INTRODUCTION
C ataract surgery is one of the most common and most successful surgical interventions today. Yet, from intra and postoperative complications to patient compliance; it is not without issues. Like other types of surgeries, cataract surgery also induces an inflammatory response, commonly anterior segment inflammation and macular oedema.
Postoperative regimens to minimise inflammation vary widely among clinicians. Studies[1-2] have evaluated the efficacy of alternative administrative routes of corticosteroids apart from topical to deliver higher and more stable intraocular concentrations and improve patient compliance. Injection of depot corticosteroids into sub-Tenon’s capsule is an established approach of treating various ocular inflammatory diseases[3-4].Postoperative topical drops such as NSAIDs (e.g. ketorolac and nepafenac)[5-6] and steroids (e.g. dexamethasone and prednisolone)[7] have been the mainstay for prophylaxis and treatment of inflammation. Unfortunately, adherence to topical medication regimens is less than ideal[8].
The current study is designed to evaluate the effects of a single subconjunctival triamcinolone acetonide (SCTA) injection to that of topical loteprednol on intraocular pressure (IOP),anterior chamber inflammation and macular oedema post cataract surgery.
SUBJECTS AND METHODS
Ethical Approval This study was conducted at a tertiary eye care centre in Southern India. Institution Ethics committee approval was obtained before commencement of the study and informed consent taken from each participant. The study was performed in accordance with the tenets of the Declaration of Helsinki.
It was designed as a randomised prospective interventional study and comprised of patients who underwent phacoemulsification surgery for cataract over duration of 6mo from July 1st 2016 to Dec 31st 2016. A consort flow diagram for recruitment and analysis of patients in this study is shown below (Figure 1). Patients were randomised 1:1 on the basis of simple coin flipping technique. Group A patients were to receive 5 mg SCTA at the end of surgery with topical ketorolac tromethamine (0.5%) and ofloxacin (0.3%) combination 4 times per day for 7d followed by 2 times per day for 2wk. Group B patients were administered tapering topical loteprednol (0.5%) 6-4-3-2-1 times per day for 7d each with ofloxacin (0.3%) and ketorolac tromethamine (0.5%) 4 times per day for 1wk followed by 2 times per day for 2wk. Patients were followed up at 1wk (visit 1), 6wk (visit 2), and 12wk(visit 3) for evaluation of IOP, anterior chamber cells/flare and macular oedema and other adverse events if any.
Inclusion/Exclusion Criteria Patients eligible for inclusion were all those who had a cataractous lens according to Lens Opacities Grading System III (LOCS III)[9]. Patients excluded were those who had cataract with grade 5, 6 nuclear colour/opalescence, a prior history of uveitis or evident intraocular inflammation, suffering from glaucoma, high myopes (axial length >25 mm), known steroid responders, previous ocular surgeries, corneal diseases. Also excluded were patients with diabetes, hypertension and asthma or on any systemic antiinflammatory therapy. In addition, those who developed any intra-operative complications such as posterior capsule rupture,zonular dialysis or those who could not complete the required follow up visits were also to be excluded.
Baseline Evaluation Patients were advised not to take any systemic anti-inflammatory drugs during the study period.Preoperative parameters measured were IOP in mm Hg by Non-contact tonometry (NCT; Topcon CT-80, Topcon Corp,Japan). Slit lamp biomicroscopy examination (Topcon SL 1E, Topcon Corp, Japan) was used to rule out the presence of cells/flare in the anterior chamber and macular edema. Best corrected visual acuity (BCVA) using Snellen visual acuity was noted for every patient.
Study Medication A vial of triamcinolone acetonide injection(Tricort 10, Cadila Pharmaceuticals) of 1 mL containing 10 mg/mL was used. A 1-mL syringe with 26 gauge needle was utilised to withdraw 0.5 mL (5 mg) which was subsequently injected subconjunctivally at the end of surgery.
Surgical Technique Routine phacoemulsification under topical anaesthesia and implantation of a foldable hydrophobic acrylic monofocal 1 piece posterior chamber intraocular lens(Technis 1, Santa Ana, California, AMO Inc., USA) was performed by a single surgeon. After the conclusion of surgery,5 mg SCTA was injected under the inferior bulbar conjunctiva in patients of Group A (Figure 2).
