INTRODUCTION
V ision impairment is a global health concern. Unfortunately,loss of vision is rising at a rapid pace due to increase in overall aging population worldwide. The aging process is often accompanied by numerous instances of age associated system disturbances especially due to hyperhomocysteinemia (HHcy).
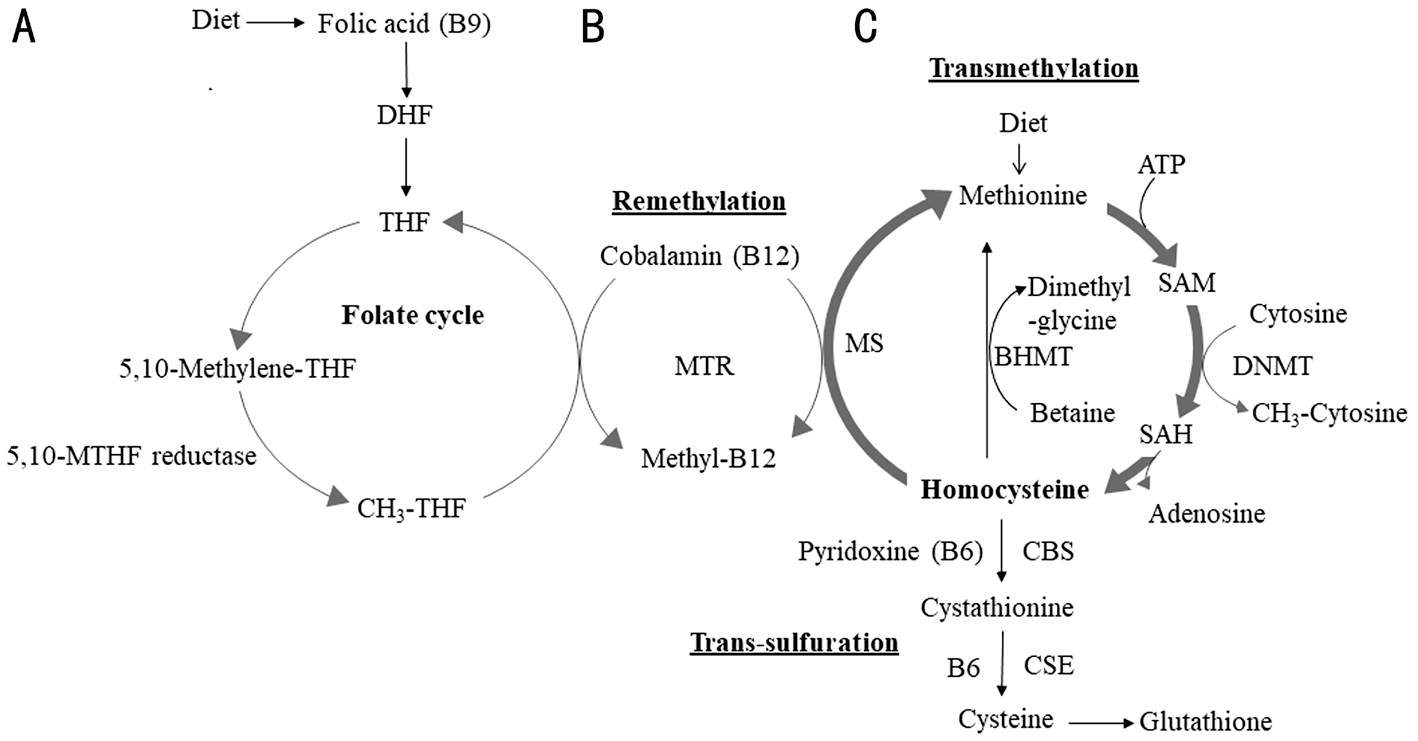
Figure 1 Methionine/homocysteine metabolism A: Dietary folic acid (Vitamin B9) enters the folate cycle after its conversion first to dihydrofolate (DHF) and then to tetrahydrofolate (THF). The 5,10-methyltetrahydrofolate reductase, MTHFR is a key enzyme that converts 5,10-methylene-THF to 5-methyl-THF. B: Cobalamin (a cofactor form of vitamin B12), together with folate (B9) is required for the conversion of Hcy to methionine by methionine synthase (MS), and is responsible for the regeneration of methionine from homocysteine (Hcy). B12 is essential for the transmethylation cycle. C: Dietary methionine is converted to Hcy through S-adenosyl methionine (SAM) and S-adenosyl homocysteine (SAH) and then back to methionine. Hcy also bifurcates to the transsulfuration pathway where it is converted to cysteine in presence of rate-limiting enzymes cystathionine-β synthase (CBS) and cystathionine-γ lyase (CSE). Cysteine generated through transsulfuration pathway is further converted to glutathione. Methionine and Hcy are written in bold while pathways are underlined.
The mechanisms of vision loss are as diverse but often rampant inflammation along with metabolic dysregulation in form of endoplasmic reticulum (ER) stress and oxidative damage (redox imbalance) that can be enhanced by conditions such as HHcy. These are the hallmarks of devastating eye conditions like age-related macular degeneration (AMD),glaucoma, retinoblastoma, and cataract that are responsible for these eye ailments. HHcy is a well-studied metabolic disorder of methionine/homocysteine (Hcy) metabolism that can lead to an excessive build-up of Hcy in the body (Figure 1)[1]. Hcy,a sulfur-containing non-proteinogenic amino acid is generated as a by-product of methyl transfer reactions during methionine metabolism[2]. Two important enzymes, cystathionine-βsynthase (CBS) and cystathionine-γ lyase (CSE) participate in Hcy metabolism and help synthesize hydrogen sulfide(H2S) endogenously. Both are present in ocular tissues suggesting their physiological relevance in the mammalian eyes[3-6]. HHcy being a high-risk factor for cardiovascular[7],neurodegenerative[8] and retinal vascular occlusive diseases[9]brings home the attention that neurovascular retina is equally susceptible to HHcy insults[10-12]. Studies have suggested that HHcy plays pathological roles in many eye diseases such as AMD[12-14], diabetic retinopathy[15-18], glaucoma[19-20] and retinal vein occlusion[21-23]. However, details of the disease mechanisms and how to effectively treat them remain largely unknown.
In recent past, it was discovered that H2S exerts powerful cytoprotective effects on multiple organ systems and that its application led to the protection of blood vessels, reduction of inflammation and the normalization of the blood pressure.Like nitric oxide and carbon monoxide, H2S has also emerged as a critical signaling molecule affecting endothelial cells,bone marrow cells[24], smooth muscle cells, inflammatory cells,mitochondria, ER and nuclear transcription factors[25-27]. We recently hypothesized that inflammation and cellular stress conditions might be treated by employing the cytoprotective nature of the H2S molecule[28]. In this work, we tested that hypothesis by employing the long-acting H2S donor,GYY4137 in a mouse model which is heterozygous (+/-) for the CBS enzyme. To further create the HHcy condition in the CBS+/- mice, we fed them with high methionine diet (HMD).The results obtained were compared with that of wild-type(C57BL/6J) mice. All groups of mice were treated the same way as that of CBS+/- mice.
