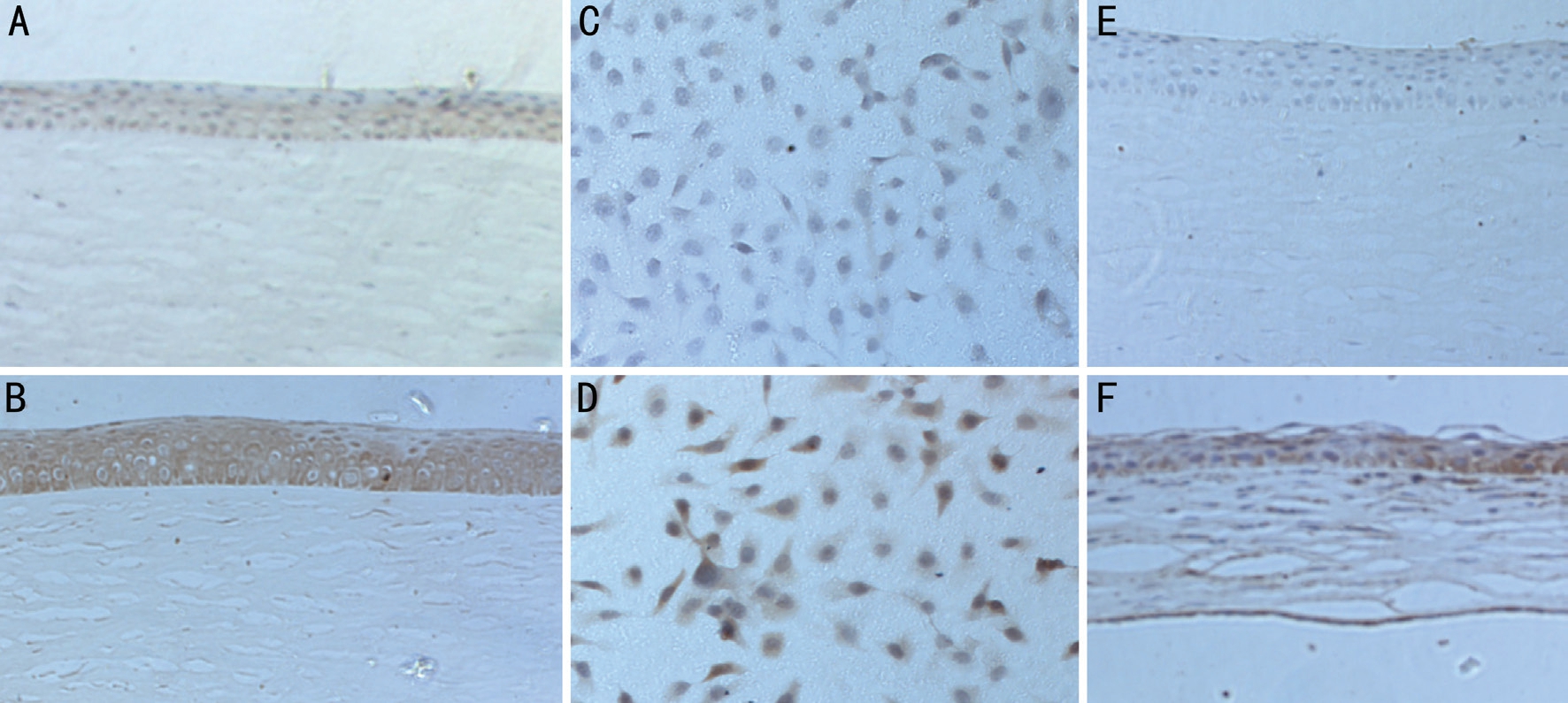
Figure 1 The results of immunohistochemistry A: MIF in the ocular trauma patients; B: MIF in the FK patients; C: MIF in normal THCEs;D: MIF in THCEs stimulated by A. fumigatus hypha suspension; E: MIF in normal rats; F: MIF in the corneas of rats injected by A. fumigatus.