Outcome Measures Follow up visits were advised at 1, 6, and 12wk and BCVA was recorded. Primary outcomes evaluated were IOP, anterior chamber inflammation and cystoid macular oedema. IOP was measured with the help of NCT with the mean of two measurements used for analysis.
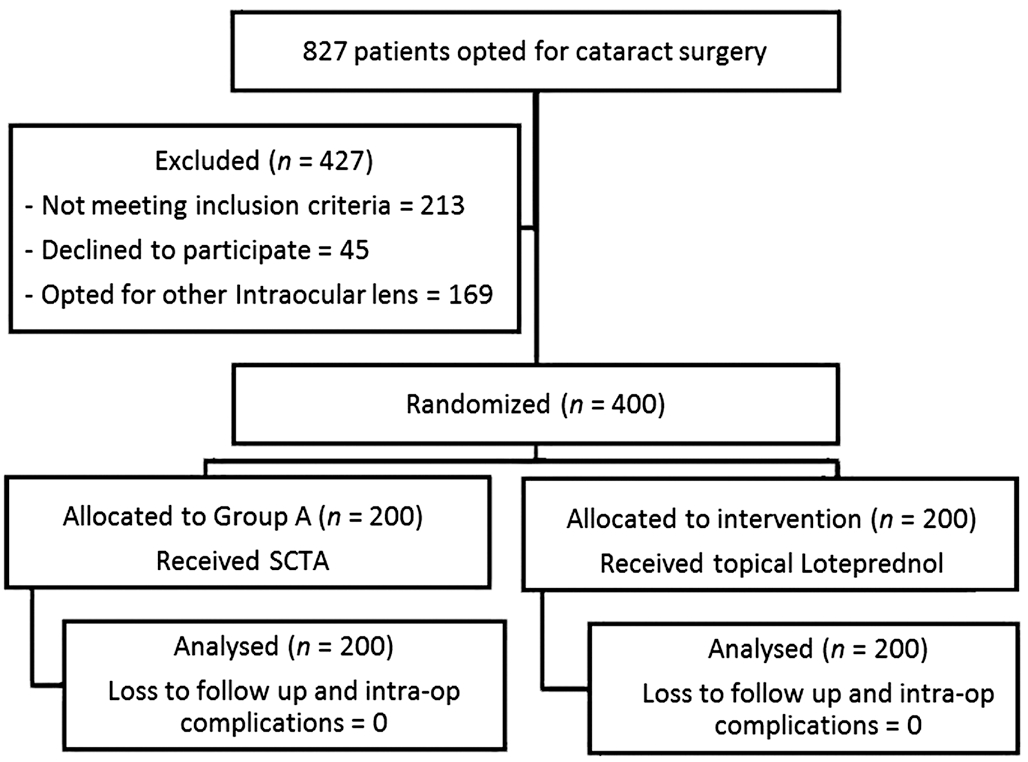
Figure 1 Study profile of patient flow.

Figure 2 A peri-operative subconjunctival injection of triamcinolone acetonide.
Anterior chamber cells/flare were evaluated on slit lamp and graded according to Standardization of Uveitis Nomenclature Working Group criteria (SUN)[10]. Macular oedema was assessed clinically and using Spectralis optical coherence tomography (Cirrus HD OCT 4000).
Secondary outcomes evaluated were the size of SCTA depot in millimetres (mm) at every visit and also local adverse effects like subconjunctival haemorrhage (SCH), chemosis,congestion or necrosis, if any.
Statistical Analysis Data was analysed using commercial software (SPSS version 14.0 Inc., Chicago, Illinois, USA).Paired t-test (within group) and unpaired t-test (between groups) were used to compare the IOP values. Pearson Chisquare test was used to analyse patient demographics and the presence of anterior chamber cells/flare and development of macular oedema. P value less than 0.05 was considered statistically significant (95% confidence interval) for all variables. Secondary outcomes except size of SCTA depot were analysed qualitatively.