MATERIALS AND METHODS
Ethical Approval The animal procedures were reviewed and subsequently approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Louisville.Further, the animal care and guidelines of the National Institutes of Health (NIH, USA) were also adhered to.
Antibodies and Reagents Antibodies for CBS, CSE, and glyceraldehyde 3 phosphate dehydrogenase (GAPDH) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA,USA), MTHFR, Hcy and superoxide dismutase 1 (SOD1)from Abcam (Cambridge, MA, USA), BiP, protein kinase R like ER kinase (PERK), CAAT enhancer binding protein homologous protein (CHOP), AMP activated protein kinase(AMPK), p-AMPK from Cell Signaling Technology (Danvers,MA, USA), GAPDH was from Boster Biological Technology(Pleasanton, CA, USA) and occludin was purchased from Novus Biologicals (Littleton, CO, USA). GYY4137 was purchased from Cayman Chemical (Ann Arbor, Michigan,USA). The meta-phosphoric acid was purchased from Millipore Sigma (St. Louis, MO, USA).
Animal Handling and Care Male and female 10-12 weeks old mice, WT (C57BL/6J) and CBS+/- (B6.129P2-Cbstm1Unc/J 002853) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). In order to create the HHcy condition, the mice were fed with an HMD having higher methionine content(1.2%) along with low folate (0.08 mg/kg), low vitamin B6(0.01 mg/kg) and B12 (10.4 μg/kg) (Harlan Laboratories) for 6wk. Control mice groups were fed the standard chow diet. All mice were allowed water ad libitum.
Genotyping of CBS+/- Mice and GYY4137 Administration The mice were weaned and genotyped according to the Jackson Laboratory’s recommendations and as previously reported by our group[29]. Tail samples were collected, and genotypic analyses were performed using polymerase chain reaction (PCR), as shown in Figure 2A. Heterozygotes CBS+/-mice produced two bands (450 and 308 bp) while CBS+/+mice presented only one band (308 bp). Unless otherwise mentioned, three to five mice were used in each group for all the experiments. GYY4137 (crystalline solid in nature) was dissolved in phosphate buffered saline (PBS) and was injected intraperitoneally (IP) to mice for 6wk (0.25 mg/Kg·d), while the control mice (without GYY4137) were given normal saline (PBS)[30]. At the end of the experiment, animals were euthanized by using 2× tribromoethanol (TBE). Mice were grouped as follows: WT+PBS (control group), WT+GYY4137(treatment group), CBS+/-+PBS (control group) and CBS+/-+GYY4137 (treatment group).
Western Blotting Analyses Preparation of mouse retinal tissue samples was carried out as per the standard protocol. In brief,retinal samples were sonicated in ice with 1× RIPA buffer (Tris-HCl 50 mmol/L, pH 7.4; NP-40, 1%; 0.25% Na-deoxycholate,150 mmol/L NaCl; 1 mmol/L EDTA; 1 mmol/L PMSF;1 μg/mL each of aprotinin, leupeptin, pepstatin; 1 mmol/L Na3VO4; 1 mmol/L NaF) containing 1 mmol/L PMSF and 1 μg of complete protease inhibitor (Sigma-Aldrich Corp., St.Louis, MO, USA). After centrifugation, the supernatants were collected; the protein amount for all samples was quantified by the Bradford method (Bio-Rad, Hercules, CA, USA) and stored at -80℃ for further use. Western blot analyses pertaining to the Hcy metabolizing enzymes’ pathway(s) (i.e. CBS, CSE,MTHFR, and Hcy), markers of oxidative stress (i.e. SOD1),hallmarks of ER stress (i.e. PERK, CHOP, BiP) and the tight junction protein (TJP; Occludin) were performed by following our previously reported protocol[31]. Equal amounts of total protein (50 μg) were resolved on SDS-PAGE and transferred to polyvinylidene membranes. The membranes were probed overnight at 4℃ with primary antibodies followed by 2h incubation in secondary antibodies. The signal capturing was done with the Bio-Rad ChemiDoc XRS+ system and Image Lab software (Bio-Rad, Hercules, CA, USA). The relative optical density of protein bands was analyzed using gel software Image Lab 3.0. The membranes were stripped and reprobed with GAPDH as a loading control.
Estimation of Glutathione and Glutamate Levels At the end of each experiment, the blood was drawn via vena cava venipuncture using a 23-gauge needle attached to the polypropylene syringe containing sodium citrate. The blood samples were transferred to Eppendorf tubes and centrifuged at 1000 g for 15min to obtain plasma supernatants. The cellular GSH levels in the experimental mice plasma samples were determined using the glutathione assay kit according to the manufacturer’s instructions, Trevigen (Gaithersburg, MD, USA). Briefly,the fresh experimental plasma samples were treated with metaphosphoric acid (MPA; final concentration 5%; Sigma-Aldrich Corp., St. Louis, MO, USA) for 15min and centrifuged at 14 000 g for 10min at 4℃ and used the supernatant for total GSH assay. The readings were measured at 405 nm with the help of SpectraMax M2 Microplate Reader. Similarly, the level of glutamate in the plasma samples was also measured using a mouse-specific glutamate assay kit by Millipore Sigma (St.Louis, MO, USA) according to their test protocol.
Measurement of Intraocular Pressure The intraocular pressure (IOP) was monitored using a Tonovet tonometer,model TV02, iCare (Raleigh, NC, USA) according to the manufacturer’s instructions. Briefly, mice were anesthetized IP with TBE; 5 mg/kg body weight. Each of the animals was placed in the prone position and keeping the probe horizontal and maintaining a probe-cornea distance about 3-5 mm with an angle of 25° limit relative to the visual axis at the corneal apex for recording[32]. Only measurements that were judged by data analytical system to be within its acceptable parameters were recorded as valid. After effectively measuring for a few times,the tonometer generated average values were used as valid IOP measurements for each eye of the animals. Every time,a new disposable probe was used for recording the pressure measurement.
Barium Sulfate Angiography and Microvascular Leakage Imaging Barium sulfate angiography was undertaken in mice as described in our previous work[33]. Briefly, barium sulfate(0.1 g/mL) was dissolved in 50 mmol/L Tris-buffer (pH 5.0)and infused slowly at a constant flow and pressure with a syringe pump through the carotid artery post-TBE anesthesia.The mouse eye globe from each of the experimental group was dissected out and placed in the X-ray chamber Kodak 4000 MM image station and angiograms were captured with the high penetrative phosphorous screen using 31 KVP X-ray exposure for 3min. Vessel density was quantified using VesSeg tool (Institute for Signal Processing, University of Luebeck, Luebeck, Germany) as previously reported[34]. The vascular permeability was measured in anesthetized mice with a modification of our previously described procedure[35].Briefly, BSA-FITC was injected via tail vein and allowed to circulate in the system for up to 20-30min. After that mice were euthanized, and eye globes were harvested for the retinal mount preparations. The retinal vascular imaging was performed with the help of the BX61WI fluorescent microscope (Olympus, Tokyo, Japan). Subsequently, the data were interpreted with the software provided with the instrument and Image-J software.
