INTRODUCTION
O cular prostheses improve the esthetic alteration that causes the loss of an eye. However, anophthalmic patients often refer chronic discharge and irritation in their sockets[1]. These annoyances lead to the frequent use of antibiotic drops, which predisposes to increase the resistance of the conjunctival flora and select the species with greater pathogenic potential[2].Several studies[3-8] have investigated the conjunctival flora in anophthalmic sockets, although most of them were published in the 1990s or earlier. These reports have generally shown higher rates of pathogens in sockets compared to the normal conjunctival flora. Moreover, some authors have suggested that the flora of the anophthalmic socket could affect the healthy eye flora[4-5,8-9]. If pathogens from the socket persist in a surgical field, the risk of developing an intraocular infection in the fellow eye is increased[10]. Therefore, these authors recommended maximizing the anti-infective prophylaxis of the fellow eye during intraocular surgery. However, the antibiotic sensitivity of the isolated microorganisms has not been analyzed in these studies.
The purpose of this paper was to characterize the conjunctival microbiota of anophthalmic patients, in both their socket and their healthy eye, and determine and compare the antibiotic susceptibility of the isolated species from the two microbial communities.
SUBJECTS AND METHODS
Ethical Approval This study protocol was approved by the Ethics Committee of the University Hospital of León (Spain) in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects before participation.
The microorganisms isolated in this research were obtained from samples taken from 60 anophthalmic patients in a previous investigation[9]. That study was designed to explore the relationship between conjunctival flora and comfort of the socket in anophthalmic patients. Inclusion criteria,exclusion criteria and the technique to obtain the specimens were thoroughly described in that publication[9]. Basically,selected subjects were unilateral anophtalmic patients with daily wearing of artificial eye, and with stable symptomatology in the last month. Patients that regularly used self-prescribed antibiotic eyedrops in their sockets were also included.Subjects with acute conjunctivitis, conjunctival cyst, orbital implant exposure, or wearing a poorly fitting prosthesis were excluded. Regard the technique to collect the samples, topical anesthetic was not used to avoid damaging the conjunctival microorganisms[9]. Sterile rayon swabs (Copan Diagnostics Inc., Murrieta, CA, USA) previously moistened with sterile brain heart infusion (BHI) culture medium were used to take three microbiological specimens of each patient: the lower conjunctival sac of 1) the healthy eye; 2) the anophthalmic socket before removing the ocular prosthesis (pre-prosthesis sample); and 3) the socket after removing the artificial eye using a suction cup (retro-prosthesis sample). Each swab was cut off and placed in a tube (Becton, Dickinson & Company,Franklin Lakes, NJ, USA) with 4 mL of sterile BHI, and the tube was closed. Samples were taken to the clinical microbiology laboratory of our hospital within an hour after collection.
The 180 tubes with the samples were incubated at 37℃ in an aerobic atmosphere[9]. They were assessed 24h after collection and then daily for 10d before they were classified as negativecultures. If growth was noted, the broth was subcultured to different media for microorganism identification. Culture media for fungi and aerobic, facultative anaerobic and strict anaerobic bacteria were used.
Microbial identification was founded on growth in selective media, colonies morphology, and the utilization of MicroScan(Siemens, Munich, Germany) or API (bioMérieux, Marcy l’Etoile, France) systems. Staphylococci were identified by using panel 31 of the MicroScan system, Enterococci by panel 32, fermentative Gram-negative bacteria by panel 53, and nonfermentative Gram-negative bacteria by panel 54. The other microorganisms were identified by API galleries.
The antibiotic sensitivity was determined by MicroScan panels(Siemens, Munich, Germany) in those bacteria suitable for their use (Staphylococci, Enterococci and Gram-negative bacteria), and by diffusion discs in plates (Oxoid, Basingstoke,UK) in the other microorganisms. Antifungal sensitivity was not tested. The selection of antibiotics, their minimum inhibitory concentration (MIC), and inhibition zones were established according to the guidelines from the Clinical and Laboratory Standards Institute (CLSI) of 2010 and 2011. Thus,isolates were initially classified as sensitive, intermediate,or resistant for each antibiotic in accordance with CLSI break points. Subsequently, the intermediate sensitivity was considered resistant[11].
The isolated bacterial species were clustered to perform the antibiotic susceptibility analysis. Groups that settled initially were Staphylococci, Streptococci, Enterococci, Micrococcus spp., coryneform bacteria[12], and Gram-negative bacteria.Since Staphylococci were the most isolated bacteria from the conjunctival flora, they were classified in 3 new groups:coagulase-positive Staphylococci (CPS), coagulase-negative Staphylococci (CNS) excluding Staphylococcus epidermidis(S. epidermidis), and S. epidermidis which formed its own group. Moreover, microorganisms were catalogued as pathogens or saprophytes. CNS (S. epidermidis and other species),Micrococcus spp., and Coryneform bacteria were classified as saprophytic microorganisms. Other groups were considered pathogens.
Statistical Analysis Descriptive statistics were performed.The “percent susceptible” was calculated as the number of susceptible isolates over the total number of isolates times 100[13]. Group comparisons of 2 by 2 cross tables were conducted with the two-tailed Fisher’s exact test, while the Chi-square test (with Yate’s correction when appropriate) was used in the other cases. Analysis were run using SPSS for Windows version 22.0 (SPSS Inc., Chicago, IL, USA). The statistical significance level was set as P value less than 0.05.
RESULTS
A total of 251 isolates were cultured from 60 anophthalmic patients (62 isolates from healthy eye conjunctiva, 93 from pre-prosthesis space of the socket, and 96 from retro-prosthesis space). The patients were 36 male subjects and 24 female subjects. The average age of the patients was 61.9 (range 25-89y). The results of the microbiological identification are shown in Table 1.
A total of 14 different microbial species were isolated in the healthy eyes, while 35 different species were cultured from the sockets. Of the 60 samples obtained from healthy eyes, there were 9 that showed no growth. Likewise, 3 specimens had no growth among pre-prosthesis samples. On contrary, all of the specimens from retro-prosthesis space had a positive culture.Therefore, the positive culture rate was 85.0%, 95.0%, and 100.0% for healthy eye, pre-prosthesis and retro-prosthesis samples, respectively.
Statistical analysis was performed after isolates were grouped.There were not statistical differences between isolates from pre-prosthesis and retro-prosthesis space, so the strains from the socket were assessed together. The most commonly isolated species, in both healthy eyes and sockets, was S.epidermidis, followed by S. aureus (Table 1). CNS group was the main in both healthy eyes (77.4%) and sockets (56.6%).CPS accounted for 8.1% of isolates in healthy eyes and 12.1% in sockets. The remaining groups were less than 5% of cultures, except Streptococci and Gram-negative bacteria in the socket (14.3% and 11.1%, respectively). The proportion of pathogenic species in isolates from socket (40.7%; 77/189)was significantly higher than from healthy eye (16.1%; 10/62;P<0.001). When this analysis was carried out in groups,only Streptococci were significantly increased in the socket(P=0.020), but not the Gram-negative bacteria (P=0.076) and the other pathogenic groups.