Western Blot Analysis Cornea tissues and cells were collected in RIPA (Solarbio) solution containing 1 mmol/L PMSF(Solarbio, Beijing, China), and then grinded and centrifuged.The protein samples were boiled and then separated on 10%acrylamide sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After these steps, they were transferred onto PVDF membrane (Solarbio, Beijing, China).After blocked and incubated with a polyclonal rabbit antibody to GAPDH (1:3000; Bioss, Beijing, China), and polyclonal rabbit antibody to MIF (1:1000; Bioss, Beijing, China) at 4℃for 16h, membranes were incubated with secondary antibodies(1:6000, goat anti-rabbit, Santa Cruz Biotechnology, CA,USA) at 37℃ for 1h. Then the blots were developed by using chemiluminescence (ECL; Thermo Fisher Scientific, Waltham,MA, USA).
Statistical Analysis All data were presented as mean±standard deviation (SD) and analyzed by SPSS22.0. One-way ANOVA test was used to make comparison among three or more groups, and an unpaired, two-tailed Student’s t-test was used to identify between each two groups. P<0.05 was considered to be statistically significant.
RESULTS
The Staining Results of Immunohistochemistry As is shown in Figure 1, brown staining indicates the expression of MIF in the corneas and THCEs. In ocular trauma patients(Figure 1A), the brown staining was weak, while in patients diagnosed of FK (Figure 1B), the staining could be found in the cytoplasm in the whole epithelium layer nearby the ulcer area and much stronger than the control group. Figure 1C and 1D showed the staining result of cultured THCEs, from which we could find that the distribution and morphology of cells was normal and there was little staining in a few cells in the normal group (Figure 1C), but after stimulated by the hypha suspension of A. fumigatus, the density of the cells decreased and more apoptosis happened, and there were more floating cells in the culture medium, from which we could seen significantly higher proportion and degree of staining in the cytoplasm (Figure 1D). In the corneas of rats (Figure 1E and 1F), there were much stronger brown staining shown in the epithelium layer of the infected cornea (Figure 1F) than that in normal rats (Figure 1E). We could also find more inflammatory cell infiltration in the infected group.
Results of the Stimulation of Telomease-immortalized Human Corneal Epithelial Cells We stimulated cultured cells of THCEs with the hyphae suspension of A. fumigatus to detect whether MIF expressed in them, and then the cells were cultured with MIF inhibitor, 4-IPP to demonstrate its inhibitory effect on MIF. The level of TNF-α and IL-6 mRNA were detected and make a contrast among normal THCEs,hyphae stimulated THCEs and 4-IPP pretreated cells to study the influence of MIF on the expression of TNF-α and IL-6.At first, we wanted to choose a proper concentration of the hypha suspension of A. fumigatus to stimulate the THCEs.As was shown in Figure 2A, the mRNA level of MIF after 24h post-stimulation increased obviously at concentration of 3.5×106 (P<0.01) and 7.5×106 (P<0.05), but there were more cells left in the bottom of 12-well plate at 3.5×106, which one we had chosen to undergo the following tests. In Figure 2B,once fungal stimulation happened, the mRNA level of MIF began increased at 16h (P<0.01), peaked at 24h (P<0.01), then decreased by time. In order to select a suitable concentration of 4-IPP, we used three different concentration: 100, 200 and 400 μmol/L to pretreat the THCEs for 3h and found that 200 μmol/L was effective (Figure 2C). Figure 2D showed that after pretreatment of 4-IPP, the ability of the cells to generate MIF was decreased after the fungal stimulation, but after 24h,the inhibitor could not work effectively. The mRNA level of TNF-α and IL-6 were tested to verify the regulative role of MIF on their expression. From Figure 2E, we could find that fungal stimulation could cause TNF-α increased at 16h(P<0.01) and 24h (P<0.01), IL-6 increased at 12h (P<0.01),16h (P<0.01) and 24h (P<0.01). With use of 4-IPP, both expression of TNF-α and IL-6 declined markedly (Figure 2F).
