INTRODUCTION
V arious infectious diseases as well as trauma often lead to corneal opacification and vision impairment[1].However, there is a severe shortage of donor corneas worldwide.Comparatively, scarcity of allografting donor tissue has spurred the continued advancement in corneal tissue engineering.During development, constructing appropriate biomaterial scaffolds and seeding them with cells has become a main focus[2]. Ideally, these scaffolds allow cell survival and differentiation[3-5]. Previous studies have documented that biological and synthetic materials such as amniotic membrane,fibrin, electrospun nanofibres and plastic compression could serve as scaffolds for epithelial cell expansion[6-8]. Furthermore,the nanofibrous scaffolds have been shown to promote limbal stem cells to differentiate into mature corneal epithelial cells[6].However, such scaffolds have limited clinical applications due to limited tissue availability, progressive biodegradability,exogenous contamination and potential graft rejection.Whereas decellularized corneal scaffolds, originated from endogenous ocular tissue, have gained widespread attention as a substitute for fabricating biomaterial substrates by preserving the native corneal structure[9-10].
Although human corneal stroma is the most appropriate scaffolding for cornea tissue-engineering, its source is scarce.With the advent of a new generation of refractive surgery,the femtosecond laser has gained favorable reception[11].Until now, over 500 000 small incision lenticule extraction(SMILE) procedures have been performed worldwide[12].Thus, an ample amount of lenticule tissue is available for other clinical scenarios. Intrastromal lenticule removal from the SMILE procedure for myopic correction and its implantation in allogeneic subjects with hypermetropia and keratoconus has been investigated with encouraging preliminary results[13-16].Recently, Yam et al[17] have reported that decellularized human stromal refractive lenticules could promote corneal stromal fibroblast growth in vitro. Additionally, SMILE-derived lenticules have been used to treat corneal microperforation and lamellar corneal defects[18-19]. Similarly, Yin et al[20] used SMILE-derived decellularized lenticules with fibrin glue as a scaffold for rabbit cornea implant, and found that the grafts presented re-epithelialization. Hence, decellularized lenticule scaffolds possess great therapeutic potential in generating corneal tissue for transplantation.
Mesenchymal stem cells (MSCs) are adult nonhematopoietic precursor cells that reside primarily in the bone marrow.These cells can be extensively expanded in vitro and are able to differentiate into multiple lineages such as bone, cartilage,and adipose[21-22]. The pluripotent nature of MSCs makes them ideal alternative sources for tissue engineering and regenerative medicine. Studies have revealed that MSCs are capable of differentiating into corneal epithelial-like cells and expressing cytokeratin 3 (CK3), a marker of mature corneal epithelium[23-24]. Moreover, MSCs derived from embryonic stem cells (ESCs) have been shown robust proliferation and multipotent differentiation[25]. Recently, Alio del Barrio et al[26] reported that human adipose-derived MSCs seeded on acellular human corneal matrix sheets could differentiate into functional keratocytes. Consequently, in this study, we investigated whether MSCs derived from human ESCs after being seeded on the decellularized SMILE-derived lenticules can differentiate into corneal epithelial cells.
SUBJECTS AND METHODS
Ethical Approval Permission to use human corneal tissue
was granted by the Ethics Committee of Xiangya Hospital(No.201512539). Patients provided the written informed consent in accordance with the Declaration of Helsinki.
Culture and Characterization of Mesenchymal Stem Cells from Embryonic Stem Cells The purchased H1 human
ESCs (WA01, Wicell, USA) were cultured on matrigel-coated plates with daily change of mTeSR1 medium (Stem Cell Technologies, Canada) for several passages. These cells were subsequently trypsinized, harvested, and expanded in 25 cm2 flasks (Corning, USA), in low-glucose Dulbecco’s modified eagle’s medium (L-DMEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA) and 100 U/mL penicillin-streptomycin (Gibco, USA) at a concentration of 5×106 cells per millilitre. Cells were maintained at 37℃ in a humidified 5% CO2 incubator. Medium was changed every 3d. The culture was continually passaged when reaching about 90% confluence to generate MSCs with fibroblast-like morphology.For the analysis of cell surface markers, human ESC-derived MSCs at passage 3 were evaluated using flow cytometry. These cells were harvested in single-cell suspension, and then stained with monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE): CD45-PE,CD34-FITC, CD73-PE, and CD105-PE (all four antibodies from eBioscience, USA). At least 10 000 events were acquired using the FC500MCL flow cytometer (Beckman Coulter, Brea,CA, USA).