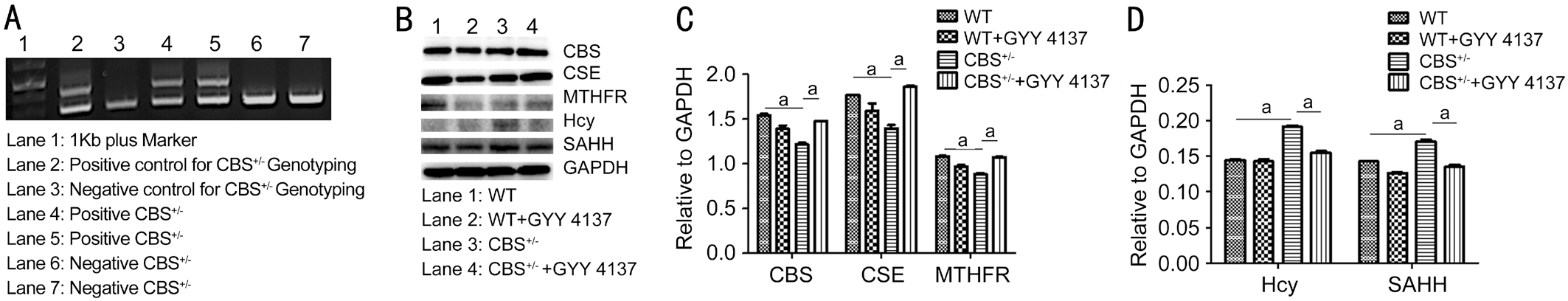
Figure 2 Genotyping of CBS mice and expression profiling of target enzymes/metabolites involved in methionine/homocysteine metabolism CBS deficiency can lead to HHcy. GYY4137 was able to alleviate the effects of HHcy in the CBS+/- mice. A: Genotyping analysis from mice tail clips. Samples from positive CBS+/- show double bands while negative CBS+/- show single band. B: Western blot analysis for CBS, CSE, MTHFR, Hcy and SAHH expression in mouse retinae. Decreased CBS, CSE, and MTHFR and increased Hcy and SAHH levels of proteins/metabolites were observed compared to WT mice. C, D: Quantitative estimation of protein expressions after normalization with GAPDH. aP < 0.05.
Assessment of the Vision Guided Behavior In order to assess the vision-guided behavior in mice, we relied upon the protocol for performing the novel object recognition test(NORT)[36]. Briefly, mice were subjected to a training session comprising of two identical objects in an open-field arena for 10min. After completion of the training, mice underwent a testing session wherein they were presented with one familiar stimulus (i.e. Object 1) and with another but a novel stimulus for the duration of 5min. The amount of time spent exploring the stimulus during the training and test sessions were recorded using a computerized algorithm. The relative exploration time during the test trial was expressed as a discrimination index[D.I.=(time novel-time familiar)/(time novel+time familiar)].Similarly, the light-dark box test (LDBT) analyses were performed according to the modified procedure[37]. Briefly, the latency of the initial time taken from the white (illuminated) to the dark (non-illuminated) areas was recorded after the initial 20s of initial exploration. All groups of animals were subjected individually to NORT and LDBT assessments.
Statistical Analyses Data analyses and graphical presentations were performed with the help of GraphPad InStat 3 and GraphPad Prism, version 6.07 (GraphPad Software, Inc., La Jolla, CA). Data are represented as mean values±standard error (SE) from 5 independent experiments in all cases. The experimental groups were compared by one-way analyses of variance (ANOVA) if the values were sampled from Gaussian distributions. For a set of data, if ANOVA indicated a significant difference (P<0.05), Tukey-Kramer multiple comparison tests were used to compare group means. Post-test was only performed if P<0.05. If the value of Tukey-Kramer‘Q’ is less than 4.046, then the P-value is less than 0.05 and considered statistically significant.
RESULTS
Genotyping of Progeny Mice and Evaluation of H2S Donor, GYY4137 Effects in CBS Deficient Mice Crossbreeding of male and female CBS+/- mice yielded about 10%CBS-/-, 60% CBS+/- and 25% CBS+/+ progeny. Figure 2A shows genotyping results wherein two animals (lanes 4, 5)turned out to be CBS+/- heterozygous deficient from one of the litters. Equal numbers of animals for CBS+/- and normal WT(C57BL/6J) were selected for the experiments. To examine the effects of H2S on metabolic regulation of methionine/Hcy in CBS+/- and WT mice strains, we studied the enzymes that are involved in methionine/Hcy metabolism including CBS, CSE and MTHFR. Findings indicate that CBS, CSE,and MTHFR expressions were decreased in CBS+/- mice with respect to WT group but GYY4137 treatment was able to rescue their respective levels in the CBS+/-+GYY4137 treated group as compared to untreated CBS+/- mice (Figure 2B). To examine the effects of GYY4137 in treated and untreated mice groups, we used anti-Hcy and anti-SAHH antibodies. Data indicated elevated Hcy and SAHH amounts in the CBS+/- mice in comparison to the WT group, however, GYY4137 treatment could reduce the Hcy and SAHH amounts in the CBS+/- treated group (Figure 2B). The quantitative representation of the data is depicted in Figure 2C and 2D. Collectively, the results suggest that GYY4137 intervention improved the levels of CBS, CSE, MTHFR, and SAHH, highlighting the protective role afforded by GYY4137 during HHcy condition.
GYY4137 Mitigates ER and Oxidative Stress Responses in CBS Deficient Mice To evaluate the effects of H2S on oxidative and ER stress responses, we examined the key stress markers’ expression profiling via Western blotting in the experimental animal groups. Analysis of ER stress condition markers such as BiP, CHO, and PERK (the hallmarks of ER stress) showed an increased in their expressions in the CBS+/-mice in comparison to WT group (Figure 3A and 3B). Data also indicated that the levels of oxidative stress marker SOD1 were significantly upregulated in the CBS+/- group as compared to the WT mice (Figure 3A and 3B). The stressful effects were successfully mitigated by GYY4137 (Figure 3A and 3B).These results clearly demonstrate that H2S intervention can help alleviate cellular stress during inflammatory conditions.To further confirm the existence of the redox imbalance in CBS+/- mutant mice, we determined the GSH contents in their plasma. The results of the GSH assay confirmed that there was a significant decrease in the levels of total GSH in CBS+/-mice in comparison to WT group (Figure 3C). Interestingly,GYY4137 intervention could bring the total GSH levels closer to the normal levels in the CBS+/-+GYY4137 treated group indicating that H2S plays an important role towards maintaining an antioxidant environment in the body.

Figure 3 Hyperhomocysteinemia causes ER and oxidative stress responses A: Expression of ER stress markers (BiP, CHOP, PERK)and oxidative stress marker (SOD1) were upregulated while glutathione levels were downregulated in CBS+/- mice compared to WT group.GYY4137 was able to rescue their levels during the treatment. B, C: Bar graphs showing a quantitative estimation of key proteins after normalization with GAPDH. aP<0.05.