Table 1 Isolated microorganisms from anophthalmic patients n (%)
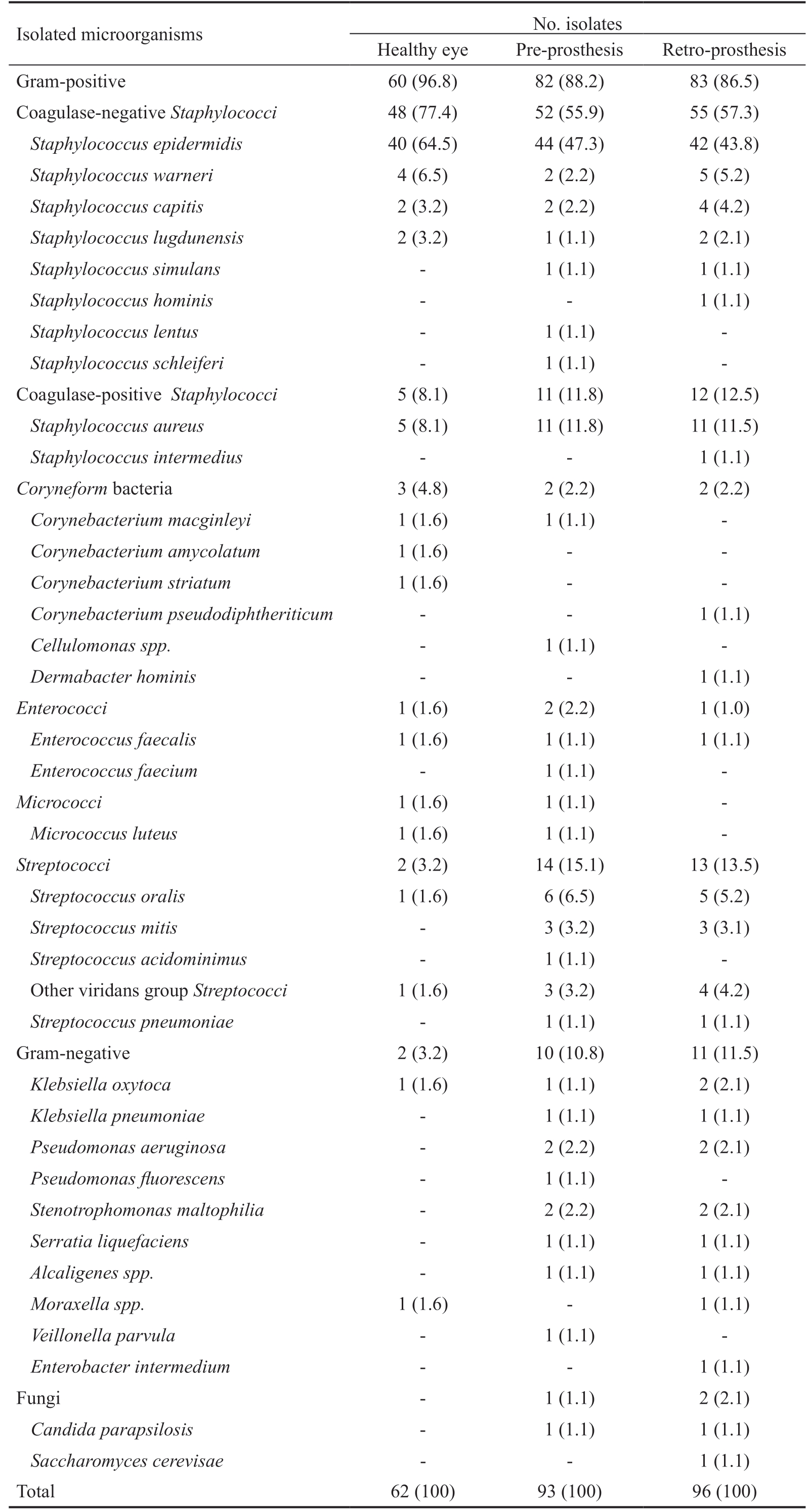
Isolated microorganisms No. isolates Healthy eye Pre-prosthesis Retro-prosthesis Gram-positive 60 (96.8) 82 (88.2) 83 (86.5)Coagulase-negative Staphylococci 48 (77.4) 52 (55.9) 55 (57.3)Staphylococcus epidermidis 40 (64.5) 44 (47.3) 42 (43.8)Staphylococcus warneri 4 (6.5) 2 (2.2) 5 (5.2)Staphylococcus capitis 2 (3.2) 2 (2.2) 4 (4.2)Staphylococcus lugdunensis 2 (3.2) 1 (1.1) 2 (2.1)Staphylococcus simulans - 1 (1.1) 1 (1.1)Staphylococcus hominis - - 1 (1.1)Staphylococcus lentus - 1 (1.1) -Staphylococcus schleiferi - 1 (1.1) -Coagulase-positive Staphylococci 5 (8.1) 11 (11.8) 12 (12.5)Staphylococcus aureus 5 (8.1) 11 (11.8) 11 (11.5)Staphylococcus intermedius - - 1 (1.1)Coryneform bacteria 3 (4.8) 2 (2.2) 2 (2.2)Corynebacterium macginleyi 1 (1.6) 1 (1.1) -Corynebacterium amycolatum 1 (1.6) - -Corynebacterium striatum 1 (1.6) - -Corynebacterium pseudodiphtheriticum - - 1 (1.1)Cellulomonas spp. - 1 (1.1) -Dermabacter hominis - - 1 (1.1)Enterococci 1 (1.6) 2 (2.2) 1 (1.0)Enterococcus faecalis 1 (1.6) 1 (1.1) 1 (1.1)Enterococcus faecium - 1 (1.1) -Micrococci 1 (1.6) 1 (1.1) -Micrococcus luteus 1 (1.6) 1 (1.1) -Streptococci 2 (3.2) 14 (15.1) 13 (13.5)Streptococcus oralis 1 (1.6) 6 (6.5) 5 (5.2)Streptococcus mitis - 3 (3.2) 3 (3.1)Streptococcus acidominimus - 1 (1.1) -Other viridans group Streptococci 1 (1.6) 3 (3.2) 4 (4.2)Streptococcus pneumoniae - 1 (1.1) 1 (1.1)Gram-negative 2 (3.2) 10 (10.8) 11 (11.5)Klebsiella oxytoca 1 (1.6) 1 (1.1) 2 (2.1)Klebsiella pneumoniae - 1 (1.1) 1 (1.1)Pseudomonas aeruginosa - 2 (2.2) 2 (2.1)Pseudomonas fluorescens - 1 (1.1) -Stenotrophomonas maltophilia - 2 (2.2) 2 (2.1)Serratia liquefaciens - 1 (1.1) 1 (1.1)Alcaligenes spp. - 1 (1.1) 1 (1.1)Moraxella spp. 1 (1.6) - 1 (1.1)Veillonella parvula - 1 (1.1) -Enterobacter intermedium - - 1 (1.1)Fungi - 1 (1.1) 2 (2.1)Candida parapsilosis - 1 (1.1) 1 (1.1)Saccharomyces cerevisae - - 1 (1.1)Total 62 (100) 93 (100) 96 (100)
Table 2 Antibiotic sensitivities of S. epidermidis according to the area of isolationa
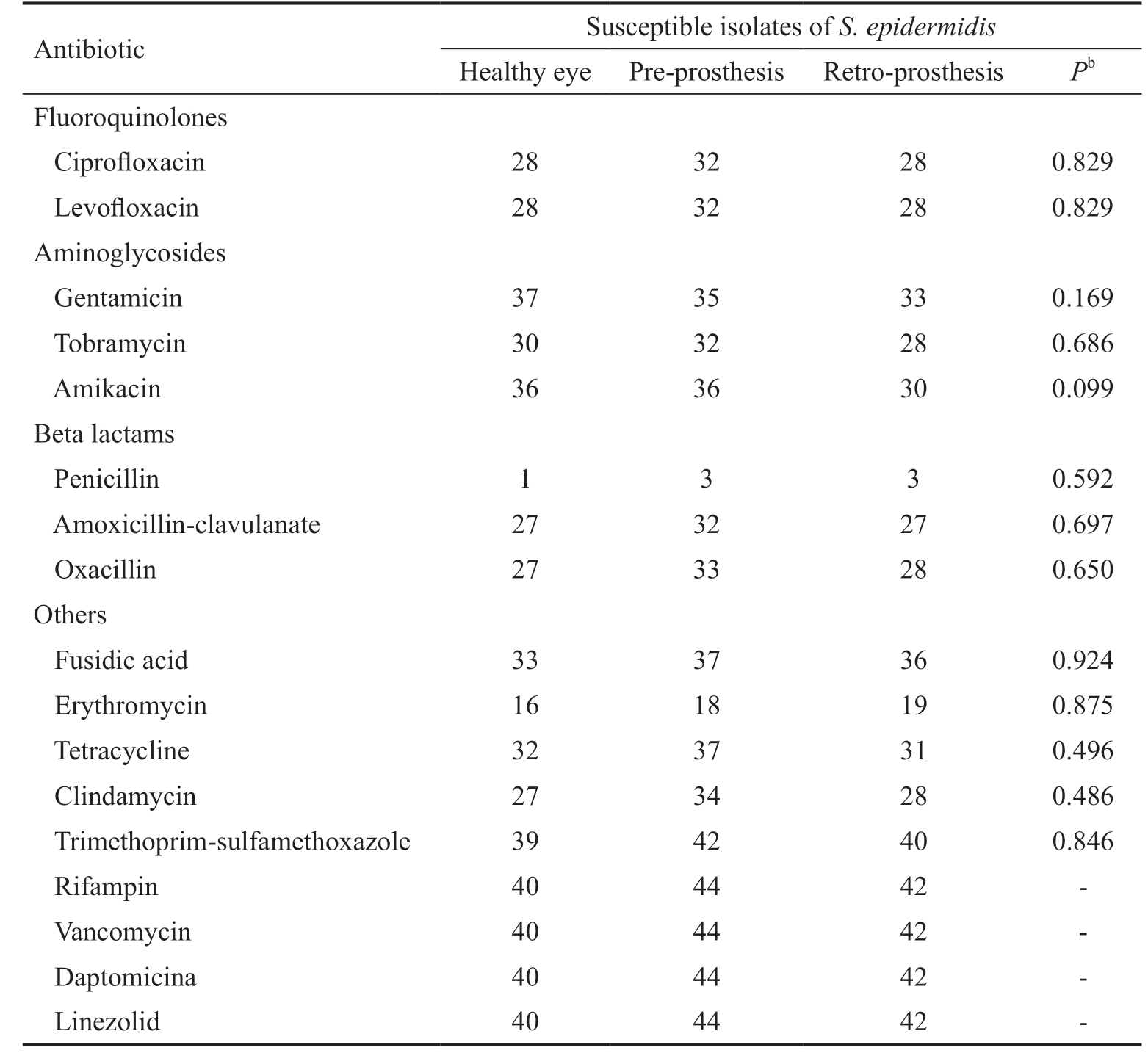
aS. epidermidis isolates were 40, 44, and 42 from healthy eyes, pre-prosthesis and retro-prosthesis spaces, respectively. All isolates of S. epidermidis were tested for all antibiotics. bPearson’s Chi-square test.