Removal of Small Incision Lenticule Extraction-Derived Lenticules Ten myopic patients (20 eyes, 3 males and 7 females) who underwent the SMILE procedure using the VisuMax femtosecond laser (Carl Zeiss Meditec, Jena,Germany) on the same day were enrolled in this study. The patients had a mean age of 22.4±3.7y (range: 18 to 29y),with a mean spherical equivalent refraction of -5.48±1.17 diopter (D). The single same surgeon (Wen D) performed all the operations. In brief, the femtosecond laser created an intrastromal lenticule by photoablative incisions, both anterior and posterior interfaces were then dissected with a flap separator. Finally, the lenticule was extracted manually through a 2-mm incision. Parameters during the SMILE procedure were as follows: repetition rate 500 kHz, pulse energy 120 nJ,lenticule side-cut angle 90 degrees, lenticule diameter 6.0 mm,lenticule thickness 80 to 120 μm.
Decellularization of Small Incision Lenticule Extraction-Derived Lenticules The extracted stromal lenticules were divided into two groups of 10 lenticules each. One group was set aside in McCarey-Kaufman medium storage(fresh lenticules in MSCs-A group), and the other group was decellularized according to the previous protocol(decellularized lenticules in MSCs-B group)[27]. Briefly, the acellular lenticules were prepared in the process of enzymatic digestion, freezing and thawing and lyophilization, and their histological structure were observed. The samples were digested with 0.25% trypsin (Gibco, USA) on an orbital shaker(500 rpm) at 37℃ for 30min. Later, the sheets were thoroughly washed with three-fold-distilled water with continuous shaking at room temperature. The sheets were frozen in liquid nitrogen of -196℃ for 15min and then thawed quickly at 37℃. The procedures of freezing and thawing were repeated four times.To remove DNA, the sheets were treated with DNAase (Roche,Switzerland) at 37℃ for 1h, followed by 3 washes with threefold-distilled water for 30min with continuous shaking at room temperature. Subsequently, the sheets were pre-frozen in a -80℃ refrigerator for 4h, and then dried in vacuum in a lyophilizer for 16h. Finally, the lyophilized acellular lenticules were sealed into a sterile plastic envelope, sterilized by Cobalt-60 and then preserved for later use. The cell removal was verified by 4’,6-diamidino-2-phenylindole (DAPI, Sigma)staining of serial sections of the sheets.
Table 1 List of primer sequences for qRT-PCR
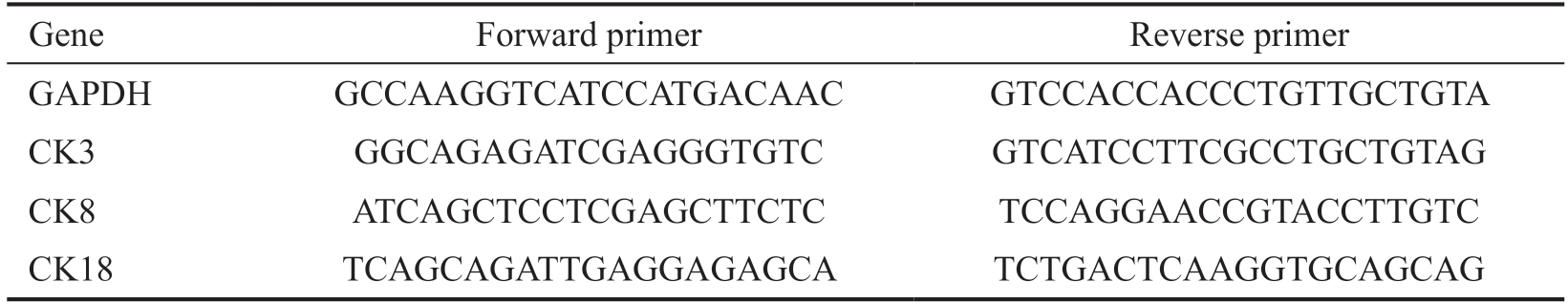
Gene Forward primer Reverse primer GAPDH GCCAAGGTCATCCATGACAAC GTCCACCACCCTGTTGCTGTA CK3 GGCAGAGATCGAGGGTGTC GTCATCCTTCGCCTGCTGTAG CK8 ATCAGCTCCTCGAGCTTCTC TCCAGGAACCGTACCTTGTC CK18 TCAGCAGATTGAGGAGAGCA TCTGACTCAAGGTGCAGCAG
MSCs Seeded on Small Incision Lenticule Extraction-Derived Lenticules The MSCs at passage 5 were harvested and resuspended with L-DMEM containing 10% FBS, 100 IU/mL penicillin-streptomycin, and then incubated with 0.4%1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI, Beyotime, China) at 37℃ with 5% CO2 for 20min, to fluorescently label cellular membranes. The lenticules in MSCs-A and MSCs-B groups were soaked in L-DMEM supplemented with 10% FBS at 37℃ for 48h to recover their tenacity before seeding. These lenticules were subsequently washed twice with phosphate buffered saline(PBS) by centrifugation at 200×g for 5min, and then a 250 μL suspension of MSCs was gently seeded on the surface, in the 48-well plate (Corning, USA) with a concentration of 3×104 cells per well. MSCs without lenticules were cultured as a control for comparative study. Culture medium was changed every 2d.All cultures were incubated at 37℃ in 5% CO2 atmosphere.
