INTRODUCTION
G laucoma is the first reported irreversible blindness disease in the world[1]. Currently, it can be treated by consistent use of glaucoma medication, laser, or surgery[2].Long-term usage of hypotensive eye drops might be safe but need to be used several times a day. The ocular surface may be harmed by the preservative, and the reduction of the intraocular pressure (IOP) is limited to 30%, with IOP fluctuations occurring[3]. Surgery can achieve a lower IOP;however, there are risks and complications associated with surgery[4]. Laser therapy is more widely used because of its efficacy and safety[5-8].
Laser trabeculoplasty (LTP) for open angle glaucoma (OAG)includes Argon laser trabeculoplasty (ALT), selective laser trabeculoplasty (SLT), micropulse laser trabeculoplasty (MLT),and Titanium-Sapphire Laser Trabeculoplasty[5-10]. MLT technology, which was innovated 10 years ago, uses a dutycycle algorithm that delivers subthreshold treatment to ocular tissues without scar formation[11]. MLT has been applied to the treatment of macular edema in retinal vein occlusion, diabetic retinopathy, and central serous chorioretinopathy[12-14], and is now used for the treatment of OAG[5-9]. MLT has a theoretical advantage over other laser therapies by not destroying the pigmented trabecular meshwork cells[11,15-17].
The purpose of this retrospective study was to evaluate the efficacy and safety of MLT on primary open angle glaucoma(POAG) patients. The IOP and glaucoma medications were compared before and after MLT.
SUBJECTS AND METHODS
Ethical Approval The study was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of Peking University Third Hospital (PUTH; No.2014166). All patients had been fully informed of the purpose and methods of the present study and provided written informed consent from themselves.
Subjects A series of Chinese POAG patients treated with MLT at Peking University Third Hospital Ophthalmology Department from June 2016 to November 2017 were enrolled.One eye of each patient was randomly treated by MLT.
This retrospective study included 72 eyes of 72 POAG patients, including newly diagnosed cases and cases with prior glaucoma medication. Inclusion criteria were as follows: 1)age>18y; 2) IOP>21 mm Hg; 3) open angle on gonioscopy(Shaffer grading >1 in 270); and 4) glaucomatous visual field loss and optic nerve fiber defects[11,15-17]. Cases were excluded if they involved angle closure, corneal pathologies, or if the patients had received prior laser trabeculoplasty.
Micropulse Laser Trabeculoplasty Therapy and Post Laser Management MLT was performed with the IRIS Medical OcuLight SLx 532 IQ Laser System (IRIDEX Corporation,Mountain View, CA, USA). Two drops of topical anesthesia(0.4% oxybuprocaine hydrochloride eye drops; Santen Pharmaceutical Co., Ltd., Osaka, Japan) was applied prior to MLT. No IOP-lowering drugs were used prior to MLT procedure. MLT was performed by the first author (Hong Y) with the same laser settings. Patients were seated at the slit lamp and a special MLT lens was placed on the eye to be treated with an inner face guide that allowed the surgeon to deliver exactly 10 confluent laser shots per clock hour. The MLT setting was 300 μm spot size diameter, 1 W power, 300ms duration with 15% duty cycle. The laser was carefully focused on the anterior trabecular meshwork and 120 laser spots were evenly distributed around 360° in the trabecular meshwork.
The IOPs at 2h, 1d, 1, 4, 12 and 24wk after MLT were recorded. IOP was always measured between 08:00 and 10:00 a.m. to minimize the effects of diurnal variations. Glaucoma medication was recorded at follow-up time points and adjusted by the IOP from 4wk post-MLT. The complications during and after MLT were observed. The complications included cornea side effects, hyphema, trabecular meshwork burn, peripheral anterior synechiae, and IOP spikes. An IOP spike was defined as an IOP increase of at least 5 mm Hg after MLT[18-19].
Statistical Analysis Statistical analyses were performed using SPSS, version 22.0 software (SPSS Inc., Chicago, IL, USA).The IOP and the numbers of antiglaucoma eye drops were presented as the mean±standard deviation (SD). Repeated measurement analysis of variance (ANOVA) was performed to compare the mean IOP at different follow-up time points to baseline. Post hoc LSD t-tests were performed to compare all pairs of independent variables. Paired samples t-tests were performed for the antiglaucoma eye drops before and after MLT. P values <0.05 were considered statistically significant.
RESULTS
Patients’ Characteristics There were 72 eyes of 72 patients enrolled, including 42 male patients and 30 female patients.