Lowering of Glutamate Levels by GYY4137 in CBS Deficient Mice To evaluate the potential effects of HHcy on glutamate levels, its impact on the retinae of CBS+/- mice and also its modulation by H2S donor GYY4137, we performed a biochemical assay on plasma samples from experimental mice groups to measure the glutamate concentration. Analysis revealed significantly higher levels of glutamate in the CBS+/-untreated group when compared to WT mice group (Figure 4).Interestingly, the GYY4137 intervention could bring the overall glutamate levels closer to the normal ones.
Normalization of the Altered IOP in CBS Deficient Mice by GYY4137 Eye is one of the most metabolically active organs in our body and requires strict maintenance of physiological homeostasis to function properly. Higher concentrations of Hcy can lead to disruption of metabolism causing ocular damage and malfunctioning of various cell types in the eye.
We measured the effect(s) of Hcy IOP and our results show that HHcy condition significantly triggered the IOP on the upper side in the CBS+/- mice group in comparison to the WT group (Figure 5). Treatment with GYY4137 could bring the ocular pressure back to the baseline levels in CBS+/-+GYY4137 treatment group suggesting that H2S donor could help maintain homeostatic regulation of the IOP during the hyperhomocysteinemic conditions.

Figure 4 HHcy induces higher levels of glutamate in the CBS+/-mice Increased levels of glutamate in the plasma of CBS+/- mice were observed in comparison to WT group. Treatment with GYY4137 was able to decrease the amount of excessive glutamate in CBS+/- mice.Data are presented as mean±SEM. aP<0.05, n = 3-5 mouse/group.

Figure 5 Effect of GYY4137 on IOP in CBS+/- mice HHcy induced higher IOP in CBS +/- mice compared to WT and it is normalized (or rescued) by GYY4137 treatment. aP<0.05.
Improvement in Blood-Retinal Barrier Functions by GYY4137 in CBS Deficient Mice To determine whether GYY4137 treatment can also mitigate the Blood-retinal barrier(BRB) permeability in the HHcy CBS+/- mice, we performed barium sulfate and BSA-FITC angiographies individually to examine the retinal vasculature and blood vessels permeability and compared the results with that of the control WT mice.Intra-carotid barium sulfate angiography revealed the presence of more vessels in the eyes of CBS+/- mice than the WT mice(Figure 6A, 6B and 6C) while BSA-FITC infusion exemplified an increased retinal vascular permeability in the CBS+/- mice in comparison to the WT group (Figure 6D). In the GYY4137 treatment group, there was less vessels’ density (Figure 6A,6B and 6C) and permeability (Figure 6D). The TJP, occludin was also probed via the Western blotting analysis. The results showed that HHcy caused a significant decrease in occludin contents and this decrease in the TJP could be prevented by GYY4137 treatment of the CBS+/- mice group (Figure 6E and 6F).

Figure 6 Angiographic imaging of mice eyes and their retinae A: HHcy induced microvasculature changes in mice eyes (imaging with barium sulphate). B: Quantitation of vessels’ density from A. C: BSA-FITC images showing leakage of vessels (arrow) as observed on retinal flat mounts of CBS+/- treated mice group. GYY4137 could halt leakage of the retinal vasculature in CBS+/- mice. D: Graph represents the percent change in vascular density in different mice groups. E: Decreased expression of TJP occludin in the CBS+/- mice was rescued by GYY4137 treatment. F: The bar graph showing the quantitative estimation of occludin after normalization with GAPDH, data presented as mean ± SEM.
aP <0.05, n = 3-5 eye globes/retinae/group. aP <0.05.
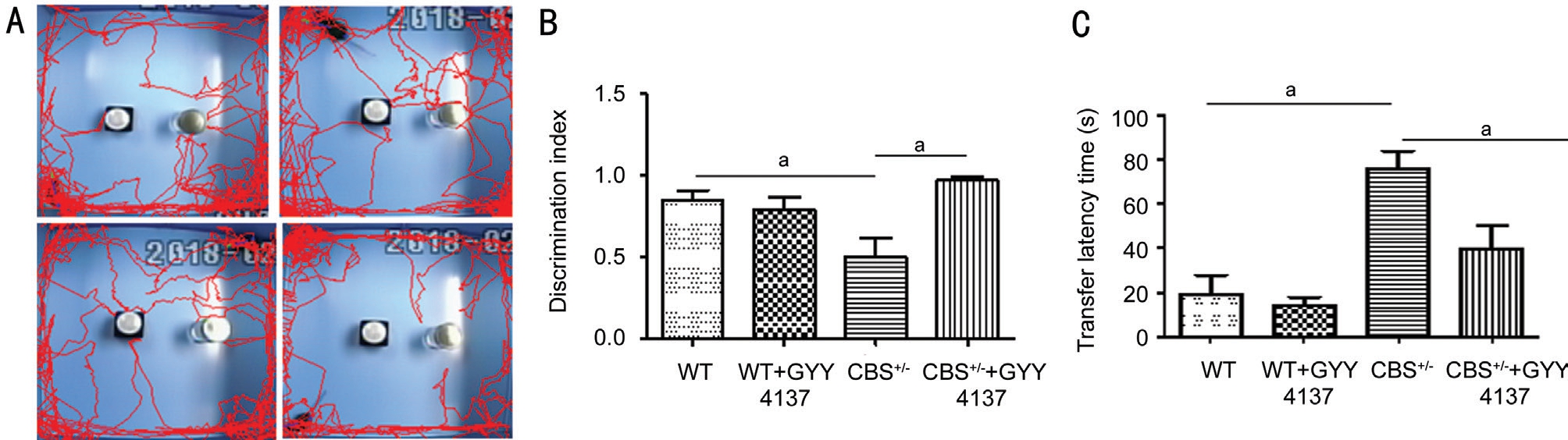
Figure 7 Effect of GYY4137 treatment on vision-guided behaviors during HHcy in CBS+/- mice A: Representative images of mice behavior were recorded employing the NORT; B: Graph showing discrimination index; C: Graph showing LDBT analysis. Graph data represent the visually guided behavior of experimental mice as transfer latency time. Values are mean±SEM of four to six separate experiments. aP<0.05,when compared with WT. aP < 0.05.
Improvement in Visual Guided Behavioral Functions in CBS Deficient Mice by GYY4137 The NORT and the LDBT were performed to test vision guided behaviors in the experimental mice groups. NORT performance relies upon a novel object recognition task by the mouse’s natural exploratory instinct or behavior. Figure 7A displays the schematic representation of the experimental protocol conducted in which mice were habituated to an open-field apparatus for 3 consecutive days. The mice were then allowed to explore two identical objects. On day 3rd, one hour after the last habituation, the individual mice were presented with a familiar and a new object respectively. The mice in the CBS+/-group exhibited significantly impaired novel object recognition performance during the task relative to control WT mice in exploring the novel object. The discrimination indices (DI) as calculated by the computerized algorithm in the CBS+/- mice were significantly reduced in comparison to the WT group(Figure 7A and 7B). CBS+/-+GYY4137 group mice exhibited improvement in their recognition preference between a novel and the familiar object when compared to the CBS+/- mice.Similarly, the LDBT data showed an increased transfer latency time in the CBS+/- group in comparison to the WT mice and that was a significant improvement in the CBS+/-+GYY4137 treated group (Figure 7C).