Cell Proliferation Analysis Within 24h after being seeded,most DiI labeled MSCs attached on the lenticules in MSCs-A and MSCs-B groups started to spread. Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8; Dojindo,Japan) according to the manufacture’s protocol. Briefly, 25 μL of CCK-8 solution was added to each well of three groups(MSCs, MSCs-A, and MSCs-B groups) and incubated for 4h at 37℃, and in 5% CO2 atmosphere, after which the optical density (OD) at 450 nm was measured by a microplate reader.The wells without allocating cells served as the control group.
Immunofluorescence For immunofluorescent staining, the spreading MSCs on the surface of lenticules in MSCs-A and MSCs-B groups at day 10 were harvested and then fixed with 4% paraformaldehyde for 15min at room temperature, washed twice, then permeabilized with 0.5% Triton X-100 in PBS for 5min. Subsequently, the cells were incubated with the primary antibody (CK3, 1:1000, Abcam, UK) containing 1%bovine serum albumin (BSA) at 1h at room temperature. After washing three times with PBS to remove unbound antibody,the secondary antibody (goat anti-mouse Alexa Fluor 488,1:1000, Invitrogen, USA) was applied for detection at room temperature for 2h on an orbital shaker. Later, the cells were thoroughly washed with PBS, and finally, DAPI was used for nuclear staining. The cells were examined by using a confocal fluorescence microscope (Zeiss, Germany).
Analysis of mRNA Expression by Quantitative Reverse Transcription Polymerase Chain Reaction To further assess corneal epithelial marker expression, mRNA levels of CK3, CK8, and CK18 were selected to compare. By day 10 after initial seeding, total RNA was isolated from MSCs in each group (MSCs, MSCs-A, and MSCs-B) using TRIZOL(Invitrogen, USA) following the manufacturer’s protocol.About 1 μg of total RNA was reverse-transcribed in a 10 μL reaction solution to prepare cDNA. For quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis,the SYBR Green Master Mix (TaKaRa, Japan) was used with cDNA samples and primers to determine the target mRNA transcripts. The sequences of the primers were listed in Table 1. The relative target gene mRNA expression levels were analyzed using the formula 2-ΔΔCt in reference to the expression level of glyceraldehype-3-phosphate dehydrogenase (GAPDH) gene.All assays were performed in triplicate for each primer set.
Statistical Analysis Analysis of variance (ANOVA) with Bonferroni’s correction was used for comparisons between multiple groups. Analyses were done using SPSS software(IBM Corp., Armonk, NY, USA) and GraphPad Prism software(GraphPad, Inc., San Diego, CA, USA) was used. Data are presented as the mean±SEM of at least three experiments. P<0.05 were considered statistically significant.
RESULTS
Characterization of Mesenchymal Stem Cells from Human Embryonic Stem Cells The human ESCs exhibited a uniform undifferentiated phenotype as spheres in suspension when cultured on matrigel-coated plates (Figure 1A). While the human ESC-derived MSCs were cultured using the plastic adherence method, most of cells presented a characteristic spindle shape with a fibroblast-like morphology after 3 subculture passages (Figure 1B). To further assess the phenotype of the cells, cell surface markers CD34, CD45,CD105, and CD73 were analyzed by flow cytometry. As shown in Figure 1, greater than or equal to 95% of the MSCs derived from human ESCs at passage 3 were positive for CD105 and CD73, which are the MSC-associated specific markers, and were negative for CD34 and CD45, which are the hematopoietic markers.

Figure 1 Characterization of human ESC-derived MSCs A-B: Human ESCs grew as a uniform undifferentiated colony and MSCs differentiated into the adherent cell population with a fibroblastoid, spindle-shaped morphology (scale bar=200 μm); C-F: Flow cytometry analysis of human ESC-derived MSCs at passage 3. The data showed that 99.84% cells were negative for CD34, 99.8% cells were negative for CD45, 97% cells were positive for CD105, and 95% cells were positive for CD73.
