INTRODUCTION
G laucoma refers to a specific pattern of retinal ganglion cell (RGC) death that can result in severe vision loss and blindness. Glaucoma can be subtle in onset, allowing for substantial RGC damage to occur before visual dysfunction is apparent[1]. Thus, identification of glaucoma risk factors is of great importance. Surveillance of known glaucoma risk factors allows physicians to closely monitor high-risk individuals and to initiate treatment early in the disease, which aids in the prevention of blindness.
Human immunodeficiency virus (HIV) infection is an additional public health problem in the United States and worldwide. The prevalence of HIV infection in the US is more than 1.1 million people[2]. Treatment of HIV infection with combination active antiretroviral therapy (ART) has increased patient survival,reduced opportunistic infections, and increased the number of HIV positive patients living with other chronic medical conditions[3-4].
HIV plasma viral load and CD4 cell count are surrogate markers of immune function in HIV positive patients.
These values are used as part of the routine clinical care of HIV positive patients to evaluate response to treatment and monitor for disease progression. With initiation of ART therapy, the CD4 cell count generally increases as the HIV plasma viral load is controlled. CD4 cell count is the strongest predictor of subsequent disease progression and survival according to findings from clinical trials and cohort studies[5-6].Additionally, the measurement is used to determine the need for opportunistic infection prophylaxis.
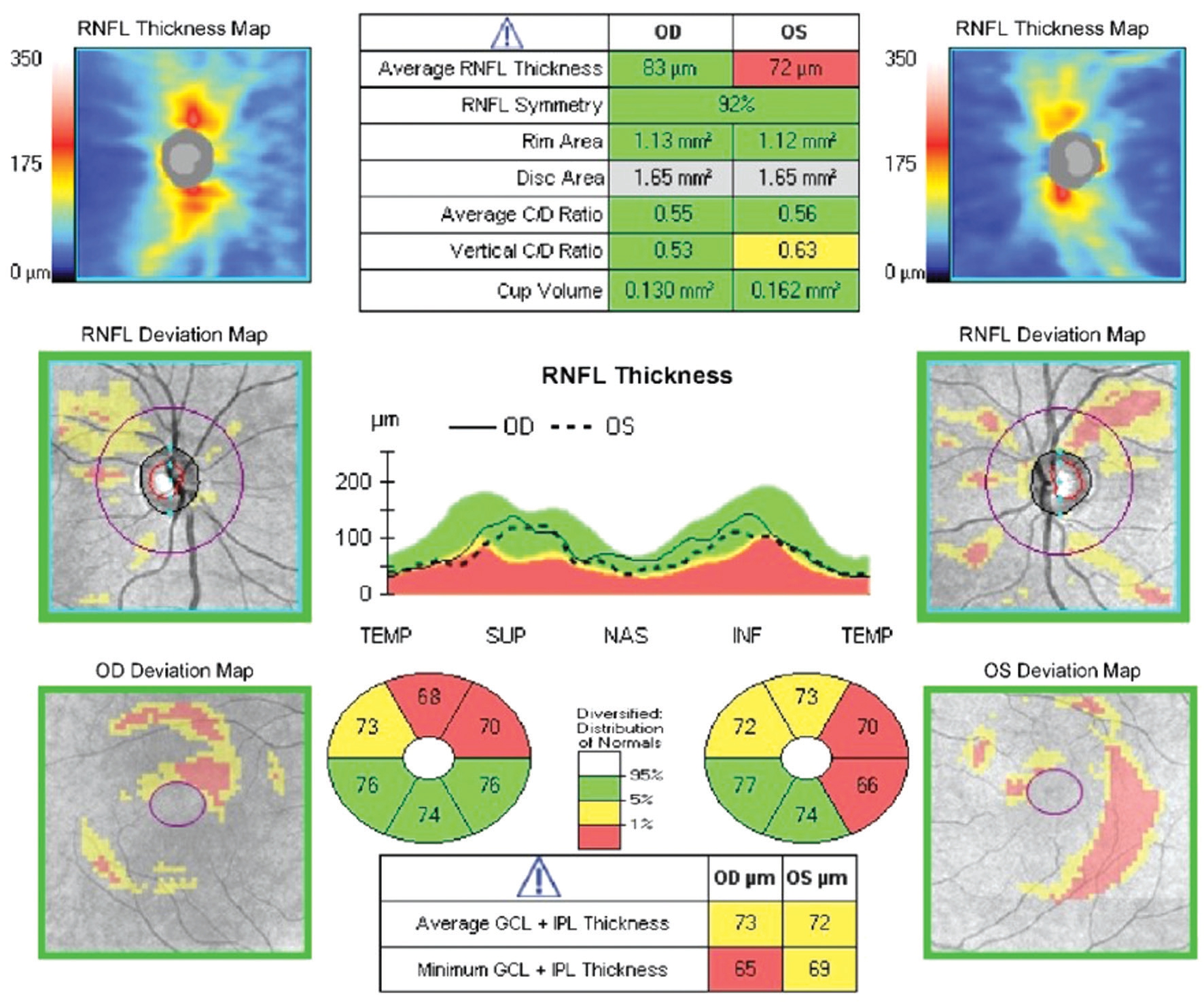
Figure 1 Example patient A 39-year-old emmetropic male, CD4 count 369 at time of imaging, followed for suspicion of glaucoma primarily due to optic nerve head cupping and RNFL appearance. OCT reveals average RNFL thickness of 83 µm in the right eye and 72 µm in the left eye. Average macular ganglion cell-inner plexiform layer thickness was 73 µm in the right eye and 72 µm in the left eye.
Despite advancements in therapy, patients with HIV infection experience structural and functional damage to the visual system[7-8]. Optical coherence tomography (OCT) and scanning laser polarimetric studies of the peripapillary retinal nerve fiber layer (RNFL) show evidence of inner retinal damage,which appears cumulative in HIV patients with a history of low