RESULTS
This study enrolled 400 patients. Each treatment group comprised of 200 patients. There was no loss to follow up for any patient in either group. The demographics of the study population have been presented in Table 1.
Figure 3 depicts the mean IOP and their standard deviations(SD) at baseline, postoperative 1, 6, 12wk in Group A receiving SCTA and Group B receiving topical loteprednol.The mean IOP values at each postoperative visit were not statistically significant between Group A and Group B at visit 1 (P=0.14), visit 2 (P=0.06) and visit 3 (P=0.12). Nine patients in Group A had an IOP>21 mm Hg on visit 1, which reduced to 3 patients on visit 2 and to 2 patients on visit 3. There was also no statistical difference in the mean preoperative and postoperative IOP values within Group A (P=0.82) and within Group B (P=0.61) at visit 1. Similar findings were noted at visit 2 where the difference in IOP values were not significant for both Group A (P=0.57) and Group B (P=0.33) compared to baseline values. Visit 3 also revealed similar results for Group A (P=0.65) and Group B (P=0.39). Two patients who had consistent IOP values of greater than 25 mm Hg at all visits underwent excision of SCTA after 12wk follow up.
Five percent patients among Group A developed anterior chamber cells/flare by visit 1 compared to 6% among Group B. The incidence of cells/flare reduced to 1% and 1.5% of total patients in Groups A and B respectively by visit 2. There was complete resolution of cells/flare in both groups by visit 3 as seen in the data given in Table 2. The difference in anterior chamber cells/flare scores between both the groups were not statistically significant at both visit 1 and visit 2 (P=0.82).
Among both the groups receiving treatment, there was no evidence of postoperative macular oedema on slit lamp examination which was then confirmed on optical coherence tomography (OCT). There weren’t any other abnormal clinically significant ophthalmic findings.
Mean size of the SCTA depot at the time of injection was 6.5 mm.On follow up the mean sizes were 3.5 mm at visit 1, 2.25 mm at visit 2 and 1.15 mm at visit 3. Minimal SCH was noted in 5 patients on visit 1 which completely resolved by visit 2. No other local adverse effects like chemosis, congestion, necrosis or ulceration were seen in any of the follow up visits.
DISCUSSION
With improvement in cataract surgical techniques, the incidence of postoperative inflammation has declined but still persists. To counter it, various modalities are being tested one of which is the subconjunctival injection of steroids immediately after cataract surgery. With increasing incidence of cataracts in recent times, lack of compliance to postoperative topical medications from patients, increasing cost, and patient inconvenience etc. a single subconjunctival injection of steroid could be a viable alternative to postoperative topical steroid use. Our study shows that a single injection of SCTA at the end of an uneventful cataract surgery is equally effective if not more to prolonged topical steroid use.
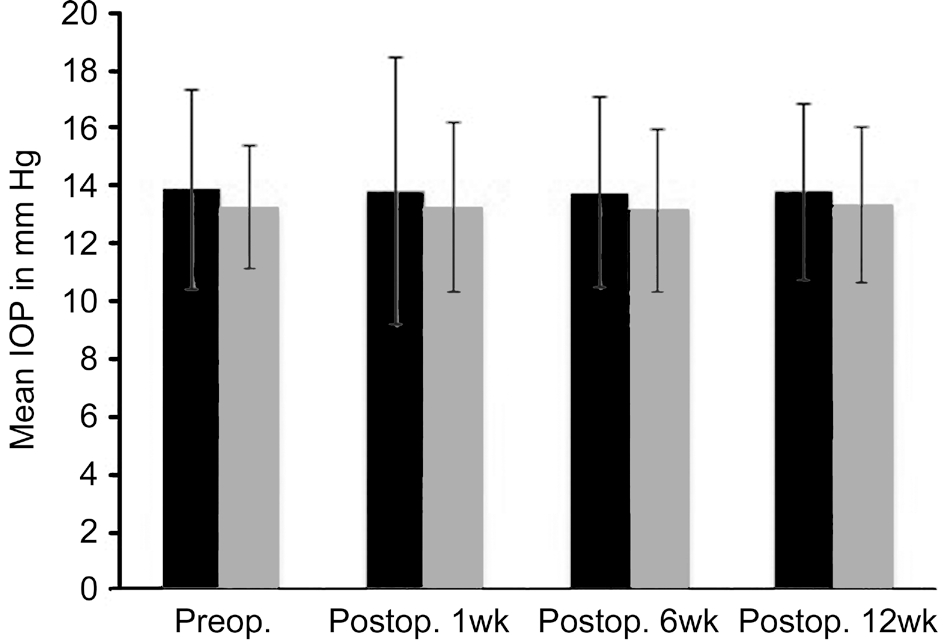
Figure 3 Comparison of IOP values between patients who received peri-operative SCTA (Group A) and patients receiving postoperative topical loteprednol (Group B).