Antibiotic Susceptible isolates of S. epidermidis Healthy eye Pre-prosthesis Retro-prosthesis Pb Fluoroquinolones Ciprofloxacin 28 32 28 0.829 Levofloxacin 28 32 28 0.829 Aminoglycosides Gentamicin 37 35 33 0.169 Tobramycin 30 32 28 0.686 Amikacin 36 36 30 0.099 Beta lactams Penicillin 1 3 3 0.592 Amoxicillin-clavulanate 27 32 27 0.697 Oxacillin 27 33 28 0.650 Others Fusidic acid 33 37 36 0.924 Erythromycin 16 18 19 0.875 Tetracycline 32 37 31 0.496 Clindamycin 27 34 28 0.486 Trimethoprim-sulfamethoxazole 39 42 40 0.846 Rifampin 40 44 42 -Vancomycin 40 44 42 -Daptomicina 40 44 42 -Linezolid 40 44 42 -
The antibiotic sensitivities of each microbiological group(S. epidermidis, other CNS, CPS, Coryneform bacteria,Enterococci, Micrococci, Streptococci, and Gram-negative bacteria) were compared according to the site of isolation(healthy eye, pre-prosthesis or retro-prosthesis space). The results of isolated S. epidermidis were exposed in Table 2.
There were no significant differences in antibiotic sensitivities between strains of S. epidermidis from healthy eyes and sockets (Table 2). The same analysis was performed on the other bacterial groups (with the antibiotics tested in each group), and no differences regarding to the isolation site were demonstrated in any case. Based on these results and according to the microbiological group, the isolates from sockets and healthy eyes were joined to perform the following antibiotic sensitivities.
The rates of susceptible staphylococcal cultures were showed in Table 3. All isolates of Staphylococcus genus were tested for the same antibiotics, as all of them were identified by using the panel 31 of the MicroScan system. The three groups of Staphylococci (S. epidermidis, other CNS, and CPS) were poorly susceptible to penicillin (5.6%, 24.1%, and 10.7%,respectively) and erythromycin (42.1%, 75.9%, and 78.6%,respectively). CNS isolates different of S. epidermidis exhibited high susceptibilities to most other antibiotics,except for tetracycline (65.5%). In contrast, the resistance rates of S. epidermidis and CPS to different antibiotic groups such as aminoglycosides, fluoroquinolones, and beta lactams were much higher. There were no significant differences in aminoglycosides susceptibility betweenaCultured Staphylococci from healthy eyes and sockets were 126 isolates of S. epidermidis, 29 of other CNS, and 28 of CPS. All staphylococcal isolates were tested for all antibiotics; bFor each microbiological group, the number of susceptible isolates over the total number of isolates times 100.