Table 1 Pretreatment patient characteristics
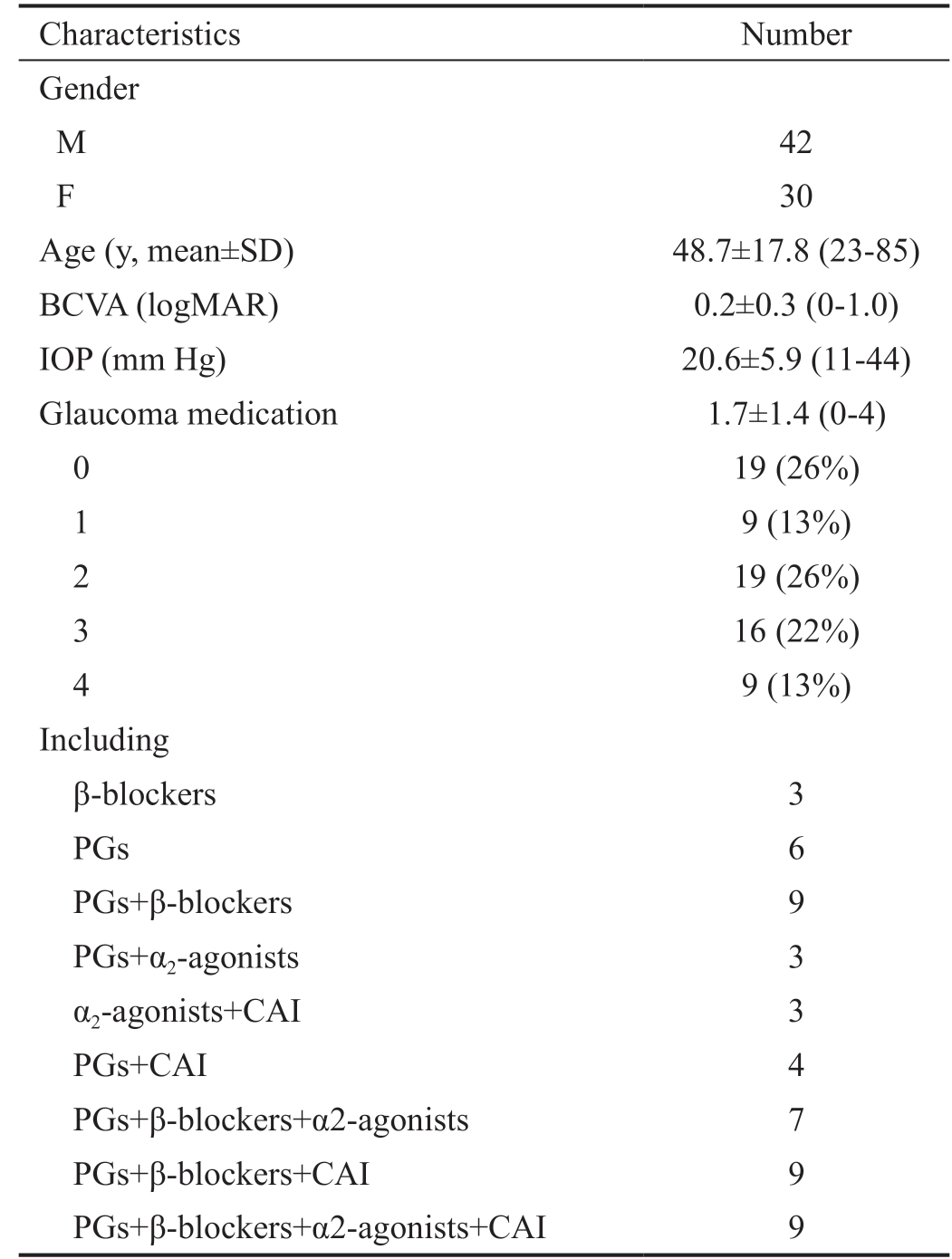
Characteristics Number Gender M 42 F 30 Age (y, mean±SD) 48.7±17.8 (23-85)BCVA (logMAR) 0.2±0.3 (0-1.0)IOP (mm Hg) 20.6±5.9 (11-44)Glaucoma medication 1.7±1.4 (0-4)0 19 (26%)1 9 (13%)2 19 (26%)3 16 (22%)4 9 (13%)Including β-blockers 3 PGs 6 PGs+β-blockers 9 PGs+α2-agonists 3 α2-agonists+CAI 3 PGs+CAI 4 PGs+β-blockers+α2-agonists 7 PGs+β-blockers+CAI 9 PGs+β-blockers+α2-agonists+CAI 9
BCVA: Best corrected visual acuity; PGs: Prostaglandins; CAI:Carbonic anhydrase inhibitors.