Table 1 Patient characteristics
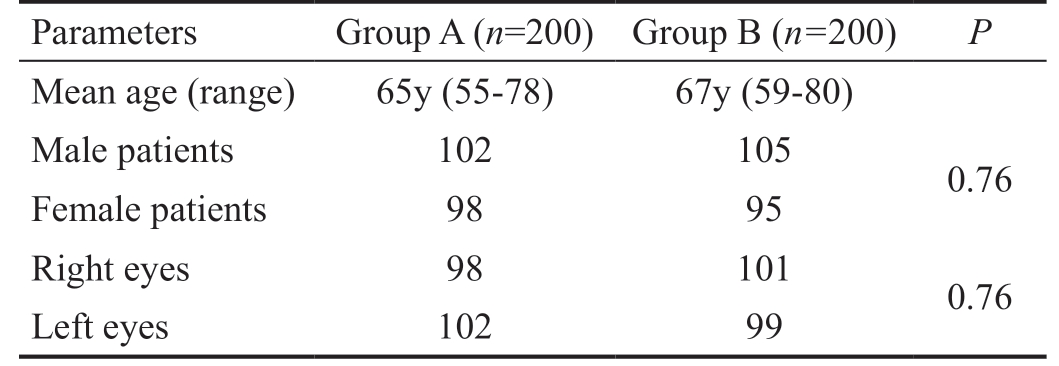
Parameters Group A (n=200) Group B (n=200) P Mean age (range) 65y (55-78) 67y (59-80)Male patients 102 105 0.76 Female patients 98 95 Right eyes 98 101 0.76 Left eyes 102 99
Table 2 Number of patients having anterior chamber cells/flare
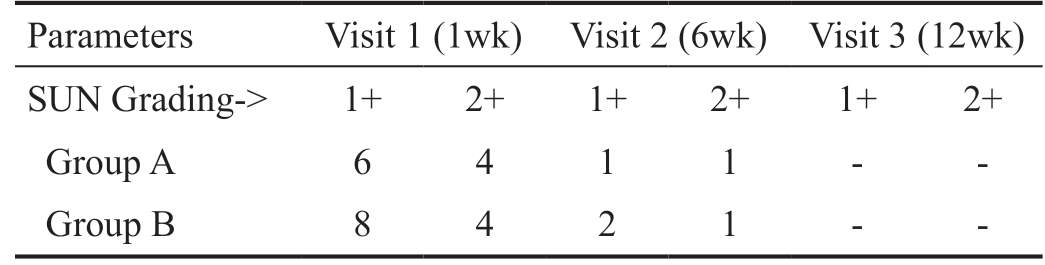
SUN: Standardization of uveitis nomenclature.
Parameters Visit 1 (1wk) Visit 2 (6wk) Visit 3 (12wk)SUN Grading-> 1+ 2+ 1+ 2+ 1+ 2+Group A 6 4 1 1 - -Group B 8 4 2 1 - -
Weijtens et al[11] suggested that periocular injections of corticosteroids induce a higher concentration of the steroidal agent than topical administration. Of particular concern, is the increase in IOP after administration of systemic corticosteroids[12].