DISCUSSION
Declining of one’s vision or losing the eyesight leaves patients with tortuously robbing feeling. Several chronic inflammatory eye diseases with complex pathophysiologies have no available cure and can lead to blindness if suitable treatment options are not explored. The aim of the current study was to understand the beneficial effect(s) of the GYY4137, a slow H2S donor compound in a well-characterized mouse model of HHcy-induced metabolic derangements. As reported by our group[11-12,28,30,38] and others[39-46] lack or diminished levels of CBS activity can result into panoply of disorders since CBS is hailed as a critical enzyme in the transsulfuration pathway.It is responsible for the synthesis of cystathionine from serine and Hcy and as we know that cystathionine is a precursor to amino acid cysteine. More importantly, CBS is responsible for the synthesis of H2S from cysteine. Thus, alterations in the CBS enzyme’s activities cause Hcy levels to rise giving rise to HHcy. Elevated Hcy is associated with an increased risk of neurovascular and ocular diseases[10,47-50]. The results from this study reveal that when CBS+/- mice are fed with HMD they had significantly higher Hcy in comparison to the WT group leading to HHcy condition inducing alterations in the expression of enzymes that are involved in Hcy metabolism such as CBS, CSE, and MTHFR (Figure 2B, 2C, and 2D).The administration of GYY4137 mitigated these changes most likely by stabilizing CBS and CSE factors that might be involved in the generation of endogenous H2S and the production of GSH (Figure 3C). H2S can easily penetrate cells’ plasma membrane and thus induce a wide spectrum of signaling cascades in the target cells. As mentioned earlier,endogenous H2S acts as a potent regulator of various biological processes which are especially related to vasomotor functions,anti-oxidant and anti-inflammatory responses.
HHcy induced metabolic dysregulation coupled with glutamate cytotoxicity can potentially affect intracellular calcium homeostasis. This can trigger another level of cellular injuries as a result of the ER and oxidative stress responses especially in neural cells[51]. ER-derived stress signals are decoded by the cellular machinery to implement adaptive response strategies that can bring back the homeostasis[52-53]. Chronic or excessive ER stress and redox imbalance with a failure to mount appropriate responses to the stressful environment can have dramatic consequences for the affected cells[54-57]and retinal diseases[58-60]. Consistent with these observations,we observed increased expressions of markers like BiP,CHOP, PERK and SOD1 in the CBS+/- mice group, although H2S intervention was able to normalize these markers in the CBS+/-+GYY treated mice group further demonstrating that GYY4137 can boost cellular defense system that can help alleviate the harmful HHcy mediated effects. Glutamate is an important neurotransmitter serving the nervous system by playing roles in neuronal plasticity, memory-related behavior and cell death[61]. Excess glutamate can overstimulate these receptors resulting into excitotoxicity[62-63]. Our results showed an increase in the levels of glutamate in CBS+/- mice possibly reelecting glutamate excitotoxicity like condition. This might be the result ensuing from the Ca2+ ion entrance at a higher rate than usual into the affected cells by virtue of overstimulated postsynaptic glutamate receptors[64]. The excess amount of intracellular Ca2+ levels can increase the burden of ER for handling Ca2+, thereby inducing ER damage and reactive oxygen species (ROS) generation[65-67].
Overproduction of ROS leads to disturbance in redox homeostasis. Normally there is a sophisticated antioxidant defense mechanism in cells that help in coping with ROS levels under normal physiological conditions[68] but under certain conditions, such as excessive ROS and inflammation,excessive Ca2+ can cause cellular dysfunction and remodeling too[69-71]. H2S is a scavenger of ROS and dilates retinal vessels. These salubrious effects may protect the retinal ganglionic cells (RGCs) from pressure over-load and oxidative stress-induced RGC loss thus serving as a novel neuroprotectant in glaucomatous conditions[72]. In related studies, the neuroprotectant and IOP lowering effects of H2S in animal models of experimental glaucoma have been described wherein it reduced IOP and prevented IOP induced retinopathies[73]. Detailed studies of the pharmacological actions of H2S using several compounds (both fast- and slowreleasing donors) on anterior uveal tissues revealed an effect on sympathetic neurotransmission and the ability of the H2S to relax precontracted iris and ocular vascular smooth muscles,responses that were blocked by inhibitors of CSE, CBS, and KATP channels. Also, in the retina, there is evidence that H2S can inhibit excitatory amino acid neurotransmission and can protect retinal cells from a wide variety of cellular insults.Furthermore, exogenous application of H2S compounds was reported to increase aqueous humor outflow in an ex vivo model of the porcine ocular anterior segment and lowered IOP in both normotensive and glaucomatous animals.Looking at the progress made in the pharmacological actions and applications of H2S, it appears that it is involved in the regulation of hydrodynamics pertaining to IOP maintenance[74].Taken together, the finding that H2S compounds can lower IOP and can serve a neuroprotective role in the eyes suggests that H2S based drugs/prodrugs could be used as important tools or therapeutic agents in diseases of the eyes such as glaucoma[75-77]. In our study, it is not surprising to find the IOP lowering properties of GYY4137 in CBS+/- mice which have higher IOP than that of WT mice.
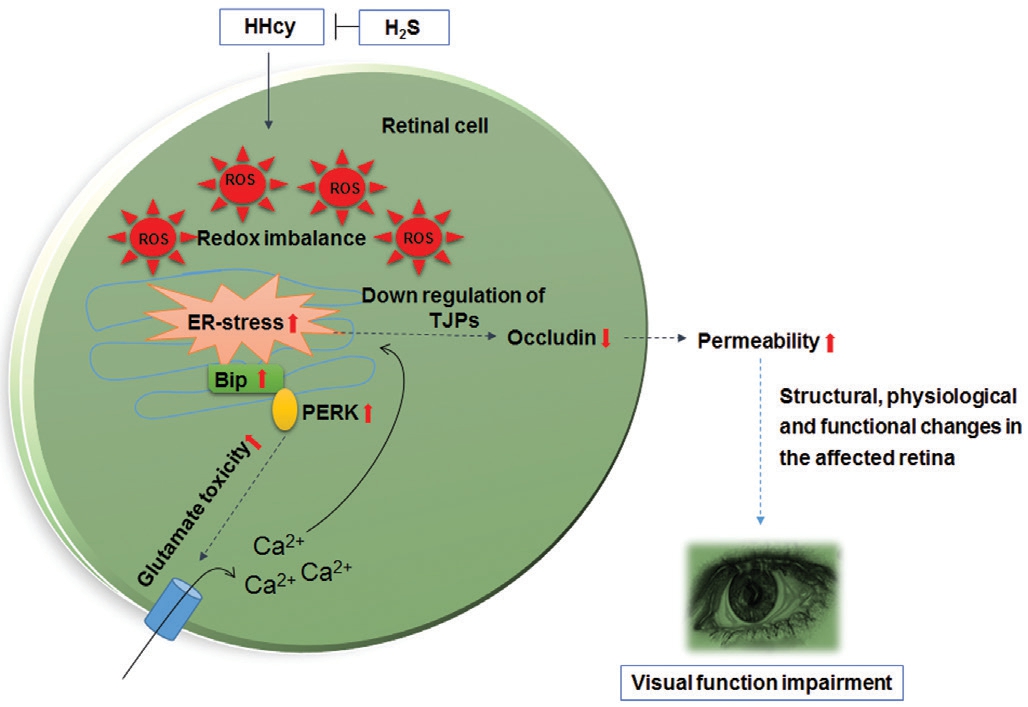
Figure 8 Overall working model HHcy induced oxidative and ER stress conditions in the eyes of CBS+/- mice indicating the presence of ROS through the upregulation of BiP, PERK and downregulation of TJPs such as occludin respectively. These metabolic changes are generally accompanied by the excess production of glutamate. Such physiological alterations can contribute to the downregulation TJPs making blood vessels permeable which can lead to the loss of ocular homeostasis followed by vision impairment. GYY4137 treatment could potentially mitigate these harmful effects.