CD4 cell counts without any evidence of infectious retinitis[9-10]. Additionally, abnormalities in color vision and contrast sensitivity have been reported in HIV, contributing to the complex referred to as HIV-associated neuroretinal disorder (NRD)[11-12]. Proposed mechanisms for NRD include damage to the optic nerve and related structures by the HIV virus itself, destruction from the immune response to HIV infection, and tissue damage from virus-associated chronic microvasculopathy and hematologic abnormalities[7,12].
There are numerous reports of RNFL damage[13-16], visual field abnormalities[17-18], and optic nerve head morphology changes[19]in HIV positive patients. Clinically, we have observed that these changes in HIV positive patients often resemble those seen in patients under evaluation for glaucoma suspect (Figure 1).Accordingly, many HIV positive patients undergo thorough glaucoma suspect workup, frequent surveillance visits and testing, and sometimes treatment for glaucoma. There is a dearth of evidence regarding how to distinguish the complex of HIV-associated NRD from glaucoma suspect or early glaucoma. Existing literature has been variable in observing a relationship between CD4 cell count and ophthalmic variables such as RNFL thickness[7,10,13-14,20]. While structural or functional progression in a glaucomatous pattern often points toward the diagnosis of glaucoma, we were motivated to look at patients under evaluation for glaucoma suspect to better understand fundamental characteristics that might distinguish patients with HIV-associated NRD and patients at risk for developing glaucoma.
We sought to perform an exploratory study investigating relationships between historic nadir CD4 count, RNFL thickness, and ganglion cell layer thickness to better guide clinical management of our complex cohort of patients with HIV infection, many of whom are long-term disease survivors.
SUBJECTS AND METHODS
Ethical Approval The study protocol was approved by the Institutional Review Board and Clinical Study Evaluation Committee at Weill Cornell Medical College (WCMC), and the study was exempt from informed consent because of the retrospective nature. All procedures and data collection were performed in accordance with compliance guidelines outlined by the Health Insurance Portability and Accountability Act and the tenants of the Declaration of Helsinki.