Results of the Experiments of the Rats After dealt with the hypha suspension of A. fumigatus, the corneas of the rats were slightly edematous at 4h post-infection. As time went on, the lesions of corneas were more and more serious with gradually aggravated inflammation reaction and edema, and the ulceration could be seen at 24h (Figure 3C). At 3d, the clinical grading peaked, with larger ulceration, hyper edema, corneal neovascularization, and some of them even perforated (Figure 3D). At 5d, the degree of inflammation decreased (Figure 3E).With pretreatment of 4-IPP, we could observe a markedly reduced inflammatory response, which proved that suppression of MIF would play a protective role in FK (Figure 3B and 3F).After stimulated by fungi, the relative mRNA and protein level of MIF in collected corneas were detected. There was no significant difference between the control group and normal rats. Results in Figure 4A indicated that MIF mRNA levels were up-regulated in infected rats’ corneas at 1, 3, 5d (P<0.01,P<0.01, P<0.01, respectively).
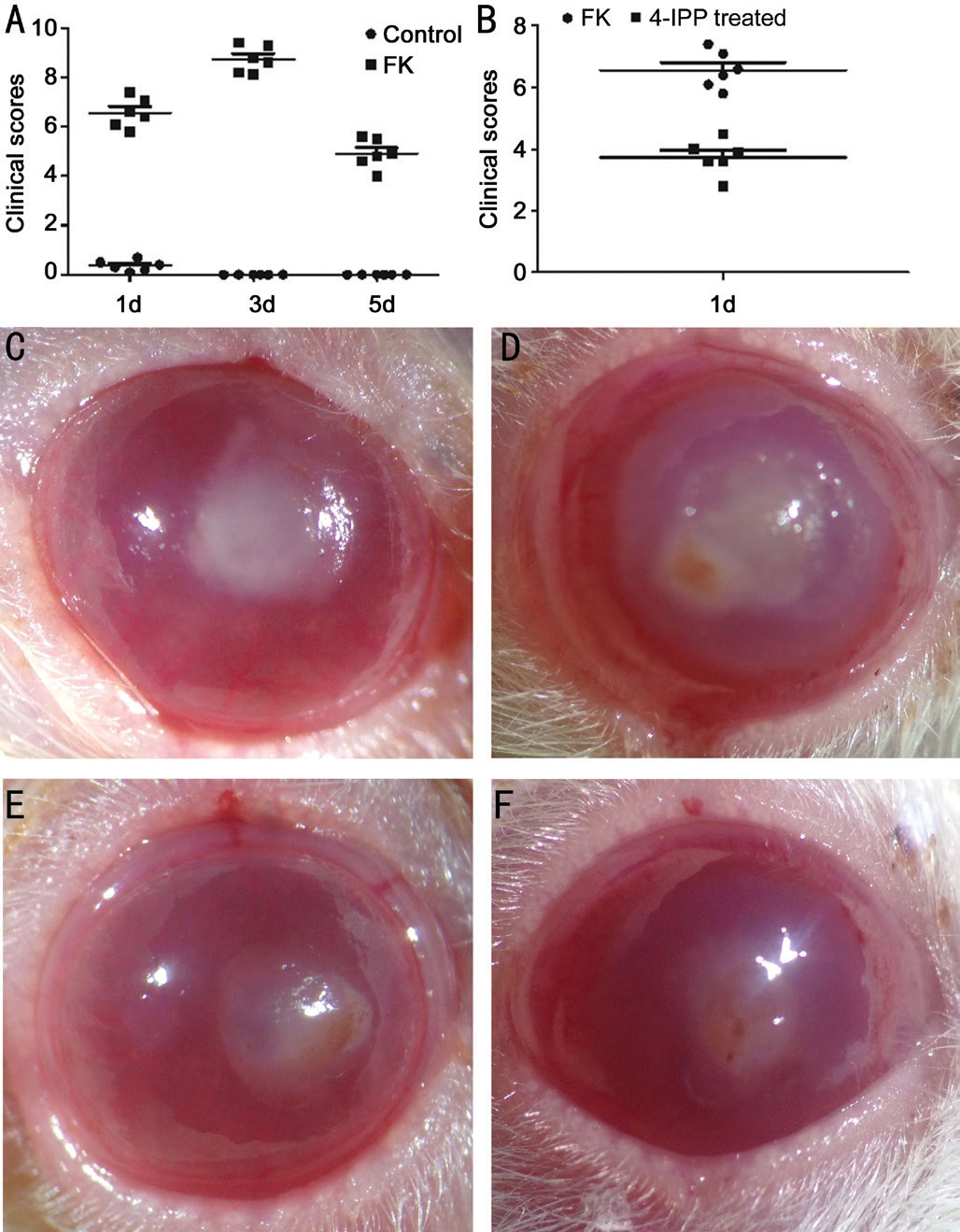
Figure 3 The clinical outcomes of rats after A. fumigatus infection A: The clinical scores of rats after A. fumigatus infection (F=49.74,P<0.01); B: After pretreated with 4-IPP, the clinical scores of the treated group were significantly decreased than the FK group(P<0.01); C-E: The corneas at 1, 3, 5d after fungi infection; F: The cornea with pretreatment of 4-IPP at 1d after fungi infection.