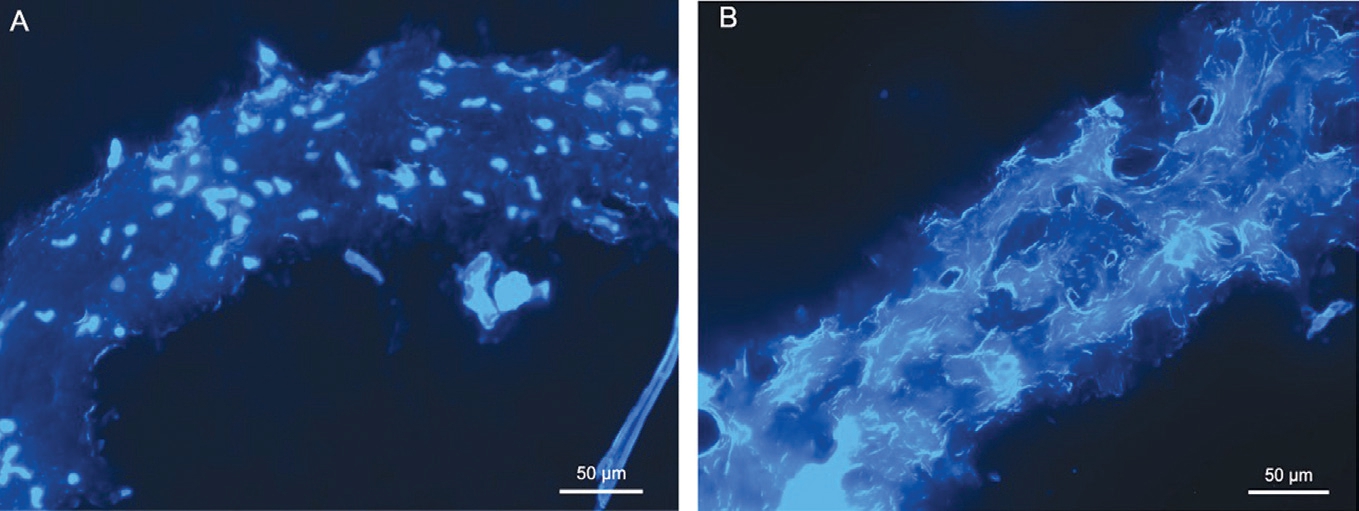
Figure 2 Histological changes of decellularized lenticule A: Fluorescence image of a fresh lenticule stained with DAPI; B: Fluorescence image of a decellularized lenticule stained with DAPI, an absence of cellular nuclei and empty cell space were found inside the lenticule. The scale bar is 50 μm.
Structure of Decellularized Lenticules The removal of cells was confirmed by staining with DAPI for nucleic acid.Many keratocyte nuclei were observed in the fresh lenticule(Figure 2A). When compared to control lenticules, no cellular nuclei were shown in the decellularized lenticule (Figure 2B). Furthermore, the arrangement of the collagens in the decellularized lenticule was meshy and slightly loosened.Hence, the decellularization protocol carried out in this study completely removed the cells and retained the overall tissue matrix structure.
Mesenchymal Stem Cells Proliferation after Seeding on Lenticules As regards cell proliferation, the CCK-8 assay(Figure 3) showed that the proliferation capacity of MSCs-A and MSCs-B groups were significantly higher than that in the control group (P=0.02 for MSCs-A, P=0.001 for MSCs-B,respectively). There was no significant difference between MSCs-A and MSCs-B groups (P=0.083). These results suggested that both fresh and decellularized lenticules play an important role in promoting MSCs cell proliferation.
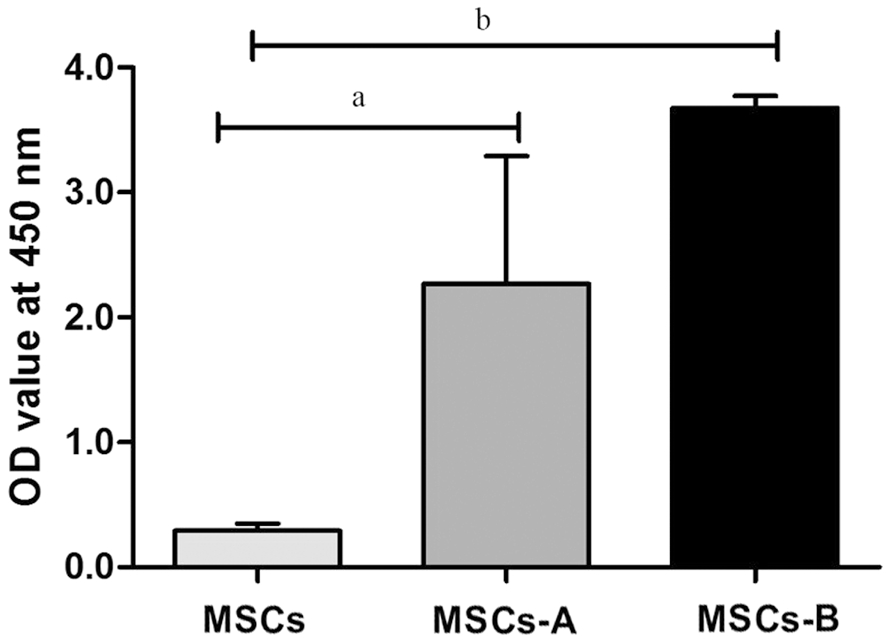
Figure 3 Comparison of the proliferative activity of MSCs seeded on lenticules in MSCs-A and MSCs-B at 24h Cell proliferation was analyzed using CCK-8. OD: Optical density. The data represent the mean±SEM from three experiments (aP<0.05, bP<0.01, cP<0.001).