But changes in IOP, our main outcome measure were not significant in the group receiving SCTA in comparison to the baseline values. Nine patients had an IOP>21 mm Hg on visit 1 (among those 4 had an IOP>30 mm Hg). Follow up visits showed improvement in IOP values and by visit 3,IOP values of almost all patients returned to their baseline levels, except only 2 patients among Group A who had an IOP>25 mm Hg. Surgical excision of depot injection has been suggested and may be required in patients who have received sub-Tenon’s capsule injections of corticosteroids and are not responding to maximal anti-glaucoma therapy[13]. Considering the above, those 2 patients underwent excision of SCTA under topical anaesthesia, the indication being uncontrolled IOP.Post excision, the IOP in these patients promptly reduced to the normal range without requiring any further topical antiglaucoma therapy.
Coming to anterior chamber cells/flare, which were graded on the basis of SUN for reporting clinical data; the results showed almost equal distribution with no statistical difference in patients having cells/flare postoperatively in between both Group A and B during visit 1 and visit 2 (P=0.82). Complete resolution of cells/flare was noted by visit 3. Study conducted by Shah and Spalton[14] showed similar course in resolution of cells/flare to almost nil by 12wk. Laser flare meter has
been used for the objective assessment of anterior chamber inflammatory activity[15]. Studies conducted by Tan et al[16]and Negi et al[17] using the above mentioned method showed similar results. The postoperative cells/flare counts in our study were clinically insignificant and thus did not warrant any extra treatment in both groups.
Macular oedema, a common complication of cataract surgery is best detected using OCT and in particular using the center point thickness parameter[18-19]. Since OCT can achieve a highresolution measurement of retinal thickness of up to 10 mm,it may be able to detect subtle changes in retinal thickness
which is not clinically evident[20]. Macular oedema mostly peaks at around 4 to 6wk after cataract surgery and can also develop several months postoperatively[21]. Macular oedema has been defined as a relative increased diffuse thickening,presenting with or without cystoid abnormalities and with or without visual loss. Among both groups, no patient developed postoperative macular oedema at any follow up visit.
Size of SCTA depot at the time of injection was around 6-7 mm.By visit 3 the depot had completely disappeared in greater than 95% patients and the rest had a depot of less than 2 mm.Location and size of the depot was cosmetically acceptable.Conjunctival ischemia and necrosis following periocular/intraocular injection of triamcinolone acetonide had been reported in adult patients[22-23]. In our study, minimal SCH was noted in 5 patients at visit 1 which completely resolved by visit 2. Apart from SCH, no other local adverse effects like chemosis, congestion or necrosis/ulceration were noted at any follow up visits.
Limitations of our study include lack of laser flare meter measurements for assessment of flare postoperatively, need for longer follow up especially for analysing macular edema.Wider group of patients not assessed in this study e.g. patients falling in our exclusion criteria; for us to confidently advocate safety and efficacy in various scenarios. An evaluation of subjective patient satisfaction or comfort score to assess the patient’s perspective regarding the therapeutic modalities could have added value to this study. Advantage of this study included large sample size with a proper follow up of each and every patient.
In our opinion, a single subconjunctival injection of triamcinolone acetonide at the end of a routine uneventful phacoemulsification surgery can be a useful alternative to prolonged tapering topical steroid use in preventing intraocular inflammation and macular oedema. Since the number,frequency and duration of instilling eye drops is significantly reduced in the SCTA group it definitely contributes to faster rehabilitation coupled with greater patient comfort. Further studies are needed for detailed analysis and to cover a broader spectrum of patients.
ACKNOWLEDGEMENTS
Conflicts of Interest: Reddy JK, None; Chaitanya V,None; Shah N, None; Guduru VP, None; Khan S, None;Kuttupalayam S, None.
1 Watson D, Noble MJ, Dutton GN, Midgley JM, Healey TM. Penetration of topically applied dexamethasone alcohol into human aqueous humor.Arch Ophthalmol 1988;106(5):686-687.
2 Chang DF, Wong V. Two clinical trials of an intraocular steroid delivery system for cataract surgery. Trans Am Ophthalmol Soc 1999;97:261-279.