Table 3 Antibiotic sensitivities of Staphylococcal isolatesa
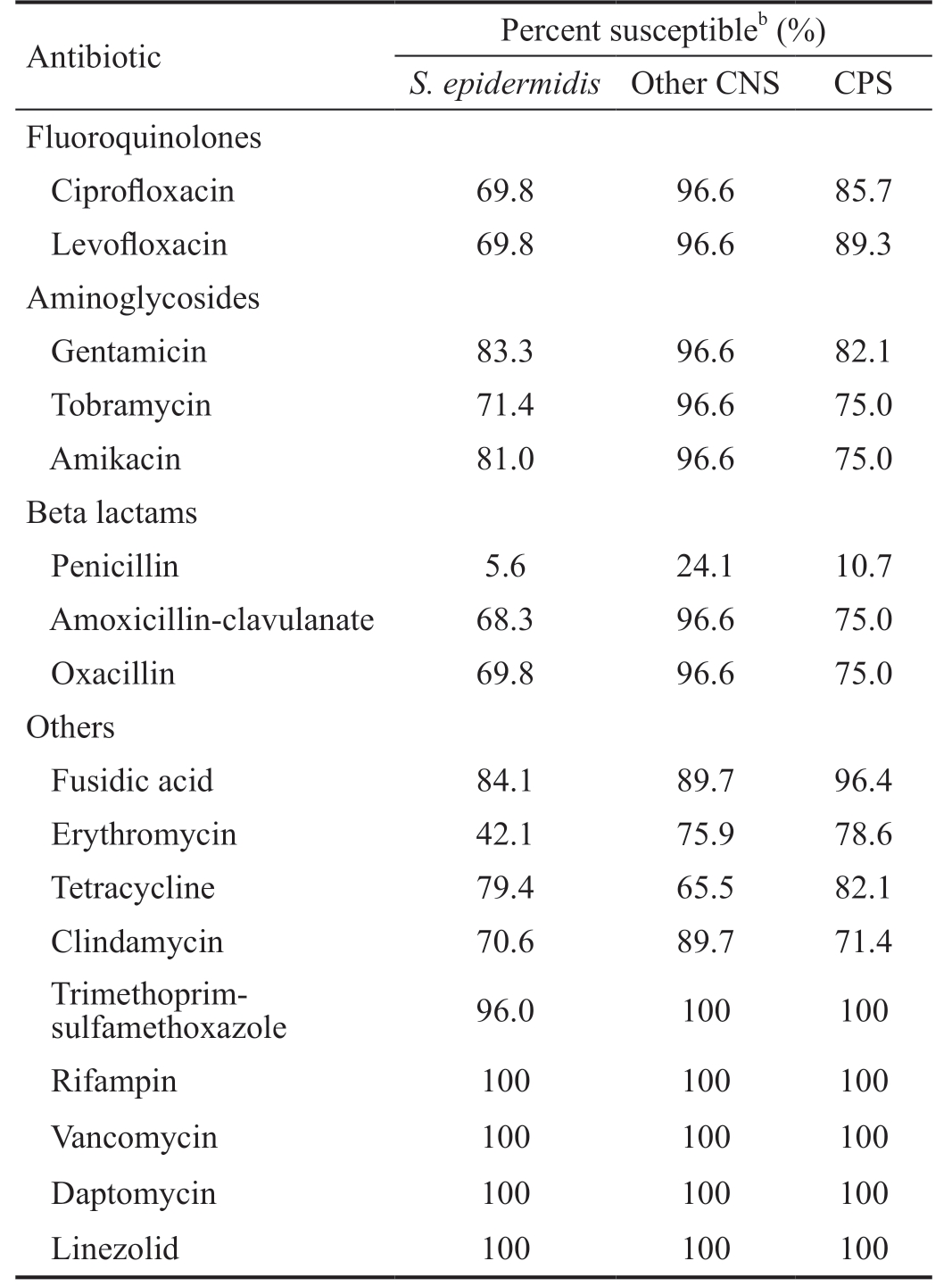
Antibiotic Percent susceptibleb (%)S. epidermidis Other CNS CPS Fluoroquinolones Ciprofloxacin 69.8 96.6 85.7 Levofloxacin 69.8 96.6 89.3 Aminoglycosides Gentamicin 83.3 96.6 82.1 Tobramycin 71.4 96.6 75.0 Amikacin 81.0 96.6 75.0 Beta lactams Penicillin 5.6 24.1 10.7 Amoxicillin-clavulanate 68.3 96.6 75.0 Oxacillin 69.8 96.6 75.0 Others Fusidic acid 84.1 89.7 96.4 Erythromycin 42.1 75.9 78.6 Tetracycline 79.4 65.5 82.1 Clindamycin 70.6 89.7 71.4 Trimethoprimsulfamethoxazole 96.0 100 100 Rifampin 100 100 100 Vancomycin 100 100 100 Daptomycin 100 100 100 Linezolid 100 100 100
S. epidermidis and CPS. Nevertheless, there was a higher susceptibility to levofloxacin of CPS (89.3%; 25/28) compared to S. epidermidis (69.8%; 88/126; P=0.036). Ciprofloxacin susceptibility rates were comparable to levofloxacin (85.7% for CPS and 69.8% for S. epidermidis), although in this case the statistical significance was not reached (P=0.104). The oxacillin resistant rate for S. epidermidis (30.2%; 38/126) and CPS (25.0%;7/28) were similar (P=0.653), while there was a significantly lower susceptibility to erythromycin of S. epidermidis (42.1%;53/126) compared to CPS (78.6%; 22/28; P=0.001).
Gram positive non-staphylococcal isolates (29 Streptococci, 7 Coryneform bacteria, 4 Enterococci and 2 Micrococci) showed high susceptibility to ampicillin (94.7%) and cefotaxime(97.2%; Table 4). Gentamicin was only tested in isolates of Coryneform bacteria and Micrococci, and all of them were susceptible. Oxacillin was tested in 19 isolates of viridans group Streptococci, of which 9 were resistant (47.4%). There was not any resistant Gram-positive (neither Staphylococcus nor no-Staphylococcus) to rifampin, vancomycin, daptomycin or linezolid (Tables 3 and 4).Regarding the Gram-negative isolates, they were mainly susceptible to beta lactams, specifically to cephalosporins(Table 5). All the tested strains to cefoxitin, cefotaxime,and cefepime were susceptible. Although these isolates did not include Pseudomonas spp. in the case of cefoxitin and cefotaxime, cefepime was tested in 6 isolates of Klebsiella spp., 5 Pseudomonas spp., 2 Serratia liquefaciens, and 1 Alcaligenes spp. In contrast, the antibiotic susceptibility rates to cefuroxime(88.9%) and ceftazidime (78.9%) were lower (Table 5).