Figure 4 Confocal microscopic images of immunofluorescence at day 10 after seeding MSCs A: DAPI labeled cell nuclei; B: DiI labeled cellular membranes; C: CK3 labeled corneal epithelial cells; D: Merge: triple staining of CK3 (green), Dil (red), and DAPI (blue); E: CK3 expression was detected in the MSCs-A group; F: CK3 expression was detected in the MSCs-B group, the scale bar is 100 and 50 μm.
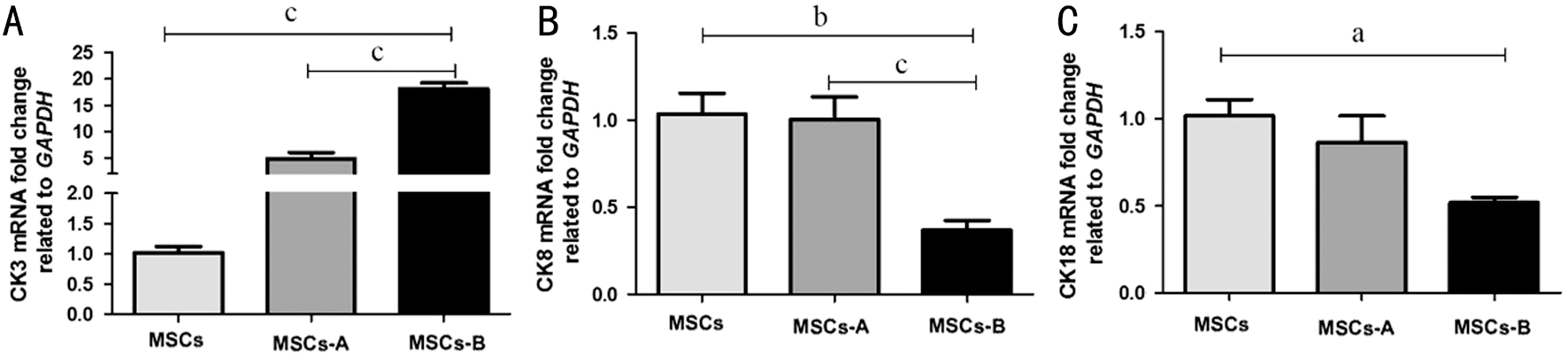
Figure 5 Expression of CK3, CK8, and CK18 in the seeded cells in each sample The expression level was normalized against GAPDH expression. The data represent the mean±SEM from three experiments (aP<0.05, bP<0.01, cP<0.001).
Immunofluorescent Staining of Differentiated Mesenchymal Stem Cells To confirm these differentiated MSCs cells,we used DAPI for nuclear staining (Figure 4A), DiI labeled the seeded MSCs membranes (Figure 4B). In addition,the expression of CK3 (Figure 4C) was investigated with immunofluorescent staining at day 10 after cellular seeding.Double immunostaining (orange) for CK3 (green) and DiI(red) revealed that MSCs differentiated into corneal epithelial cells. As illustrated in Figure 4E-4F, positive CK3 staining was detected both in MSCs-A and MSCs-B groups. The result showed that both fresh and decellularized lenticules might be suitable for MSCs to differentiate into corneal epithelial cells.
Quantitative Reverse Transcription Polymerase Chain Reaction Analysis of Differentiated Mesenchymal Stem Cells To further determine the extent of differentiation, qRTPCR was used to evaluate the total population of differentiated MSCs (Figure 5A-5C). The expression of different types of cytokeratin in the samples was evaluated. At the mRNA level,CK3 expression increased 5-fold in MSCs-A group and 18-fold in MSCs-B group compared with that in MSCs group. There was a significant difference in the expression of CK3 between MSCs-A and MSCs-B groups (P<0.001). Both CK8 and CK18 were preferentially expressed in MSCs-A and MSCs groups(P>0.05). In contrast, the MSCs-B group exhibited a weaker expression of CK8 (P=0.001) and CK18 (P=0.077).
DISCUSSION
The majority of previous research has comprehensively investigated xenogeneic cellular corneal scaffolds in animal models[28-31], as they are not limited by donor availability.Presently, few studies employ the human fresh corneal scaffolding to facilitate tissue regeneration. Nevertheless,xenogeneic materials may possess more immunological and regulatory barriers compared to human tissue. In this study,we have discovered MSCs seeded on human SMILE-derived lenticules could differentiate into corneal epithelial cells for the first time. As sufficient SMILE-derived surplus lenticules are readily available, this may open a way to new corneal substitute for reconstructing ocular surfaces.
The application of MSCs in tissue engineering relies primarily on an appropriate scaffold to support their proliferation and differentiation[32]. The decellularized cornea scaffolds have been considered to pose great therapeutic potential due to the native matrix ultrastructure, good porosity, regenerative capacity and long-term biocompatibility[9,33-34]. The commonly used decellularization methods consist of the removal of cellular components as well as antigen materials and the preservation of corneal extracellular matrix structure[35]. It is worth stressing that most of the decellularization methods retain small amounts of cellular materials, while physical or chemical methods combined with enzymatic agents allow an optimal decellularized scaffold[36].