Figure 2 The results of THCEs tests A: The mRNA level of MIF of 24h after stimulation by different concentrations of A. fumigatus hypha suspension; B: The mRNA level of MIF changes at different time after stimulation; C: Stimulated THCEs with three different concentrations of 4-IPP; D: The influence of 4-IPP on the mRNA level of MIF; E: The change of TNF-α mRNA level after fungal stimulation with or without 4-IPP treatment; F: The change of IL-6 mRNA level after fungal stimulation with or without 4-IPP treatment. aP<0.05; bP<0.01.
MIF protein was examined by Western blot, from which we could see an obviously elevated MIF level in infected rats’ corneas at 1d (Figure 4C). And there was low protein level detected in the normal corneas, in keeping with the staining results of immunohistochemistry.After treated with 4-IPP, compared with the infection group and DMSO control group, the level of MIF gene was significantly lower (P<0.01; Figure 4B). The protein level shown in Figure 4D also indicated that MIF protein was suppressed by 4-IPP.
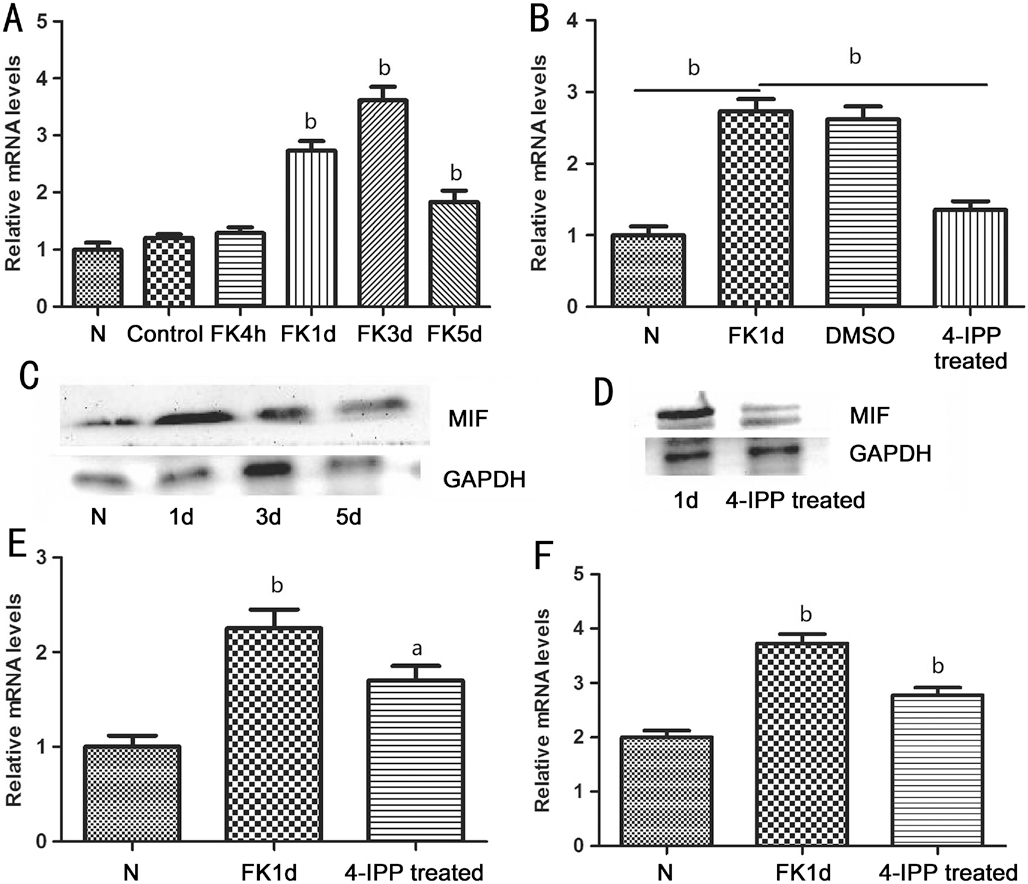
Figure 4 The results of the experiments of the rats A: Changes of the relative MIF mRNA levels after stimulated by fungi; B: The influence of 4-IPP on the MIF mRNA level; C: The changes of MIF protein levels; D: The influence of 4-IPP on the MIF protein levels;E and F: The influence of 4-IPP on the TNF-α and IL-6 mRNA level after fungal stimulation. aP<0.05; bP<0.01.