TJPs help maintain the structural integrity of the BRB.Previous studies have indicated that alterations in TJPs are associated with increased permeability[78], cardiovascular diseases[79], retinal diabetic complications[80], gliomas[81]and the ocular disorders[82-83]. In our study, we show that HHcy condition resulted in down-regulation of TJP occludin suggesting that TJP expression plays key role in BRB integrity and any alteration can lead to BRB dysfunction.The fluorescence microscopy analysis revealed increased retinal vascular permeability in the CBS+/- group as compared to the WT group together with increased vessel density in CBS deficient mice. Additionally, GYY4137 treatment could stabilize occludin; vessel density and leakage of the retinal vasculature meaning that increased bioavailability of H2S and augmented biosynthesis of GSH in combination activate the cellular protective measures in the eyes as outlined in Figure 8.Although, the finer details of the cytoprotective mechanisms remain to be discovered at the genomic/epigenomic levels such as regulation of gene expressions. These are supposed to be governed by circular RNA molecules as recently reported in the CBS+/- mouse strain[84] and in human retinal cells[85-86]. Despite these limitations, the findings from this study emphasize that H2S could still be developed as a potential therapeutic target for serious ocular indications wherein chronic inflammation,redox imbalance and stressful environment underlie disease pathogenesis in serious eye conditions.
ACKNOWLEDGEMENTS
Part of this work was presented at the FASEB 2018 Annual Meeting in San Diego, California, USA. We thank all members of the laboratory for their continued help and support.
Foundations: Supported by NIH Heart, Lung, and Blood Institute (No.HL-74815); Institute of Neurological Disorders and Stroke (No.NS-084823).
Conflicts of Interest: George AK, None; Homme RP, None;Majumder A, None; Laha A, None; Metreveli N, None;Sandhu HS, None; Tyagi SC, None; Singh M, None.
1 Majumder A, Behera J, Jeremic N, Tyagi SC. Hypermethylation:causes and consequences in skeletal muscle myopathy. J Cell Biochem 2017;118(8):2108-2117.
2 Škovierová H, Vidomanová E, Mahmood S, Sopková J, Drgová A,Červeňová T, Halašová E, Lehotský J. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci 2016;17(10). pii: E1733.
3 De Luca G, Ruggeri P, Macaione S. Cystathionase activity in rat tissues during development. Ital J Biochem 1974;23(6):371-379.
4 Persa C, Osmotherly K, Chao-Wei Chen K, Moon S, Lou MF. The distribution of cystathionine beta-synthase (CBS) in the eye: implication of the presence of a trans-sulfuration pathway for oxidative stress defense.Exp Eye Res 2006;83(4):817-823.
5 Pong WW, Stouracova R, Frank N, Kraus JP, Eldred WD. Comparative localization of cystathionine beta-synthase and cystathionine gammalyase in retina: differences between amphibians and mammals. J Comp Neurol 2007;505(2):158-165.
6 Kulkarni M, Njie-Mbye YF, Okpobiri I, Zhao M, Opere CA, Ohia SE. Endogenous production of hydrogen sulfide in isolated bovine eye.Neurochem Res 2011;36(8):1540-1545.
7 Lai WK, Kan MY. Homocysteine-induced endothelial dysfunction. Ann Nutr Metab 2015;67(1):1-12.
8 Sharma M, Tiwari M, Tiwari RK. Hyperhomocysteinemia: impact on neurodegenerative diseases. Basic Clin Pharmacol Toxicol 2015;117(5):287-296.
9 Cahill M, Karabatzaki M, Meleady R, Refsum H, Ueland P, Shields D,Mooney D, Graham I. Raised plasma homocysteine as a risk factor for retinal vascular occlusive disease. Br J Ophthalmol 2000;84(2):154-157.
10 Ajith TA, Ranimenon. Homocysteine in ocular diseases. Clin Chim Acta 2015;450:316-321.
11 Singh M, Tyagi SC. Homocysteine mediates transcriptional changes of the inflammatory pathway signature genes in human retinal pigment epithelial cells. Int J Ophthalmol 2017;10(5):696-704.
12 Singh M, Tyagi SC. Hyperhomocysteinemia and age-related macular degeneration: role of inflammatory mediators and pyroptosis; a proposal.Med Hypotheses 2017;105:17-21.
13 Huang P, Wang F, Sah BK, Jiang J, Ni Z, Wang J, Sun X. Homocysteine and the risk of age-related macular degeneration: a systematic review and meta-analysis. Sci Rep 2015;5:10585.
14 Franke AA, Halm BM, Custer LJ, Tatsumura Y, Hebshi S. Isoflavones in breastfed infants after mothers consume soy. Am J Clin Nutr 2006;84(2):406-413.
15 Malaguarnera G, Gagliano C, Salomone S, Giordano M, Bucolo C,Pappalardo A, Drago F, Caraci F, Avitabile T, Motta M. Folate status in type 2 diabetic patients with and without retinopathy. Clin Ophthalmol 2015;9:1437-1442.
16 Srivastav K, Saxena S, Mahdi AA, Shukla RK, Meyer CH, Akduman L,Khanna VK. Increased serum level of homocysteine correlates with retinal nerve fiber layer thinning in diabetic retinopathy. Mol Vis 2016;22:1352-1360.
17 Bulum T, Blaslov K, Duvnjak L. Plasma homocysteine is associated with retinopathy in type 1 diabetic patients in the absence of nephropathy.Semin Ophthalmol 2016;31(3):198-202.
18 Homme RP, Singh M, Majumder A, George AK, Nair K, Sandhu HS,Tyagi N, Lominadze D, Tyagi SC. Remodeling of retinal architecture in diabetic retinopathy: disruption of ocular physiology and visual functions by inflammatory gene products and pyroptosis. Front Physiol 2018;9:1268.
19 Bleich S, Jünemann A, von Ahsen N, Lausen B, Ritter K, Beck G,Naumann GO, Kornhuber J. Homocysteine and risk of open-angle glaucoma. J Neural Transm (Vienna) 2002;109(12):1499-1504.