This is a retrospective chart review of 329 consecutive HIV positive patients under evaluation for suspicion of glaucoma at WCMC between January 1, 2008 and March 31, 2015.The study was performed in collaboration with the Center for Special Studies, a New York State Designated AIDS Center.
The electronic medical record database was queried for all patients with a diagnosis of HIV (ICD-9 V08, 042) as well as glaucoma suspect (ICD-9 365.00, 365.01, 365.05) and/or ocular hypertension (ICD-9 365.04). Inclusion criteria required open angles on gonioscopy, a best-corrected visual acuity of 20/40 or better, and an age of 18 years or older.Included patients had undergone at least two reliable Cirrus high definition-OCT measurements (Carl Zeiss Meditec, Inc.,Dublin, CA, USA) taken at least 6mo apart, defined as signal strength ≥7/10 with a focused image and proper centration of the optic disc within the scan circle, from which RNFL thickness and macular ganglion cell-inner plexiform layer(GCIPL) thickness measurements were recorded. Participants were excluded if they had a history of intraocular pressure(IOP) lowering therapy, macular pathology, active or prior infectious retinitis, prior intraocular surgery, refractive error≥±5.00 diopters, non-glaucomatous optic neuropathy, or any other ocular or systemic disease that could affect the optic nerve. Charts were culled for demographic information (age,gender, self-identified race/ethnicity, presence of diabetes mellitus, history of smoking tobacco), HIV parameters (year since HIV diagnosis, treatment status, laboratory measured CD4 T-lymphocyte counts and viral load levels dating back to January 1990, nadir CD4 count and date), and glaucoma/ophthalmic variables (visual acuity in each eye, refractive error, gonioscopy, central corneal thickness (CCT), IOP at first“glaucoma suspect” visit and maximum measured IOP).
Data were described as n (%) for categorical measures and as mean (standard deviation) for continuous measures.Demographic and clinical characteristics between nadir CD4 cell count groups (<200 cells/mm3 and ≥200 cells/mm3) were compared by Chi-squared/Fisher’s Exact tests or independent two-sample t-tests/Wilcoxon rank-sum tests. Similar tests were used to compare individual eye characteristics (RNFL thickness and GCIPL thickness as measured by OCT) by nadir CD4 cell count groups. All P-values were two-sided with statistical significance evaluated at the 0.05 alpha levels.Analyses were performed in R version 3.4.3 (Vienna, Austria).
Table 1 Study population

CCT: Central corneal thickness; IOP: Intraocular pressure;HIV: Human immunodeficiency virus; SD: Standard deviation.
Demographic data for study population organized by nadir CD4 cell
counts below and above 200 cells/mm3.
Demographic and clinical characteristics Nadir CD4<200 cells/mm3 P Nadir CD4≥200 cells/mm3 Patients, n 23 32 Average age at presentation, y 49.5 48.6 0.728 Male gender, n (%) 16 (69.6) 27 (84.3) 0.208 Self-reported race/ethnicity, n (%)African-American 14 (60.8) 14 (43.8)Caucasian 3 (13.0) 3 (9.4)Hispanic/Latino 3 (13.0) 5 (15.6)Other/Declined 3 (13.0) 10 (31.3)Family history of glaucoma, n (%) 12 (52.1) 10 (31.3) 0.199 History of diabetes, n (%) 3 (13.0) 9 (28.1) 0.321 History of smoking, n (%) 13 (56.5) 20 (62.5) 0.867 Mean CCT, microns (SD) 536.4 (30.0)549.3 (41.7) 0.196 Mean IOP max, mm Hg (SD) 16.8 (3.3) 16.9 (3.2) 0.875 Average duration of HIV diagnosis, y 13 14 0.719
RESULTS
Participant Characteristics In total, 110 eyes of 55 patients met inclusion criteria, of which 46 eyes (42%) were from subjects with a nadir CD4 cell count <200 cells/mm3 and 64 (58%) were from subjects with nadir CD4 cell count≥200 cells/mm3. At the time of initial ophthalmology visit, the most recent median CD4 cell count was 500±227 cells/mm3,and 78% of subjects had an undetectable viral load. Among all participants, 48 of 55 were on ART at time of initial OCT.