Table 4 Antibiotic sensitivities of non-Staphylococcal Grampositive isolatesa
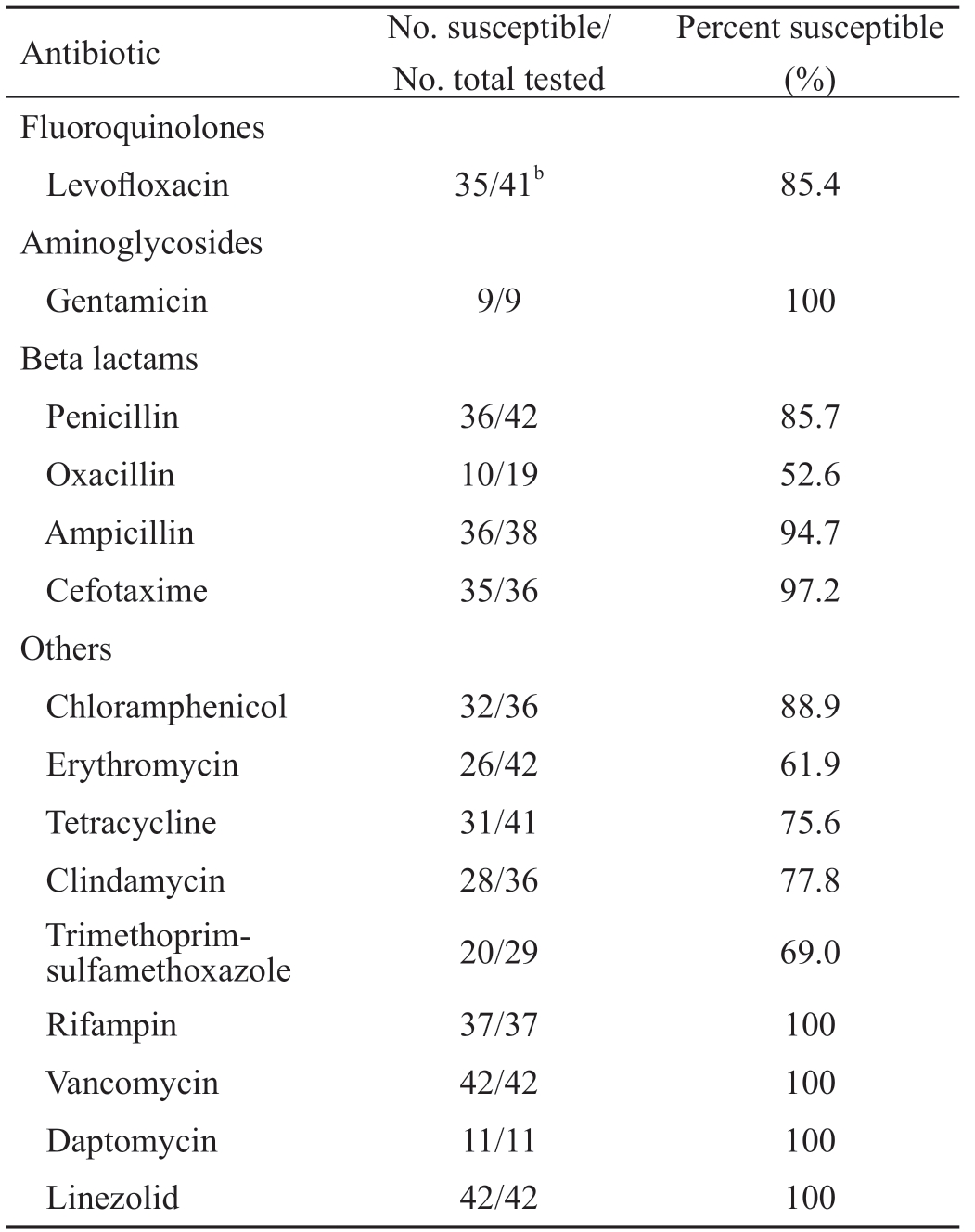
aTotal non-Staphylococcal Gram-positive isolates=42 (29 Streptococci;7 Coryneform bacteria; 4 Enterococci; and 2 Micrococci); bIn this case, levofloxacin sensitivities were performed on 41 of the 42 non-Staphylococcal Gram-positive isolates (levofloxacin sensitivity was not performed on 1 Coryneform bacterium).
Percent susceptible(%)Fluoroquinolones Antibiotic No. susceptible/No. total tested Levofloxacin 35/41b 85.4 Aminoglycosides Gentamicin 9/9 100 Beta lactams Penicillin 36/42 85.7 Oxacillin 10/19 52.6 Ampicillin 36/38 94.7 Cefotaxime 35/36 97.2 Others Chloramphenicol 32/36 88.9 Erythromycin 26/42 61.9 Tetracycline 31/41 75.6 Clindamycin 28/36 77.8 Trimethoprimsulfamethoxazole 20/29 69.0 Rifampin 37/37 100 Vancomycin 42/42 100 Daptomycin 11/11 100 Linezolid 42/42 100
Nine patients recognized to use self-prescribed antibiotic eye drops in their socket during the last month (6 patients use tobramycin, 2 ofloxacin, and 1 terramycin). However, a total of 36 isolates (17 from the healthy eye, 14 from the pre-prosthesis space, and 15 from the retro-prosthesis area) were recovered from these subjects. These isolates were 31 Gram-positive microorganisms (26 Staphylococci isolates: 20 S. epidermidis,5 S. aureus, 1 S. capitis; 3 viridans group Streptococci; and 1 isolate each of Enterococcus faecalis and Dermabacter hominis); 2 Gram-negative bacteria (both Klebsiella pneumoniae from the pre-prosthesis and retro-prosthesis space of the socket of the patient No.39); and 3 fungi (2 Candida parapsilosis and 1 Saccharomyces cerevisae, all from the socket of patient No.48). Gram-positive microorganisms from patients that used antibiotic eyedrops in their socket showed higher resistance to aminoglycosides (gentamicin,tobramycin, and amikacin) compared to Gram-positive isolates from patients who did not use antibiotics. This situation was observed in Gram-positive strains from both healthy eyes and sockets (Table 6). In addition, Gram-positive bacteria from healthy eyes of patients with antibiotic self-prescription were more resistant to tetracycline (P=0.034), although this difference was not found in socket isolates (P=0.790).