We also examined the expression of inflammatory cytokines:TNF-α and IL-6 with or without pretreatment of 4-IPP. From Figure 4E, we could confirm that at 1d after injection, TNF-α increased significantly (P<0.01), and 4-IPP could diminish its expression (P<0.05). IL-6 had a similar result with TNF-α(P<0.01, P<0.01).
DISCUSSION
Macrophages play an important role in the defense system which could response quickly on injection. There are little macrophages in the normal cornea. While infected, a large number of macrophages aggregate in the central cornea and the limbus, which may cause an excessive immune response.Activated macrophages played a critical role in regulating immune response of corneas infected by F. solani[10]. After eliminated macrophage by repeated subconjunctival injection of Cl2MDP-LIP in BALB/c mice with FK, the production of Th1 and Th2 cytokines was cut down, which showed that local existence of macrophages may help produce the proper immune response and control the degree of FK[11]. Macrophage MIF can inhibit the random macrophage migrating. High level of MIF expression on the macrophage help sustaining the innate immune responses of neonates[12]. In macrophages,there are a lot of MIF synthesized in advance. When the infection happens, MIF protein release rapidly and play a direct role in pro-inflammation by activating the expression of some cytokines such as TNF-α, IL-6, IL-8 and matrix metalloproteases (MMPS). After inhibition of MIF, the release of IL-1α, IL-1β, and IL-18 was regulated via activation of the NLRP3 inflammasome, while not by impacting on the transcription or translation of these cytokines[13].