The average age was 48.7±17.8y with a range of 23-85y. The best corrected vision acuity (BCVA) was logMAR 0.2±0.3(range 0-1.0), the IOP was 20.6±5.9 (range 11-44) mm Hg,with 1.7±1.4 glaucoma medications (range 0-4). The IOP was measured between 8:00 and 10:00 a.m. Glaucoma medications included β-receptor blockers, α-agonists, carbonic anhydrase inhibitors (CAI) and prostaglandins. Fixed combination medications were counted as two types of glaucoma medications(Table 1).
Intraocular Pressure IOP was 20.6±5.9 mm Hg before MLT and 20.8±6.8 mm Hg at 2h after MTL. The IOP at 1d, 1, 4,12 and 24wk was 17.9±4.4, 18.0±4.3, 17.5±3.4, 17.0±2.7 and 16.5±2.9 mm Hg, respectively. The IOP before and after MLT demonstrated a statistically significant difference by ANOVA analyses (F=5.797, P<0.001). LSD t-tests showed there was no statistically significant difference between pre-MLT IOP at 2h after MLT (P=0.207) which indicated there was no IOP spike after MLT. The statistically significant difference was confirmed between the pre-MLT IOP at 1d, 1,4, 12 and 24wk after MLT (P=0.006, 0.009, 0.001, <0.001,<0.001, respectively). However, the comparisons between the IOPs at 1d, 1, 4, 12 and 24wk after MLT did not show any difference (P=0.866, 0.693, 0.386, 0.165, respectively). The results indicated that the IOP was reduced 1d after MLT and was maintained at a 19.9% IOP reduction at the 6mo follow-up(Table 2).
Table 2 The IOP before and after MLT

Parameters Pre-MLT 2h post-MLT 1d post-MLT 1wk post-MLT 4wk post-MLT 12wk post-MLT 24wk post-MLT Mean IOP (mm Hg) 20.6 20.8 17.9 18.0 17.5 17.0 16.5 SD (mm Hg) 5.9 6.8 4.4 4.3 3.4 2.7 2.9 Range (mm Hg) 11-44 11-45 10-32 11-31 12-30 12-23 10-22 P 0.207 0.006 0.009 0.001 <0.001 <0.001
Table 3 The summary of recent research
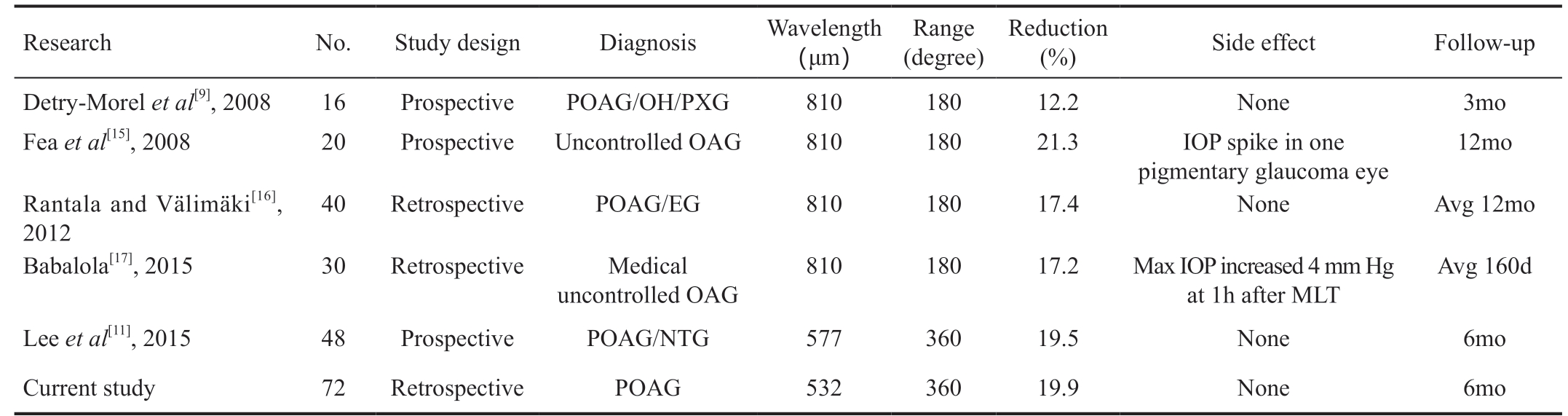
OH: Ocular hypertension; PXG: Pseudoexfoliation glaucoma; POAG: Primary open angle glaucoma; EG: Exfoliation glaucoma; NTG: Normal tension glaucoma.