20 Vessani RM, Ritch R, Liebmann JM, Jofe M. Plasma homocysteine is elevated in patients with exfoliation syndrome. Am J Ophthalmol 2003;136(1):41-46.
21 Minniti G, Calevo MG, Giannattasio A, Camicione P, Armani U,Lorini R, Piana G. Plasma homocysteine in patients with retinal vein occlusion. Eur J Ophthalmol 2014;24(5):735-743.
22 Ganapathy PS, Moister B, Roon P, Mysona BA, Duplantier J, Dun Y, Moister TK, Farley MJ, Prasad PD, Liu K, Smith SB. Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-beta-synthase mutant mouse. Invest Ophthalmol Vis Sci 2009;50(9):4460-4470.
23 Chang HH, Lin DP, Chen YS, Liu HJ, Lin W, Tsao ZJ, Teng MC,Chen BY. Intravitreal homocysteine-thiolactone injection leads to the degeneration of multiple retinal cells, including photoreceptors. Mol Vis 2011;17:1946-1956.
24 Cheng Z, Garikipati VN, Nickoloff E, Wang C, Polhemus DJ, Zhou J, Benedict C, Khan M, Verma SK, Rabinowitz JE, Lefer D, Kishore R.Restoration of hydrogen sulfide production in diabetic mice improves reparative function of bone marrow cells. Circulation 2016;134(19):1467-1483.
25 Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 2014;114(4):730-737.
26 Prabhudesai S, Koceja C, Dey A, Eisa-Beygi S, Leigh NR,Bhattacharya R, Mukherjee P, Ramchandran R. Cystathionine β-synthase is necessary for axis development in Vivo. Front Cell Dev Biol 2018;6:14.
27 Saha S, Chakraborty PK, Xiong X, Dwivedi SK, Mustafi SB, Leigh NR, Ramchandran R, Mukherjee P, Bhattacharya R. Cystathionine β-synthase regulates endothelial function via protein S-sulfhydration.FASEB J 2016;30(1):441-456.
28 George AK, Singh M, Homme RP, Majumder A, Sandhu HS,Tyagi SC. A hypothesis for treating inflammation and oxidative stress with hydrogen sulfide during age-related macular degeneration. Int J Ophthalmol 2018;11(5):881-887.
29 Tyagi N, Qipshidze N, Sen U, Rodriguez W, Ovechkin A, Tyagi SC.Cystathionine beta synthase gene dose dependent vascular remodeling in murine model of hyperhomocysteinemia. Int J Physiol Pathophysiol Pharmacol 2011;3(3):210-222.
30 John AMSP, Kundu S, Pushpakumar S, Fordham M, Weber G,Mukhopadhyay M, Sen U. GYY4137, a hydrogen sulfide donor modulates miR194-dependent collagen realignment in diabetic kidney. Sci Rep 2017;7(1):10924.
31 Kamat PK, Kalani A, Givvimani S, Sathnur PB, Tyagi SC, Tyagi N. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice.Neuroscience 2013;252:302-319.
32 Morrison JC, Jia L, Cepurna W, Guo Y, Johnson E. Reliability and sensitivity of the TonoLab rebound tonometer in awake Brown Norway rats. Invest Ophthalmol Vis Sci 2009;50(6):2802-2808.
33 Givvimani S, Sen U, Tyagi N, Munjal C, Tyagi SC. X-ray imaging of differential vascular density in MMP-9-/-, PAR-1-/+, hyperhomocysteinemic(CBS-/+) and diabetic (Ins2-/+) mice. Arch Physiol Biochem 2011;117(1):1-7.
34 Machens HG, Grzybowski S, Bucsky B, Spanholtz T, Niedworok C, Maichle A, Stöckelhuber B, Condurache A, Liu F, Egana JT,Kaun M, Mailänder P, Aach T. A technique to detect and to quantify fasciocutaneous blood vessels in small laboratory animals ex vivo. J Surg Res 2006;131(1):91-96.
35 George AK, Behera J, Kelly KE, Mondal NK, Richardson KP, Tyagi N. Exercise mitigates alcohol induced endoplasmic reticulum stress mediated cognitive impairment through ATF6-Herp signaling. Sci Rep 2018;8(1):5158.
36 Jadavji NM, Deng L, Malysheva O, Caudill MA, Rozen R. MTHFR deficiency or reduced intake of folate or choline in pregnant mice results in impaired short-term memory and increased apoptosis in the hippocampus of wild-type offspring. Neuroscience 2015;300:1-9.
37 Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci U S A 2008;105(41):16009-16014.
38 Sen U, Moshal KS, Singh M, Tyagi N, Tyagi SC. Homocysteineinduced biochemical stress predisposes to cytoskeletal remodeling in stretched endothelial cells. Mol Cell Biochem 2007;302(1-2):133-143.
39 Wang HJ, Xu X, Xie RH, Rui YY, Zhang PA, Zhu XJ, Xu GY. Prenatal maternal stress induces visceral hypersensitivity of adult rat offspring through activation of cystathionine-β-synthase signaling in primary sensory neurons. Mol Pain 2018;14:1744806918777406.
40 Hendrix P, Foreman PM, Harrigan MR, et al. Association of cystathionine beta-synthase polymorphisms and aneurysmal subarachnoid hemorrhage. J Neurosurg 2018;128(6):1771-1777.
41 Yuan YQ, Wang YL, Yuan BS, Yuan X, Hou XO, Bian JS, Liu CF,Hu LF. Impaired CBS-H2S signaling axis contributes to MPTP-induced neurodegeneration in a mouse model of Parkinson’s disease. Brain Behav Immun 2018;67:77-90.
42 Yuan X, Zhang J, Xie F, Tan W, Wang S, Huang L, Tao L, Xing Q,Yuan Q. Loss of the protein cystathionine β-synthase during kidney injury promotes renal tubulointerstitial fibrosis. Kidney Blood Press Res 2017;42(3):428-443.
43 Tomuschat C, O’Donnell AM, Coyle D, Puri P. Reduction of hydrogen sulfide synthesis enzymes cystathionine-β-synthase and cystathionine-γlyase in the colon of patients with Hirschsprungs disease. J Pediatr Surg 2018;53(3):525-530.
44 Kruger WD. Cystathionine β-synthase deficiency: of mice and men.Mol Genet Metab 2017;121(3):199-205.
45 Vicente JB, Colaço HG, Malagrinò F, Santo PE, Gutierres A,Bandeiras TM, Leandro P, Brito JA, Giuffrè A. A clinically relevant variant of the human hydrogen sulfide-synthesizing enzyme cystathionine β-synthase: increased CO reactivity as a novel molecular mechanism of pathogenicity? Oxid Med Cell Longev 2017;2017:8940321.
46 Jacobs RL, Jiang H, Kennelly JP, Orlicky DJ, Allen RH, Stabler SP, Maclean KN. Cystathionine beta-synthase deficiency alters hepatic phospholipid and choline metabolism: post-translational repression of phosphatidylethanolamine N-methyltransferase is a consequence rather than a cause of liver injury in homocystinuria. Mol Genet Metab 2017;120(4):325-336.