3 Helm CJ, Holland GN. The effects of posterior subtenon injection of triamcinolone acetonide in patients with intermediate uveitis. Am J Ophthalmol 1995;120(1):55-64.
4 Zamir E, Read RW, Smith RE, Wang RC, Rao NA. A prospective evaluation of subconjunctival injection of triamcinolone acetonide for resistant anterior scleritis. Ophthalmology 2002;109(4):798-805; discussion 805-807.
5 Flach AJ, Jaffe NS, Akers WA. The effect of ketorolac tromethamine in reducing postoperative inflammation: double-mask parallel comparison with dexamethasone. Ann Ophthalmol 1989;21(11):407-411.
6 Cervantes-Coste G, Sánchez-Castro YG, Orozco-Carroll M, Mendoza-Schuster E, Velasco-Barona C. Inhibition of surgically induced miosis and prevention of postoperative macular edema with nepafenac. Clin Ophthalmol 2009;3:219-226.
7 Diestelhorst M, Aspacher F, Konen W, Krieglstein GK, Hilgers RD.Effect of dexamethasone 0.1% and prednisolone acetate 1.0% eye drops on the blood-aqueous barrier after cataract surgery: a controlled randomized fluorophotometric study. Graefes Arch Clin Exp Ophthalmol 1992;230(5):451-453.
8 Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology 2009;116(11 Suppl):S30-S36.
9 Hall NF, Lempert P, Shier RP, Zakir R, Phillips D. Grading nuclear cataract: reproducibility and validity of a new method. Br J Ophthalmol 1999;83(10):1159-1163.
10 Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140(3):509-516.
11 Weijtens O, Schoemaker RC, Romijn FP, Cohen AF, Lentjes EG, van Meurs JC. Intraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphate. Ophthalmology 2002;109(10):1887-1891.
12 Herschler J. Increased intraocular pressure induced by repository corticosteroids. Am J Ophthalmol 1976;82(1):90-93.
13 Akduman L, Kolker AE, Black DL, Del Priore LV, Kaplan HJ.Treatment of persistent glaucoma secondary to periocular corticosteroids.Am J Ophthalmol 1996;122(2):275-277.
14 Shah SM, Spalton DJ. Changes in anterior chamber flare and cells following cataract surgery. Br J Ophthalmol 1994;78(2):91-94.
15 el-Maghraby A, Marzouki A, Matheen TM, Souchek J, Van der Karr M. Reproducibility and validity of laser flare/cell meter measurements as an objective method of assessing intraocular inflammation. Arch Ophthalmol 1992;110(7):960-962.
16 Tan DT, Chee SP, Lim L, Lim AS. Randomized clinical trial of a new dexamethasone delivery system (Surodex) for treatment of post-cataract surgery inflammation. Ophthalmology 1999;106(2):223-231.
17 Negi AK, Browning AC, Vernon SA. Single perioperative triamcinolone injection versus standard postoperative steroid drops after uneventful phacoemulsification surgery: Randomized controlled trial. J Cataract Refract Surg 2006;32(3):468-474.
18 Kim SJ, Belair ML, Bressler NM, Dunn JP, Thorne JE, Kedhar SR, Jabs DA. A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina 2008;28(6):870-876.
19 Kim SJ, Equi R, Bressler NM. Analysis of macular edema after cataract surgery in patients with diabetes using optical coherence tomography. Ophthalmology 2007;114(5):881-889.
20 Hee MR, Puliafito CA, Wong C, Duker JS, Reichel E, Rutledge B,Schuman JS, Swanson EA, Fujimoto JG. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol 1995;113(8):1019-1029.
21 Rotsos TG, Moschos MM. Cystoid macular edema. Clin Ophthalmol 2008;2(4):919-930.
22 Rubinstein A, Hanson RJ, Chen SD, Porter N, Downes SM.Conjunctival ischaemia subsequent to posterior subtenon’s triamcinolone acetonide injection. Eye (Lond) 2006;20(3):388-389.
23 Agrawal S, Agrawal J, Agrawal TP. Conjunctival ulceration following triamcinolone injection. Am J Ophthalmol 2003;136(3):539-540.