Research No. Study design Diagnosis Wavelength(μm)Range(degree)Reduction(%) Side effect Follow-up Detry-Morel et al[9], 2008 16 Prospective POAG/OH/PXG 810 180 12.2 None 3mo Fea et al[15], 2008 20 Prospective Uncontrolled OAG 810 180 21.3 IOP spike in one pigmentary glaucoma eye 12mo Rantala and Välimäki[16],2012 40 Retrospective POAG/EG 810 180 17.4 None Avg 12mo Babalola[17], 2015 30 Retrospective Medical uncontrolled OAG 810 180 17.2 Max IOP increased 4 mm Hg at 1h after MLT Avg 160d Lee et al[11], 2015 48 Prospective POAG/NTG 577 360 19.5 None 6mo Current study 72 Retrospective POAG 532 360 19.9 None 6mo
Glaucoma Medications The number of glaucoma medications before MLT was 1.7±1.4 (range 0-4), including β-blockers, α2-agonists, prostaglandins, and CAI. Among them,19 cases were initial POAG patients without any medication,9 cases used one glaucoma medication, 19 cases had 2 medications, 16 cases had three medications, and 9 cases had 4 glaucoma medications. Six months after MLT, the number of glaucoma medications was 1.5±1.4 (range 0-4). Among them,the number of patients without glaucoma medications was 25, and the numbers with 1, 2, 3, or 4 kinds of glaucoma medications were 12, 9, 19, and 7 patients, respectively.The number of glaucoma medications was decreased after MLT with a significantly statistical difference (t=2.219,P=0.031).
Complications No intra- or postoperative complication occurred. In this study, the IOP of the patients’ 2h post-MLT was 20.8±6.8 mm Hg without IOP spikes. There was no intraocular pain, postoperative inflammation, corneal infection, hemorrhage,or trabecular meshwork burning reported in our study.
DISCUSSION
Currently, LTP is one of the treatment options for POAG.Both ALT and SLT reduce the IOP and reduce the number of glaucoma medications used, so that some patients can eliminate the risk of surgery. However, ALT and SLT both may induce injury of the local trabecular meshwork, and IOP spikes after laser treatment may occur in some patients[5-8]. Recently, a newer laser therapy named MLT has been shown to reduce the IOP of POAG patients by 12.2%-21.3%[9,11,15-17].
The current study is a retrospective study in a single university hospital. In our study, the IOP before MLT was 20.6±5.9 mm Hg,and the mean IOP 2h post-laser therapy was almost the same,with a baseline IOP of 20.8 mmHg (P=0.207). No IOP spike after MLT treatment was confirmed, which differed from other laser trabeculoplasty reports showing that 7%-27% of the patients experienced IOP spikes after ALT and SLT[20-22].
The IOP decreased significantly from 1d after MLT and was stable until the 24-week follow-up. The number of glaucoma medications was decreased from 1.7 to 1.5, with a significant difference (P=0.031). These results suggest that MLT is effective in the treatment of POAG, and that there are no complications, including IOP spikes after MLT, which further indicate the safety of MLT. Recent MLT studies showed the efficacy of MLT in variable OAG ranged from 12.2% to 21.3%[9,11,15-17]. Detry-Morel et al[9] demonstrated that MLT may decrease the IOP of OAG patients up to 12.2%, while Fea et al[15] confirmed that the reduction of IOP was up to 21.3%after a 12-month follow-up in patients with uncontrolled OAG, including POAG and pigmentary glaucoma. The study of Rantala and Välimäki[16] showed MLT decreased the IOP of POAG and exfoliation glaucoma to 17.4% at a 6-month follow-up. The study of Babalola[17] found the IOP decreased 17.2% around 5mo after MLT. Lee et al[11] showed that 6mo after MLT for OAG and normal tension glaucoma, the IOP was reduced to 19.5%. The aforementioned studies showed variable IOP reductions, which might be induced by the different types of glaucoma and laser wavelengths (810 nm or 577 nm).
There was almost no IOP spike or other laser complication in similar studies which is in accordance to our results (Table 3).
However, an IOP spike was the most common complication after SLT, which was not found in our study[20].