Studies have found that in acute or chronic lung infection and intervertebral disc degeneration, MIF could promote inflammatory reaction by MIF binding with CD74 receptors on macrophages and activating a series of downstream cytokines,release of nitric oxide (NO) and prostaglandin E2 (PGE2) and expression of MMPs[3,14]. Beyond macrophages, MIF also play different roles on the other immune cells, like neutrophils,eosinophils[15] and monocytes[16]. Blocking MIF could decrease their accumulation and function and regulate the immune response. MIF is also involved in immune response in Alzheimer’s disease (AD), and glucose modified and oxidised MIF could be a molecular link between hyperglycaemia and the dysregulation of the innate immune system in AD[17]. There are already some studies about the role of MIF in cornea.Overexpression of MIF in the UV-induced corneal injury could shorten the recovery period[18]. In bacterial P. aeruginosa keratitis, MIF could promote the proliferation of bacteria and aggravate inflammatory reaction, suggested that we could consider inhibition of MIF as a new direction to treat P.aeruginosa keratitis[5]. Zaidi et al[19] further found that blocking CD74, the specific receptor of MIF, was beneficial to reduce inflammatory response of P. aeruginosa keratitis. All the above studies have showed that MIF could play different roles in different types of corneal injury. A. fumigatus is an opportunistic pathogen which could infect many organs and systems throughout our body, and MIF has been proved to take an important part in the regulation of innate and adaptive immune response in A. fumigatus infection, for it could regulate the expression of many cytokines. In our study, we found that in patients diagnosed of FK, there was much stronger expression of MIF than control group. In cultured THCEs stimulated by A. fumigatus, there were more expression of MIF, and after pretreated with MIF specific inhibitor (4-IPP), MIF was reduced while production of TNF-α and IL-6 was decreased markedly. In rats with A. fumigatus keratitis, MIF increased,and blocked it could reduce degree of inflammation and release of TNF-α and IL-6 in accordance with those in THCEs.After MIF inhibited, there was lower NF-κB activation and enhanced Th2 responses, potentially causing the reduction of cytokines[20]. Some studies found that the deficiency of MIF in A. fumigatus infection may be harmful, which was contrary to our findings. In hematogenously disseminated aspergillosis,MIF contributed to host resistance against progressive invasive fungi by regulating downstream pro-inflammatory versus antiinflammatory cytokine production[6]. And in lethal or sublethal systemic A. fumigatus infections in spleen and other tissues,inhibition of MIF could cause impaired fungal clearance,MPO, ROS and Th1/Th17 immunity, which identified MIF as a resistance factor of anti-fungal defense[7]. The adverse effect between their and our reports was possibly caused by the difference in the organization and type of infection, for cornea is a unique immune privilege site.The mechanism of MIF in fungal corneal infections has not been reported till now. In summary, our data presented here indicated that MIF was significantly elevated in the cornea after the stimulation of A. fumigatus in both human and rats,which could effectively be inhibited by 4-IPP. Inhibition of MIF decreased inflammatory response of the infected rats and expression of TNF-α and IL-6. These data suggest that MIF may play a proinflammatory role during A. fumigatus keratitis.Deficiency of MIF shows a protective role in our study,which further verified the complex roles of MIF in infectious diseases. We need to study MIF on a deeper level and explore its possibility as a possible therapeutic target for FK further.
ACKNOWLEDGEMENTS
Foundations: Supported by National Natural Science Foundation of China (No.81470609; No.81870632); Youth Project of National Natural Science Foundation of China(No.81500695; No.81700800; No.81800800); Natural Science Foundation of Shandong Province (No.ZR2017MH008); Youth Project of Natural Science Foundation of Shandong Province(No.ZR2013HQ007); Doctor Project of Natural Science Foundation of Shandong Province (No.ZR2017BH025)
Conflicts of Interest: Xu Q, None; Hu LT, None; Wang Q,None; Lin J, None; Jiang N, None; Li C, None; Zhao GQ,None.
1 Peng XD, Zhao GQ, Lin J, Jiang N, Xu Q, Zhu CC, Qu JQ, Cong L,Li H. Fungus induces the release of IL-8 in human corneal epithelial cells, via Dectin-1-mediated protein kinase C pathways. Int J Ophthalmol 2015;8(3):441-447.
2 Weiser JN, Roche AM, Hergott CB, LaRose MI, Connolly T, Jorgensen WL, Leng L, Bucala R, Das R. Macrophage migration inhibitory factor is detrimental in pneumococcal pneumonia and a target for therapeutic immunomodulation. J Infect Dis 2015;212(10):1677-1682.
3 Takahashi K, Koga K, Linge HM, Zhang YZ, Lin XC, Metz CN, Al-Abed Y, Ojamaa K, Miller EJ. Macrophage CD74 contributes to MIFinduced pulmonary inflammation. Respir Res 2009;10:33.
4 Chuang YC, Chen HR, Yeh TM. Pathogenic roles of macrophage migration inhibitory factor during dengue virus infection. Mediators Inflamm 2015;2015:547094.