In the present study, freeze-thaw cycles and lyophilization were employed in combination with liquid nitrogen to repopulate seeding cells by increasing the porosity of the decellularized lenticules. In fact, porosity is relevant to the biological properties of seeding cells, such as proliferation,differentiation, adherence, and migration, as previously described[34]. In this study, the MSCs cultured with lenticules proliferated more than those in control, but no significant difference was observed in cell proliferation between fresh and decellularized lenticules. Possible factors may account for these differences. On the one hand, MSCs are profoundly influenced by microenvironmental factors such as the extracellular matrix, cell-cell interactions, and the intrinsic biological cues within the structure of the lenticules. Zhang et al[37] reported that MSCs seeded on the acellular corneal matrix could express growth factors, epidermal growth factor and transforming growth factor-beta1, which improved cellular growth. On the other hand, the decellularization methods used in this study disrupted some ultrastructure of the extracellular matrix, which may interfere with proliferation. Meanwhile the keratocytes remaining in the fresh lenticules may prevent MSCs from adhering and migrating. Additionally, the seeding density played a vital role in controlling cell proliferation.Hwang et al[38] demonstrated that a density of 2×104 cells per cm2 was required for MSCs to maintain relatively high proliferation capacity and multilineage differentiation potential. In our study,a seeding density of 1×104 and 3×104 cells per well were analyzed for cell proliferation. The results showed that the latter one improved cellular proliferation (data not shown).
Previous studies have specifically reported corneal epitheliallike cells generation from MSCs[23,39-40]. Cytokeratins form filaments that are responsible for the integrity of the epithelial cell structure, and their patterns of expression depend on the epithelial cell type[41-43]. Thus, these proteins could be used as differentiation markers. For example, CK3 is a specific marker for terminally differentiated corneal epithelial cells and absent in the basal layers of the limbal epithelium, while CK8 is highly expressed in the limbus, and has been identified as a limbal marker in the adult cornea epithelium[44-45]. In addition, CK18 is widely expressed in simple epithelia such as conjunctiva and limbal epithelia[46]. Our immunofluorescent staining analysis revealed that MSCs seeded on both fresh and decellularized lenticules were positive for CK3, a marker for epithelial cells. However, the qRT-PCR results further showed that the expression of CK3 was significantly different between these lenticules. Furthermore, some differences were observed between the expression profiles of CK8 and CK18, which were evenly expressed in the fresh lenticules, but barely expressed in the decellularized ones. Thus, these differences indicated that the decellularized SMILE-derived lenticules were superior to the fresh ones for MSCs to differentiate into the pure terminally corneal epithelial cells.
There are several limitations in the present study. Firstly,the study lacks lone term results, although MSCs had been induced into corneal epithelial cells. It is unknown how this differentiation reprogramming was precisely processed. For instance, what is the key switch that controls this process?What is the key transcription signal that regulate this process?Further studies are needed to answer these questions. Secondly,since the scaffolds support growth of seeding and corneal cells,the mechanical strength and biodegradability of decellularized SMILE-derived lenticules have yet to be evaluated. Finally,stromal-epithelial interactions occurred in a co-culture system deserve further investigation in future studies.In summary, the present study decellularized the SMILEderived lenticules to generate a scaffold, and then seeded them with MSCs, and eventually the scaffold facilitated MSCs to repopulate the corneal epithelial cells. To the best of our knowledge, this is the first study to investigate SMILEderived lenticules scaffolding for MSCs differentiation, which provides a significantly promising source for constructing a tissue-engineered cornea for transplantation. Based on the preliminary study, continuous molecular analysis will be exploited to treat injuries and diseases.
ACKNOWLEDGEMENTS
Authors’ contributions: Chen Y and Wen D participated in study concept and design. Chen Y, Yin YW and Zhao Y participated in data acquisition, data analysis, ethical form preparation, and prepared the manuscript. Wu XY, Young K,Song WT and Xia XB participated in critical review of the manuscript. All authors read and approved the final manuscript.
Foundations: Supported by the National Natural Science Foundation of China (No.81770927), the Natural Science Foundation of Hunan Province, China (No.2015JJ4093) and the Science and Technology Project of Changsha, China (No.kq1701079).
Conflicts of Interest: Chen Y, None; Yin YW, None; Zhao Y,None; Wu XY, None; Young K, None; Song WT, None; Xia XB, None; Wen D, None.
1 Xu SC, Chow J, Liu J, Li L, Maslin JS, Chadha N, Chen BH, Teng CC.Risk factors for visual impairment associated with corneal diseases in southern China. Clin Ophthalmol 2016;10:777-782.