Table 5 Antibiotic sensitivities of Gram-negative isolatesa
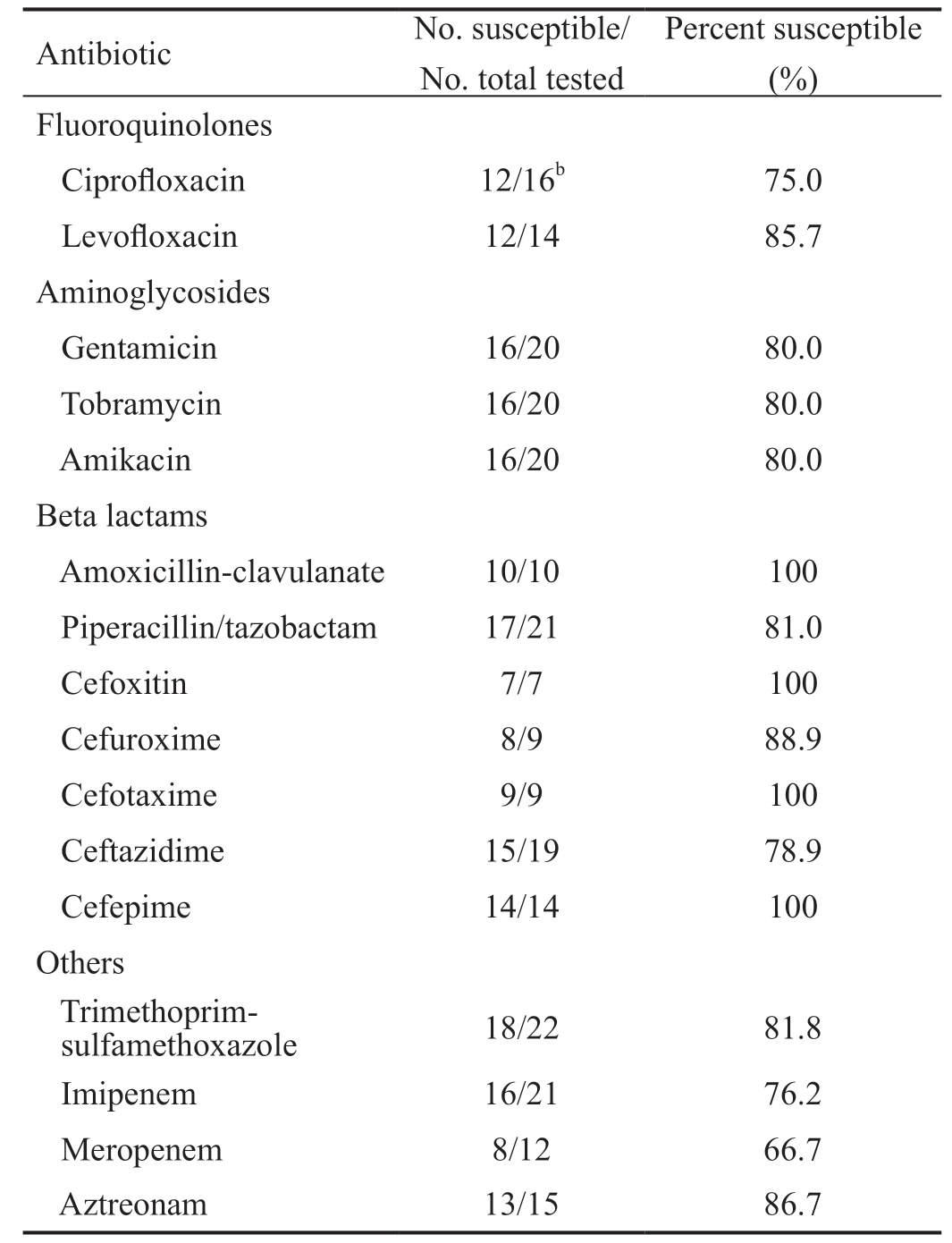
aTotal Gram-negative isolates=23 (6 Klebsiella spp., 5 Pseudomonas spp., 4 Stenotrophomonas maltophilia, 2 isolates each of Serratia liquefaciens, Alcaligenes spp., and Moraxella spp., and 1 isolate each of Enterobacter intermedium and Veillonella parvula); bIn this case,ciprofloxacin sensitivities were performed on 16 of the 23 Gramnegative isolates (ciprofloxacin sensitivities were not performed on 4 Stenotrophomonas maltophilia, 2 Moraxella spp., and 1 isolate of Veillonella parvula).
Antibiotic No. susceptible/No. total tested Percent susceptible(%)Fluoroquinolones Ciprofloxacin 12/16b 75.0 Levofloxacin 12/14 85.7 Aminoglycosides Gentamicin 16/20 80.0 Tobramycin 16/20 80.0 Amikacin 16/20 80.0 Beta lactams Amoxicillin-clavulanate 10/10 100 Piperacillin/tazobactam 17/21 81.0 Cefoxitin 7/7 100 Cefuroxime 8/9 88.9 Cefotaxime 9/9 100 Ceftazidime 15/19 78.9 Cefepime 14/14 100 Others Trimethoprimsulfamethoxazole 18/22 81.8 Imipenem 16/21 76.2 Meropenem 8/12 66.7 Aztreonam 13/15 86.7
DISCUSSION
This study has determined the microbiological spectrum and antibiotic sensitivity of conjunctival flora in anophthalmic patients. Overall positive culture rates in both healthy eyes(85%) and sockets (100%) were similar to reported by other authors[3-6]. The main difference found between healthy eyes and sockets flora was the greater isolation of pathogenic microorganisms in the sockets. The most commonly isolated bacterial organisms in our study were CNS, particularly S.epidermidis. CNS accounted for 77.4% (48/62) in healthy eyes and 56.6% (107/189) in sockets. Similar rates of CNS have been described in the literature. Studies of conjunctival flora in healthy adults have showed an isolation rate of CNS from 48.3% to 100%[14-16]. In sockets of anophthalmic patients, CNS have ranged from 25.8% to 59.1%[4-5]. This lower CNS rate in the socket corresponded to a greater isolation of pathogens.Previous reports have described an increase in the socket flora of CPS, Streptococci, and Gram-negative bacteria compared to the healthy eye flora[3-4,6]. Although our results only showed a significantly increase of Streptococci (P=0.020), classic aerobic pathogens (CPS, Streptococci and Gram-negative bacteria)accounted for less than 15% (9/62) of isolates from healthy eyes and more than 35% (71/189) of isolates from sockets(P=0.001).
This dysbiosis in the socket flora can be explained by the drastic changes in conjunctival epithelium after an enucleation or evisceration surgery. The lack of globe and the use of an artificial eye produce several modifications in the biomechanics of the ocular surface. First, the bulbar conjunctiva is no longer swept by the eyelids. Second, the artificial eye is a relatively large foreign body that can produce frictional irritation of the conjunctiva when the prosthesis moves[17]. Third, the dead space between the posterior surface of the prosthesis and the anterior surface of the socket allows the accumulation of conjunctival debris, even in custom fitted prostheses[7]. These circumstances promote the increase of mucus in the socket which in turn favors the growth of pathogenic microorganisms.Despite this remarkable disparity between the conjunctival flora of sockets and healthy eyes, we did not find differences in antibiotic sensitivities of organisms from both microbial communities. Therefore, antibiotic sensitivity analysis was performed by joining the isolates of sockets and healthy eyes.Isolated organisms (Gram-positive bacteria and Gram-negative bacteria) showed antibiotic susceptibilities comparable to previous studies on healthy conjunctival flora[14,18]. All Grampositive isolates were susceptible to vancomycin, as well as all Gram-negative isolates were susceptible to cefepime. Although the topical use of cefepime is currently limited, its stability in aqueous eyedrops has been proven[19].
As with other reports, S. epidermidis (50.2%; 126/251) and S. aureus (10.8%; 27/251) were the most frequently isolated species and exhibited a rate of oxacillin resistance of 30.2%and 22.2%, respectively. Oxacillin has replaced methicillin to identify staphylococcal isolates resistant to all beta lactam antibiotics and possibly more virulent infectious courses[13].In the present study, the percentages of oxacillin resistant S. epidermidis (ORSE) and oxacillin resistant S. aureus(ORSA) were relatively lower compared to other reports on conjunctival flora of patients undergoing cataract surgery[18,20].However, percentages of ORSE and ORSA are very variable according to age, geographical area, and antibiotic use[20].Different studies have placed the rate of ORSE from 4% to 47%; and the rate of ORSA from 0 to 64%[20-21].