5 Gadjeva M, Nagashima J, Zaidi T, Mitchell RA, Pier GB. Inhibition of macrophage migration inhibitory factor ameliorates ocular Pseudomonas aeruginosa-induced keratitis. PLoS Pathog 2010;6(3):e1000826.
6 Stojanovic I, Mirkov I, Kataranovski M, Glamoclija J, Stosic-Grujicic S.A role for macrophage migration inhibitory factor in protective immunity against Aspergillus fumigatus. Immunobiology 2011;216(9):1018-1027.
7 Mirkov I, Belij S, Kataranovski M, Zolotarevski L, Glamoclija J,Stojanovic I, Stosic-Grujicic S. The relevance of the migration inhibitory factor (MIF) for peripheral tissue response in murine sublethal systemic Aspergillus fumigatus infection. Med Mycol 2012;50(5):476-487.
8 Zhao GQ, Qiu XY, Lin J, Li Q, Hu LT, Wang Q, Li H. Co-regulation of Dectin-1 and TLR2 in inflammatory response of human corneal epithelial cells induced by Aspergillus fumigates. Int J Ophthalmol 2016;9(2):185-190.
9 Zhang J, Zhao GQ, Qu J, Che CY, Lin J, Jiang N, Zhao H, Wang XJ.Expression of S100B during the innate immune of corneal epithelium against fungi invasion. Int J Ophthalmol 2016;9(2):191-197.
10 Hu JZ, Hu YF, Chen SK, Dong CH, Zhang JJ, Li YL, Yang J, Han XL,Zhu XJ, Xu GX. Role of activated macrophages in experimental Fusarium solani keratitis. Exp Eye Res 2014;129:57-65.
11 Hu JZ, Wang Y, Xie LX. Potential role of macrophages in experimental
keratomycosis. Invest Ophthalmol Vis Sci 2009;50(5):2087-2094.
12 Roger T, Schneider A, Weier M, Sweep FCGJ, Le Roy D, Bernhagen J,Calandra T, Giannoni E. High expression levels of macrophage migration
inhibitory factor sustain the innate immune responses of neonates. Proc Natl Acad Sci USA 2016;113(8):E997-E1005.
13 Lang TL, Lee JPW, Elgass K, et al. Macrophage migration inhibitory factor is required for NLRP3 inflammasome activation. Nat Commun 2018;9(1):2223.
14 Xiong CJ, Huang B, Zhou Y, Cun YP, Liu LT, Wang J, Li CQ, Pan Y,Wang H. Macrophage migration inhibitory factor inhibits the migration of cartilage end plate-derived stem cells by reacting with CD74. PLoS One 2012;7(8):e43984.
15 de Souza H, Tortori C, Lintomen L, et al. Macrophage migration inhibitory factor promotes eosinophil accumulation and tissue remodeling in eosinophilic esophagitis. Mucosal Immunol 2015;8(5):1154-1165.
16 Müller II, Chatterjee M, Schneider M, Borst O, Seizer P, Schönberger T, Vogel S, Müller KA, Geisler T, Lang F, Langer H, Gawaz M. Gremlin-1 inhibits macrophage migration inhibitory factor-dependent monocyte function and survival. Int J Cardiol 2014;176(3):923-929.
17 Kassaar O, Pereira Morais M, Xu SY, et al. Macrophage Migration Inhibitory Factor is subjected to glucose modification and oxidation in Alzheimer’s Disease. Sci Rep 2017;7:42874.
18 Kitaichi N, Shimizu T, Yoshida K, et al. Macrophage migration inhibitory factor ameliorates UV-induced photokeratitis in mice. Exp Eye Res 2008;86(6):929-935.
19 Zaidi T, Reidy T, D’Ortona S, Fichorova R, Pier G, Gadjeva M. CD74 deficiency ameliorates Pseudomonas aeruginosa-induced ocular infection.Sci Rep 2011;1:58.
20 Damle SR, Martin RK, Cross JV, Conrad DH. Macrophage migration inhibitory factor deficiency enhances immune response to Nippostrongylus brasiliensis. Mucosal Immunol 2017;10(1):205-214.