47 Lehmann M, Gottfries CG, Regland B. Identification of cognitive impairment in the elderly: homocysteine is an early marker. Dement Geriatr Cogn Disord 1999;10(1):12-20.
48 Ravaglia G, Forti P, Maioli F, Muscari A, Sacchetti L, Arnone G, Nativio V, Talerico T, Mariani E. Homocysteine and cognitive function in healthy elderly community dwellers in Italy. Am J Clin Nutr 2003;77(3):668-673.
49 Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 2002;346(7):476-483.
50 Huang X, Yang Y, Duan Y, Kuang YQ, Lin D. Homocysteine in retinal artery occlusive disease: a meta-analysis of cohort studies. Sci Rep 2017;7(1):15708.
51 Li J, Cheng J. Apolipoprotein E4 exacerbates ethanol-induced neurotoxicity through augmentation of oxidative stress and apoptosis in N2a-APP cells. Neurosci Lett 2018;665:1-6.
52 Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol 2010;189(5):783-794.
53 Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007;8(7):519-529.
54 Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI,Ushio Y, Mori M. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ 2004;11(4):403-415.
55 Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M.Induction of neuronal death by ER stress in Alzheimer’s disease. J Chem Neuroanat 2004;28(1-2):67-78.
56 Silva RM, Ries V, Oo TF, Yarygina O, Jackson-Lewis V, Ryu EJ,Lu PD, Marciniak SJ, Ron D, Przedborski S, Kholodilov N, Greene LA, Burke RE. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem 2005;95(4):974-986.
57 Scheper W, Hoozemans JJ. Endoplasmic reticulum protein quality control in neurodegenerative disease: the good, the bad and the therapy.Curr Med Chem 2009;16(5):615-626.
58 Griciuc A, Aron L, Ueffing M. ER stress in retinal degeneration: a target for rational therapy? Trends Mol Med 2011;17(8):442-451.
59 Kroeger H, Chiang WC, Felden J, Nguyen A, Lin JH. ER stress and unfolded protein response in ocular health and disease. FEBS J 2019;286(2):399-412.
60 Kheitan S, Minuchehr Z, Soheili ZS. Exploring the cross talk between ER stress and inflammation in age-related macular degeneration. PLoS One 2017;12(7):e0181667.
61 Li F, Tsien JZ. Memory and the NMDA receptors. N Engl J Med 2009;361(3):302-303.
62 Michaels RL, Rothman SM. Glutamate neurotoxicity in vitro:antagonist pharmacology and intracellular calcium concentrations. J Neurosci 1990;10(1):283-292.
63 Mark LP, Prost RW, Ulmer JL, Smith MM, Daniels DL, Strottmann JM, Brown WD, Hacein-Bey L. Pictorial review of glutamate excitotoxicity:fundamental concepts for neuroimaging. AJNR Am J Neuroradiol 2001;22(10):1813-1824.
64 Choi DW. Glutamate neurotoxicity and diseases of the nervous system.Neuron 1988;1(8):623-634.
65 Carriedo SG, Sensi SL, Yin HZ, Weiss JH. AMPA exposures induce mitochondrial Ca(2+) overload and ROS generation in spinal motor neurons in vitro. J Neurosci 2000;20(1):240-250.
66 Carriedo SG, Yin HZ, Sensi SL, Weiss JH. Rapid Ca2+ entry through Ca2+-permeable AMPA/Kainate channels triggers marked intracellular Ca2+ rises and consequent oxygen radical production. J Neurosci 1998;18(19):7727-7738.
67 Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S,Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 1995;15(4):961-973.
68 Dröge W. Free radicals in the physiological control of cell function.Physiol Rev 2002;82(1):47-95.
69 Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest 1982;47(5):412-426.
70 Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 2011;194(1):7-15.
71 Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39(1):44-84.
72 Liu H, Anders F, Thanos S, Mann C, Liu A, Grus FH, Pfeiffer N,Prokosch-Willing V. Hydrogen sulfide protects retinal ganglion cells against glaucomatous injury in vitro and in vivo. Invest Ophthalmol Vis Sci 2017;58(12):5129-5141.
73 Özer MA, Erişgin Z, Özen S, Tekelioğlu Y, Takır S. Effects of intravitreous sodium hydrosulfide on intraocular pressure and retinopathy in ocular hypertensive rats. Biotech Histochem 2018;93(1):8-14.
74 Salvi A, Bankhele P, Jamil J, Chitnis MK, Njie-Mbye YF, Ohia SE,Opere CA. Effect of hydrogen sulfide donors on intraocular pressure in rabbits. J Ocul Pharmacol Ther 2016;32(6):371-375.
75 Ohia SE, Robinson J, Mitchell L, Ngele KK, Heruye S, Opere CA,Njie-Mbye YF. Regulation of aqueous humor dynamics by hydrogen sulfide: potential role in glaucoma pharmacotherapy. J Ocul Pharmacol Ther 2018;34(1-2):61-69.
76 Huang S, Huang P, Liu X, Lin Z, Wang J, Xu S, Guo L, Leung CK,Zhong Y. Relevant variations and neuroprotective effect of hydrogen sulfide in a rat glaucoma model. Neuroscience 2017;341:27-41.
77 Osborne NN, Ji D, Abdul Majid AS, Fawcett RJ, Sparatore A, Del Soldato P. ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Invest Ophthalmol Vis Sci 2010;51(1):284-294.
78 Wang Y, Tong J, Chang B, Wang B, Zhang D, Wang B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol Med Rep 2014;9(6):2352-2356.
79 Chistiakov DA, Orekhov AN, Bobryshev YV. Endothelial barrier and its abnormalities in cardiovascular disease. Front Physiol 2015;6:365.
80 Shin ES, Sorenson CM, Sheibani N. Diabetes and retinal vascular dysfunction. J Ophthalmic Vis Res 2014;9(3):362-373.
81 Ishihara H, Kubota H, Lindberg RL, Leppert D, Gloor SM, Errede M,Virgintino D, Fontana A, Yonekawa Y, Frei K. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J Neuropathol Exp Neurol 2008;67(5):435-448.
82 Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol 1999;14(4):240-248.
83 Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis 2007;10(2):103-117.
84 Singh M, George AK, Homme RP, Majumder A, Laha A, Sandhu HS,Tyagi SC. Circular RNAs profiling in the cystathionine-β-synthase mutant mouse reveals novel gene targets for hyperhomocysteinemia induced ocular disorders. Exp Eye Res 2018;174:80-92.
85 Singh M, George AK, Homme RP, Majumder A, Laha A, Sandhu HS, Tyagi SC. Expression analysis of the circular RNA molecules in the human retinal cells treated with homocysteine. Curr Eye Res 2019;44(3):287-293.
86 George AK, Master K, Majumder A, Homme RP, Laha A, Sandhu HS, Tyagi SC, Singh M. Circular RNAs constitute an inherent gene regulatory axis in the mammalian eye and brain. Can J Physiol Pharmacol 2018:1-10.