2 Lynch AP, Ahearne M. Strategies for developing decellularized corneal scaffolds. Exp Eye Res 2013;108:42-47.
3 Kong B, Mi SL. Electrospun scaffolds for corneal tissue engineering: a review. Materials (Basel) 2016;9(8):E614.
4 Wang B, Guo YW, Chen XF, Zeng C, Hu QK, Yin W, Li W, Xie H,Zhang BY, Huang XC, Yu FL. Nanoparticle-modified chitosan-agarosegelatin scaffold for sustained release of SDF-1 and BMP-2. Int J Nanomedicine 2018;13:7395-7408.
5 Yang X, Li YY, He W, Huang QL, Zhang RR, Feng QL. Hydroxyapatite/collagen coating on PLGA electrospun fibers for osteogenic differentiation of bone marrow mesenchymal stem cells. J Biomed Mater Res A 2018;106(11):2863-2870.
6 Sharma S, Mohanty S, Gupta D, Jassal M, Agrawal AK, Tandon R. Cellular response of limbal epithelial cells on electrospun poly-εcaprolactone nanofibrous scaffolds for ocular surface bioengineering: a preliminary in vitro study. Mol Vis 2011;17:2898-2910.
7 Levis HJ, Brown RA, Daniels JT. Plastic compressed collagen as a biomimetic substrate for human limbal epithelial cell culture. Biomaterials 2010;31(30):7726-7737.
8 Nguyen KN, Bobba S, Richardson A, Park M, Watson SL, Wakefield D,Di Girolamo N. Native and synthetic scaffolds for limbal epithelial stem cell transplantation. Acta Biomater 2018;65:21-35.
9 Shafiq MA, Gemeinhart RA, Yue BY, Djalilian AR. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng Part C Methods 2012;18(5):340-348.
10 Chen Z, You JJ, Liu X, Cooper S, Hodge C, Sutton G, Crook JM,Wallace GG. Biomaterials for corneal bioengineering. Biomed Mater 2018;13(3):032002.
11 Yan H, Gong LY, Huang W, Peng YL. Clinical outcomes of small incision lenticule extraction versus femtosecond laser-assisted LASIK for myopia: a Meta-analysis. Int J Ophthalmol 2017;10(9):1436-1445.
12 Liu YC, Rosman M, Mehta JS. Enhancement after small-incision lenticule extraction: incidence, risk factors, and outcomes. Ophthalmology 2017;124(6):813-821.
13 Pradhan KR, Reinstein DZ, Carp GI, Archer TJ, Gobbe M, Gurung R. Femtosecond laser-assisted keyhole endokeratophakia: correction of hyperopia by implantation of an allogeneic lenticule obtained by SMILE from a myopic donor. J Refract Surg 2013;29(11):777-782.
14 Ganesh S, Brar S, Rao PA. Cryopreservation of extracted corneal lenticules after small incision lenticule extraction for potential use in human subjects. Cornea 2014;33(12):1355-1362.
15 Ganesh S, Brar S. Femtosecond intrastromal lenticular implantation combined with accelerated collagen cross-linking for the treatment of keratoconus: initial clinical result in 6 eyes. Cornea 2015;34(10):1331-1339.
16 Sun L, Yao PJ, Li MY, Shen Y, Zhao J, Zhou XT. The safety and predictability of implanting autologous lenticule obtained by SMILE for hyperopia. J Refract Surg 2015;31(6):374-379.
17 Yam GH, Yusoff NZ, Goh TW, Setiawan M, Lee XW, Liu YC, Mehta JS. Decellularization of human stromal refractive lenticules for corneal tissue engineering. Sci Rep 2016;6:26339.
18 Bhandari V, Ganesh S, Brar S, Pandey R. Application of the SMILEderived glued lenticule patch graft in microperforations and partialthickness corneal defects. Cornea 2016;35(3):408-412.
19 Wu F, Jin XM, Xu YS, Yang YB. Treatment of corneal perforation with lenticules from small incision lenticule extraction surgery: a preliminary study of 6 patients. Cornea 2015;34(6):658-663.
20 Yin HF, Qiu PJ, Wu F, Zhang W, Teng WQ, Qin ZW, Li C, Zhou JJ,Fang Z, Tang QM, Fu QL, Ma J, Yang YB. Construction of a corneal stromal equivalent with SMILE-derived lenticules and fibrin glue. Sci Rep 2016;6:33848.
21 Chamberlain G, Fox J, Ashton B, Middleton J. Concise review:mesenchymal stem cells: their phenotype, differentiation capacity,immunological features, and potential for homing. Stem Cells 2007;25(11):2739-2749.
22 Li XH, Yue SJ, Luo ZQ. Mesenchymal stem cells in idiopathic pulmonary fibrosis. Oncotarget 2017;8(60):102600-102616.
23 Gu SF, Xing CZ, Han JY, Tso MO, Hong J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis 2009;15:99-107.
24 Katikireddy KR, Dana RZ, Jurkunas UV. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells 2014;32(3):717-729.
25 Brown PT, Squire MW, Li WJ. Characterization and evaluation of mesenchymal stem cells derived from human embryonic stem cells and bone marrow. Cell Tissue Res 2014;358(1):149-164.