Table 6 Antibiotic sensitivities of Gram-positive isolates according to previous use of antibiotic dropsa

aGram-positive isolates from healthy eyes and sockets of 51 patients without recent use of antibiotic drops were 53 and 141, respectively; and 7 and 24 isolates, respectively, of 9 patients with use of antibiotic drops in their sockets during the last month. bTwo-tailed Fisher’s exact test. NS:Number of susceptible isolates; NT: Total number of tested isolates; %: Percent susceptible.
Use of antibiotics in the socket Pb NS/NT (%) NS/NT (%) NS/NT (%) NS/NT (%)Fluoroquinolones Antibiotic Isolates from healthy eyes Isolates from sockets No history of recent use of antibiotics No history of recent use of antibiotics Use of antibiotics in the socket Pa Ciprofloxacin 36/47 (76.6) 4/7 (57.1) 0.358 87/113 (77.0) 13/20 (65.0) 0.268 Levofloxacin 41/53 (77.4) 4/7 (57.1) 0.351 115/141 (81.6) 16/23 (69.6) 0.259 Aminoglycosides Gentamicin 49/51 (96.1) 3/6 (50.0) 0.006 105/114 (92.1) 8/21 (38.1) 0.000 Tobramycin 39/47 (83.0) 2/6 (33.3) 0.019 92/111 (82.9) 7/20 (35.0) 0.000 Amikacin 44/47 (93.6) 3/6 (50.0) 0.015 98/111 (88.3) 7/20 (35.0) 0.000 Beta Lactams Penicillin 9/53 (17.0) 1/7 (14.3) 1.000 39/141 (27.7) 4/24 (16.7) 0.321 Amoxicillin-clavulanate 36/48 (75.0) 3/6 (50.0) 0.331 86/111 (77.5) 12/20 (60.0) 0.159 Oxacillin 36/49 (73.5) 3/6 (50.0) 0.342 95/124 (76.6) 13/23 (56.5) 0.069 Others Fusidic acid 40/50 (80.0) 6/6 (100) 0.578 100/113 (88.5) 19/20 (95.0) 0.693 Erythromycin 25/53 (47.2) 2/7 (28.6) 0.442 83/141 (58.9) 13/24 (54.2) 0.662 Tetracycline 44/53 (83.0) 3/7 (42.9) 0.034 109/141 (77.3) 17/23 (73.9) 0.790 Clindamycin 36/53 (67.9) 4/7 (57.1) 0.676 107/136 (78.7) 16/23 (69.6) 0.418 Trimethoprim-sulfamethoxazole 49/53 (92.5) 6/7 (85.7) 0.475 123/129 (95.3) 20/23 (87.0) 0.138
Regarding other antibiotic groups, S. epidermidis and CPS had a similar susceptibility to aminoglycosides (gentamicin,tobramycin, and amikacin). However, CPS (27 isolates of S. aureus and 1 isolate of S. intermedius) were slightly more susceptible to fluoroquinolones. In addition, antibiotic sensitivity to erythromycin of S. epidermidis (42.1%) compared to CPS (78.6%) was clearly lower (P=0.001). These differences in the antibiotic sensitivity between S. epidermidis and S.aureus may suggest the possible use of some antibiotics (such as macrolides and fluoroquinolones) to modify the flora in the anophthalmic sockets. In this sense, Dave et al[2] have reported a significant increase in the percentage of S. epidermidis isolated from the conjunctiva at the expense of S. aureus after repeated exposure to macrolides (azithromycin), and they have also demonstrated an increase of S. epidermidis compared to Gram-negative bacteria after exposure of conjunctiva to fluoroquinolone antibiotics (ofloxacin, gatifloxacin, and moxifloxacin). Therefore, fluoroquinolones (ciprofloxacin and levofloxacin) and macrolides (erythromycin) may be better than aminoglycosides for treating socket conjunctivitis, since they decrease pathogenic species and increase the rate of S.epidermidis.
One of the most interesting findings of this study is the clear correlation between antibiotic sensitivities of the socket flora exposed to antibiotic drops and the healthy eye flora (Table 6).Nine patients recognized that they routinely treated their socket with self-prescribed antibiotic drops to control the discharge. Tobramycin was the most commonly used antibiotic(6/9). The average rates of Gram-positive strains susceptible to aminoglycosides were 36% (gentamicin: 38%, 8/21;tobramycin: 35%, 7/20; and amikacin: 35%, 7/20) for socket cultures exposed to antibiotic and 44% for cultures from the fellow eye. On the other hand, aminoglycoside susceptibilities of cultures in patients without antibiotic use were 88% for Gram-positive organisms of the socket and 91% for healthy eye isolates. Organisms exposure to subinhibitory concentrations of tobramycin increase the resistances[22], as it happens with other antibiotics[2]. However, in patients undergoing intravitreal injections, antibiotics has been related to increase the resistant rate of organisms in the conjunctiva exposed to antibiotics[16,23],but not in the conjunctiva of the fellow eye[23].
The explanation of the change in antibiotic susceptibility in healthy eye flora of anophthalmic patients, when antibiotic drops are only used in the socket, would be a transfer of organisms from the socket to the healthy eye. Organisms cultured from the conjunctival sac are thought to reach the conjunctiva from the palpebral skin[24], so colonization of the ocular surface and surrounding tissues is a dynamic process[2].
The higher number of isolates from the socket compared to the fellow eye described in this study and by other reports[3-5]indirectly indicates a greater amount of organisms in the socket. Therefore, the socket would act as a reservoir of bacteria, which could be transferred to the conjunctiva of the healthy eye. A similar situation has been reported in fellow eyes of patients with unilateral nasolacrimal duct obstruction[25]. Although some authors have suggested the possible influence of socket organisms on the healthy eye[4-5,8,10], to our knowledge this is the first report of changes in the antibiotic sensitivity of the fellow eye flora in relation to the antibiotic treatment used in the socket.
This finding, if confirmed by other prospective studies, has a considerable clinical implication. As the main complaint of anophthalmic patients is discharge and crusting in the socket[1],antibiotics are commonly used to control these annoyances.However, antibiotic treatment does not achieve a long-term resolution[7] and favors the emergence of resistant flora in the socket. Some of the many resistant organisms in the socket can reach the conjunctiva of the fellow eye, by rubbing the eyes or manipulating the prosthesis. These organisms seriously increase the infectious risk of intraocular surgery on the healthy eye in anophthalmic patients.
There are a few limitations to our study. Our isolation technique failed in obtaining anaerobes. The initial pre-culture in conventional BHI under aerobic conditions probably prevented their further isolation. The data of MIC were not collected so bacteria were only classified as resistant or susceptible. In addition, some interesting antibiotics in the treatment of ocular infections such as azithromycin, gatifloxacin or moxifloxacin were not evaluated. Finally, the observational design of our study prevented an accurate evaluation of conjunctival flora changes based on the use of antibiotics. In this sense,a prospective, longitudinal study in anophthalmic patients is needed to describe how flora of sockets and healthy eyes change over time.