The mechanism of LTP is not fully understood. ALT could open the conduit through intervening perforations, but it causes visible histological changes including coagulation damage and scarring to the trabecular meshwork[21,23-26]. SLT was called “selective” for its targeting of pigmented trabecular meshwork cells, and histological studies showed there was minor coagulative damage and structural changes of the meshwork[24,27].
MLT uses a laser pulse with a duty-cycle algorithm to the ocular tissue without scar formation. A major advantage of MLT is the subthreshold therapy effect on the pigmented cells without the burning effect of the trabecular meshwork or damage to adjacent tissues[9]. The principle of LTP may be explained by several mechanisms, including the mechanical pulling open of the uveoscleral trabecular meshwork and Schlemm’s canal, cellular mechanisms that stimulate cell division, and biochemical mechanisms that alter cytokines and stimulate the macrophage-like capacity of trabecular lining cells[21,23]. Current research favors a cellular biomechanical cascade[28]. The threshold of the laser-induced cellular cascade is unclear, but variable cells could be activated by a nonlethal thermal insult, which constitutes the principle of laser therapy.The higher photothermal effect may damage the adjacent tissues, as was seen by ALT or SLT. The gentle photothermal effect may be enough to trigger a cellular response without visible damage or complications during or after laser therapy.
That could be why MLT reduced IOP, but without visible changes[11,15-17].
Another advantage of MLT is that it achieves its therapeutic aim by repetitive energy pulses, and not through continuous pulses, which allows the temperature of the trabecular meshwork to return to normal at the interpulse separations.There was no trabecular meshwork traction or shrinkage after ALT, or pigmented trabecular meshwork cell damage after SLT[27]. There was also no IOP spike or other complications after MLT, and the process could be repeated.
There was a lack of clinically visible morphological changes during or after MLT, which is quite different from the “bubble”appearance noted when performing SLT[11,16-17,20]. The lack of visible tissue changes constitutes a clinical challenge, because,in the absence of a visible endpoint, the treatment relies on the surgeon’s skill, which could be a variable. We chose one surgeon (Hong Y) to perform all the MLT therapies to reduce any systematic errors.
A limitation of our study was the limited number of the patients and the limited follow-up time. The cases enrolled included primary cases and cases with medical treatments. The baseline IOPs of the patients varied, and the patients were on different preoperative medical treatments. There was also an absence of a control group. In the future, we will enroll more cases and follow-up for a longer time to better evaluate the efficacy and safety of MLT.
In summary, MLT reduced the IOP of POAG patients and the number of glaucoma medications without IOP spikes.
ACKNOWLEDGEMENTS
The authors appreciate International Science Editing Language Service.
Foundation: Supported by National Natural Science Foundation of China (No.81670851).
Conflicts of Interest: Hong Y, None; Song SJ, None; Liu B,None; Hassanpour K, None; Zhang C, None; Loewen N, None.
1 Cho HK, Kee C. Population-based glaucoma prevalence studies in Asians. Surv Ophthalmol 2014;59(4):434-447.
2 Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311(18):1901-1911.
3 Alaei M, Najmi AK, Kausar H, Akhtar M. A prospective research study of anti-glaucoma drugs prescribing, utilization pattern and adverse drug reaction recording in a university hospital. Drug Res (Stuttg) 2015;65(3):164-168.
4 Giovingo M. Complications of glaucoma drainage device surgery: a review. Semin Ophthalmol 2014;29(5-6):397-402.
5 Wang W, He M, Zhou MW, Zhang XL. Selective laser trabeculoplasty versus argon laser trabeculoplasty in patients with open-angle glaucoma:a systematic review and meta-analysis. PLoS One 2013;8(12):e84270.
6 Baykara M, Hamidi NA, Akova-Budak B, Sabur H, Poroy C. Early results of selective laser trabeculoplasty in patients resistant to deep sclerectomy. Eur J Ophthalmol 2014;24(3):371-374.
7 Meyer JJ, Lawrence SD. What’s new in laser treatment for glaucoma?Curr Opin Ophthalmol 2012;23(2):111-117.
8 Tsang S, Cheng J, Lee JW. Developments in laser trabeculoplasty. Br J Ophthalmol 2016;100(1):94-97.
9 Detry-Morel M, Muschart F, Pourjavan S. Micropulse diode laser (810 nm) versus argon laser trabeculoplasty in the treatment of open-angle glaucoma: comparative short-term safety and efficacy profile. Bull Soc Belge Ophtalmol 2008(308):21-28.