26 Alio del Barrio JL, Chiesa M, Garagorri N, Garcia-Urquia N,Fernandez-Delgado J, Bataille L, Rodriguez A, Arnalich-Montiel F,Zarnowski T, Álvarez de Toledo JP, Alio JL, De Miguel MP. Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model. Exp Eye Res 2015;132:91-100.
27 Lin XC, Hui YN, Wang YS, Meng H, Zhang YJ, Jin Y. Lamellar keratoplasty with a graft of lyophilized acellular porcine corneal stroma in the rabbit. Vet Ophthalmol 2008;11(2):61-66.
28 Pang KP, Du LQ, Wu XY. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes.Biomaterials 2010;31(28):7257-7265.
29 Yoeruek E, Bayyoud T, Maurus C, Hofmann J, Spitzer MS, Bartz-Schmidt KU, Szurman P. Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts.Acta Ophthalmol 2012;90(2):e125-e131.
30 Yoeruek E, Bayyoud T, Maurus C, Hofmann J, Spitzer MS, Bartz-Schmidt KU, Szurman P. Reconstruction of corneal stroma with decellularized porcine xenografts in a rabbit model. Acta Ophthalmol 2012;90(3):e206-e210.
31 Bayyoud T, Thaler S, Hofmann J, Maurus C, Spitzer MS, Bartz-Schmidt KU, Szurman P, Yoeruek E. Decellularized bovine corneal posterior lamellae as carrier matrix for cultivated human corneal endothelial cells. Curr Eye Res 2012;37(3):179-186.
32 Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells:isolation, in vitro expansion and characterization. Handb Exp Pharmacol 2006(174):249-282.
33 Gonzalez-Andrades M, de la Cruz Cardona J, Ionescu AM, Campos A,Del Mar Perez M, Alaminos M. Generation of bioengineered corneas with decellularized xenografts and human keratocytes. Invest Ophthalmol Vis Sci 2011;52(1):215-222.
34 Xiao JH, Duan HC, Liu Z, Wu Z, Lan YQ, Zhang W, Li CY, Chen F, Zhou Q, Wang XR, Huang JQ, Wang ZC. Construction of the recellularized corneal stroma using porous acellular corneal scaffold.Biomaterials 2011;32(29):6962-6971.
35 Murphy SV, Atala A. Organ engineering: combining stem cells,biomaterials, and bioreactors to produce bioengineered organs for transplantation. Bioessays 2013;35(3):163-172.
36 Du LQ, Wu XY, Pang KP, Yang YM. Histological evaluation and biomechanical characterisation of an acellular porcine cornea scaffold. Br J Ophthalmol 2011;95(3):410-414.
37 Zhang J, Huang C, Feng Y, Li Y, Wang W. Comparison of beneficial factors for corneal wound-healing of rat mesenchymal stem cells and corneal limbal stem cells on the xenogeneic acellular corneal matrix in vitro. Mol Vis 2012;18:161-173.
38 Hwang NS, Varghese S, Lee HJ, Zhang ZJ, Ye ZH, Bae J, Cheng LZ,Elisseeff J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci USA 2008;105(52):20641-20646.
39 Ma YL, Xu YS, Xiao ZF, Yang W, Zhang C, Song E, Du YQ, Li LS.Reconstruction of chemically burned rat corneal surface by bone marrowderived human mesenchymal stem cells. Stem Cells 2006;24(2):315-321.
40 Nieto-Miguel T, Galindo S, Reinoso R, Corell A, Martino M, Pérez-Simón JA, Calonge M. In vitro simulation of corneal epithelium microenvironment induces a corneal epithelial-like cell phenotype from human adipose tissue mesenchymal stem cells. Curr Eye Res 2013;38(9):933-944.
41 Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol 2008;129(6):705-733.
42 Merjava S, Neuwirth A, Tanzerova M, Jirsova K. The spectrum of cytokeratins expressed in the adult human cornea, limbus and perilimbal conjunctiva. Histol Histopathol 2011;26(3):323-331.
43 Merjava S, Neuwirth A, Mandys V, Jirsova K. Cytokeratins 8 and 18 in adult human corneal endothelium. Exp Eye Res 2009;89(3):426-431.
44 Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA. Corneal goblet cells and their niche: implications for corneal stem cell deficiency.Stem Cells 2012;30(9):2032-2043.
45 Hashmani K, Branch MJ, Sidney LE, Dhillon PS, Verma M, McIntosh
OD, Hopkinson A, Dua HS. Characterization of corneal stromal stem cells with the potential for epithelial transdifferentiation. Stem Cell Res Ther 2013;4(3):75.
46 Merjava S, Brejchova K, Vernon A, Daniels JT, Jirsova K. Cytokeratin 8 is expressed in human corneoconjunctival epithelium, particularly in limbal epithelial cells. Invest Ophthalmol Vis Sci 2011;52(2):787-794.