The outcomes of this study describe the presence of a pathogenic flora in sockets compared to healthy eyes in anophthalmic patients. This pathogenic flora of the socket can proliferate causing discharge and annoyances, and leading to use antibiotic eyedrops to control these symptoms. In this study, socket exposition to antibiotic eyedrops has been related to a higher resistant flora not only in the socket but also in the healthy eye. This situation is a risk factor for developing a severe infection in the healthy eye of anophthalmic patients.Based on our results, quinolones (ciprofloxacin and levofloxacin)and macrolides (erythromycin) may be better therapeutic options than aminoglycosides for treating uncomplicated infections (conjunctivitis) of anophthalmic sockets, since they are more effective in eliminating pathogenic species such as S.aureus and less active against S. epidermidis.
On the other hand, in the case of a severe infection (corneal ulcer or endophthalmitis) in the healthy eye of an anophthalmic patient, vancomycin for Gram-positive organisms and cefepime for Gram-negative bacteria are the most useful antibiotics, since no resistant strain to these antibiotics has been identified in our series.
ACKNOWLEDGEMENTS
Foundations: Supported by The Dirección General de Investigación (No.SAF 2015-64306-R); The Junta de Castilla y León, Spain (No.LE283U14).
Conflicts of Interest: Toribio A, None; Marrodán T, None;Fernández-Natal I, None; Martínez-Blanco H, None;Rodríguez-Aparicio L, None; Ferrero MÁ, None.
1 Pine K, Sloan B, Stewart J, Jacobs RJ. Concerns of anophthalmic patients wearing artificial eyes. Clin Exp Ophthalmol 2011;39(1):47-52.
2 Dave SB, Toma HS, Kim SJ. Changes in ocular flora in eyes exposed to ophthalmic antibiotics. Ophthalmology 2013;120(5):937-941.
3 Christensen JN, Fahmy JA. The bacterial flora of the conjunctival anophthalmic socket in glass prosthesis-carriers. Acta Ophthalmol(Copenh) 1974;52(6):801-809.
4 Miller SD, Smith RE, Dippe DW, Lacey DR, Abel M. Bacteriology of the socket in patients with prostheses. Can J Ophthalmol 1976;11(2):126-129.
5 Patillon JC, Rousse C, Gauthier C, Guyot J, Barbier A, Royer J, Michel-Briand Y. Bacterial flora of the conjunctiva in enucleated subjects. Bull Soc Ophtalmol Fr 1978;78(11):781-787.
6 Vasquez RJ, Linberg JV. The anophthalmic socket and the prosthetic eye. A clinical and bacteriologic study. Ophthalmic Plast Reconstr Surg 1989;5(4):277-280.
7 Campos MS, Campos e Silva Lde Q, Rehder JR, Lee MB, O’Brien T, McDonnell PJ. Anaerobic flora of the conjunctival sac in patients with AIDS and with anophthalmia compared with normal eyes. Acta Ophthalmol (Copenh) 1994;72(2):241-245.
8 López-Sánchez E, España Grégori E, Roda Marzal V, Bueno I,Francés Muñoz E, Menezo JL. Conjunctival microbiological study in corneo-schleral prosthesis users. Arch Soc Esp Oftalmol 2001;76(11):669-672.
9 Toribio A, Marrodán T, Fernández-Natal I, Martínez-Blanco H,Rodríguez-Aparicio L, Ferrero MÁ. Study of conjunctival flora in anophthalmic patients: influence on the comfort of the socket. Graefes Arch Clin Exp Ophthalmol 2017;255(8):1669-1679.
10 Morris R, Camesasca FI, Byrne J, John G. Postoperative endophthalmitis resulting from prosthesis contamination in a monocular patient. Am J Ophthalmol 1993;116(3):346-349.
11 Kim SJ, Toma HS, Midha NK, Cherney EF, Recchia FM, Doherty TJ. Antibiotic resistance of conjunctiva and nasopharynx evaluation study: a prospective study of patients undergoing intravitreal injections.Ophthalmology 2010;117(12):2372-2378.
12 Suwantarat N, Weik C, Romagnoli M, Ellis BC, Kwiatkowski N,Carroll KC. Practical utility and accuracy of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of corynebacterium species and other medically relevant coryneform-like bacteria. Am J Clin Pathol 2016;145(1):22-28.
13 Gentile RC, Shukla S, Shah M, Ritterband DC, Engelbert M, Davis A, Hu DN. Microbiological spectrum and antibiotic sensitivity in endophthalmitis: a 25-year review. Ophthalmology 2014;121(8):1634-1642.
14 Tao H, Wang J, Li L, Zhang HZ, Chen MP, Li L. Incidence and antimicrobial sensitivity profiles of normal conjunctiva bacterial flora in the central area of China: a hospital-based study. Front Physiol 2017;8:363.
15 Hsu HY, Lind JT, Miller D, Tseng L. Assessment of risk factors for oxacillin-resistant ocular flora in eyes having cataract surgery. J Cataract Refract Surg 2015;41(2):387-392.
16 Grzybowski A, Brona P, Kim SJ. Microbial flora and resistance in ophthalmology: a review. Graefes Arch Clin Exp Ophthalmol 2017;255(5):851-862.
17 Pine KR, Sloan B, Jacobs RJ. Deposit buildup on prosthetic eyes and implications for conjunctival inflammation and mucoid discharge. Clin Ophthalmol 2012;6:1755-1762.
18 Barría von-B F, Chabouty H, Moreno R, Ortiz F, Barría MF. Microbial flora isolated from patient’s conjunctiva previous to cataract surgery. Rev Chilena Infectol 2015;32(2):150-157.
19 Kodym A, Pawłowska M, Rumiński JK, Bartosińska A,Kieliba A. Stability of cefepime in aqueous eye drops. Pharmazie 2011;66(1):17-23.
20 Papa V, Blanco AR, Santocono M. Ocular flora and their antibiotic susceptibility in patients having cataract surgery in Italy. J Cataract Refract Surg 2016;42(9):1312-1317.
21 Asbell PA, DeCory HH. Antibiotic resistance among bacterial conjunctival pathogens collected in the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) surveillance study. PLoS One 2018;13(10):e0205814.
22 Lin YH, Kang YC, Hou CH, Huang YC, Chen CJ, Shu JC, Hsieh PH, Hsiao CH. Antibiotic susceptibility profiles of ocular and nasal flora in patients undergoing cataract surgery in Taiwan: an observational and cross-sectional study. BMJ Open 2017;7(8):e017352.
23 Milder E, Vander J, Shah C, Garg S. Changes in antibiotic resistance patterns of conjunctival flora due to repeated use of topical antibiotics after intravitreal injection. Ophthalmology 2012;119(7):1420-1424.
24 Suto C, Morinaga M, Yagi T, Tsuji C, Toshida H. Conjunctival sac bacterial flora isolated prior to cataract surgery. Infect Drug Resist
2012;5:37-41.
25 Eshraghi B, Alemzadeh SA, Abedinifar Z. Conjunctival bacterial flora in fellow eyes of patients with unilateral nasolacrimal duct obstruction and its changes after successful dacryocystorhinostomy surgery. J Curr Ophthalmol 2017;29(1):59-62.