10 Kaplowitz K, Wang S, Bilonick R, Oatts JT, Grippo T, Loewen NA. Randomized controlled comparison of titanium-sapphire versus standard Q-switched ND:YAG laser trabeculoplasty. J Glaucoma 2016;25(7):e663-e667.
11 Lee JW, Yau GS, Yick DW, Yuen CY. MicroPulse laser trabeculoplasty for the treatment of open-angle glaucoma. Medicine (Baltimore)2015;94(49):e2075.
12 Luttrull JK, Sramek C, Palanker D, Spink CJ, Musch DC. Longterm safety, high-resolution imaging, and tissue temperature modeling of subvisible diode micropulse photocoagulation for retinovascular macular edema. Retina 2012;32(2):375-386.
13 Koss MJ, Beger I, Koch FH. Subthreshold diode laser micropulse photocoagulation versus intravitreal injections of bevacizumab in the treatment of central serous chorioretinopathy. Eye (Lond) 2012;26(2):307-314.
14 Li XY, Wang W, Zhang XL. Meta-analysis of selective laser trabeculoplasty versus topical medication in the treatment of open-angle glaucoma. BMC Ophthalmol 2015;15:107.
15 Fea AM, Bosone A, Rolle T, Brogliatti B, Grignolo FM. Micropulse diode laser trabeculoplasty (MDLT): A phase II clinical study with 12 months follow-up. Clin Ophthalmol 2008;2(2):247-252.
16 Rantala E, Välimäki J. Micropulse diode laser trabeculoplasty:180-degree treatment. Acta Ophthalmol 2012;90(5):441-444.
17 Babalola OE. Micropulse diode laser trabeculoplasty in Nigerian patients. Clin Ophthalmol 2015;9:1347-1351.
18 Zhang L, Weizer JS, Musch DC. Perioperative medications for preventing temporarily increased intraocular pressure after laser trabeculoplasty. status and date: New 2013. Available at: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010746/full.
19 Narayanaswamy A, Leung CK, Istiantoro DV, Perera SA, Ho CL,Nongpiur ME, Baskaran M, Htoon HM, Wong TT, Goh D, Su DH,Belkin M, Aung T. Efficacy of selective laser trabeculoplasty in primary angle-closure glaucoma: a randomized clinical trial. JAMA Ophthalmol 2015;133(2):206-212.
20 Latina MA, Sibayan SA, Shin DH, Noecker RJ, Marcellino G.Q-switched 532-nm Nd: YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicenter, pilot, clinical study. Ophthalmology 1998;105(11):2082-2088; discussion 2089-2090.
21 Wong MO, Lee JW, Choy BN, Chan JC, Lai JS. Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in openangle glaucoma. Surv Ophthalmol 2015;60(1):36-50.
22 Greninger DA, Lowry EA, Porco TC, Naseri A, Stamper RL, Han Y.Resident-performed selective laser trabeculoplasty in patients with openangle glaucoma. JAMA Ophthalmol 2014;132(4):403-408.
23 Samples JR, Singh K, Lin SC, Francis BA, Hodapp E, Jampel HD,Smith SD. Laser trabeculoplasty for open-angle glaucoma: a report by the american academy of ophthalmology. Ophthalmology 2011;118(11):2296-2302.
24 Kramer TR, Noecker RJ. Comparison of the morphologic changes after selective laser trabeculoplasty and argon laser trabeculoplasty in human eye bank eyes. Ophthalmology 2001;108(4):773-779.
25 Latina MA, Park C. Selective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW laser interactions. Exp Eye Res 1995;60(4):359-371.
26 Russo V, Barone A, Cosma A, Stella A, Delle Noci N. Selective laser trabeculoplasty versus argon laser trabeculoplasty in patients with uncontrolled open-angle glaucoma. Eur J Ophthalmol 2009;19(3):429-434.
27 Fudemberg SJ, Myers JS, Katz LJ. Trabecular meshwork tissue examination with scanning electron microscopy: a comparison of micropulse diode laser (MLT), selective laser (SLT), and argon laser(ALT) trabeculoplasty in human cadaver tissue. Invest Ophthalmol Vis Sci 2008;49(13):1236.
28 Alvarado JA, Alvarado RG, Yeh RF, Franse-Carman L, Marcellino GR, Brownstein MJ. A new insight into the cellular regulation of aqueous outflow: how trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm’s canal endothelial cells. Br J Ophthalmol 2005;89(11):1500-1505.