Men comprised a greater percent of the study population with nadir CD4 cell count ≥200 cells/mm3 (n=27, 84.3%) compared to those with lower nadir CD4 cell count (n=16, 69.6%;P=0.208; Table 1).
The average age of subjects was 49±10y. There was no significant difference between either group in family history of glaucoma, history of diabetes, smoking history, or years elapsed since HIV diagnosis (Table 1).
The average CCT among patients with nadir CD4 cell count<200 cells/mm3 was thinner (536.4 µm) than among those participants with higher nadir CD4 cell count (549.3 µm, Table 1)but this finding did not reach statistical significance. There was no difference in average maximum IOP between patients with nadir CD4 cell count ≥200 cells/mm3 (16.9 mm Hg) compared to those with lower nadir CD4 (16.8 mm Hg, Table 1).
Table 2 RNFL thickness by quadrant microns, mean±SD

RNFL: Retinal nerve fiber layer; OCT: Optical coherence tomography; SD: Standard deviation. Superior quadrant and temporal quadrant RNFL thickness on OCT in patients with nadir CD4 counts below and above 200 cells/mm3.
Items Nadir CD4 <200 cells/mm3 cohort Nadir CD4 ≥200 cells/mm3 cohort P Eyes, n 46 64 Mean superior quadrant RNFL thickness at initial OCT 119.7±18.6 112.8±16.8 0.048 Mean superior quadrant RNFL thickness at follow-up OCT 117.9±18.3 110.5±16.9 0.034 Mean temporal quadrant RNFL thickness at initial OCT 63.8±11.7 57.1±11.9 0.004 Mean temporal quadrant RNFL thickness at follow-up OCT 63.8±12.8 57.3±11.6 0.007
Optical Coherence Tomography Patients with a nadir CD4 cell count <200 cells/mm3 had thicker superior (119.7±18.6 µm)and temporal (63.8±11.7 µm) quadrants at time of initial OCT compared to the superior (112.8±16.8 µm) and temporal(57.1±11.9 µm) quadrants of patients with nadir CD4 cell count ≥200 cells/mm3. There was no statistically significant difference between the RNFL thickness of the inferior and nasal quadrants between the two nadir groups.
The trend toward thicker RNFL in the superior and temporal quadrants among subjects with the lower nadir CD4 persisted at the time of follow-up OCT. Participants with nadir CD4<200 cells/mm3 showed mean RNFL thickness in the superior and temporal quadrants of 117.9±18.3 µm and 63.8±12.8 µm, respectively, compared to a superior thickness of 110.5±16.9 µm and temporal thickness of 57.3±11.6 µm among those with nadir CD4≥200 cells/mm3 (Table 2). No outlier eyes were identified at either time point that could account for these findings.
Change over time between OCT1 and OCT2 was calculated for mean RNFL thickness, mean GCIPL thickness, RNFL quadrants, and GCIPL sectors. Change over time was not significantly different between the nadir CD4 cell count groups for any of these RNFL or GCIPL measurements. There was no statistically significant change over time in RNFL thickness based on HIV duration in either nadir CD4 cell count group(data not shown).
DISCUSSION
Historically, vision loss in patients with HIV resulted from retinitis due to opportunistic organisms such as cytomegalovirus and herpes viral retinitis and less commonly from infections such as toxoplasmosis, syphilis, and cryptococcosis[7].
These devastating opportunistic infections were also highly associated with mortality.
Today, the life expectancy of patients living with HIV in North America is approaching that of patients without HIV[21]. This dramatic growth in life expectancy in HIV positive individuals in the last two decades is attributed in part to antiretroviral medications with less frequent dosing and fewer side effects,improved adherence, and advancements in comorbidity management[21]. With HIV positive patients living into older age, for the first time in history clinicians are faced with the challenge of deciding how to best screen for and manage diseases of aging in this patient population. Some authors have even hypothesized that HIV results in an accelerated biological aging process[22]. Thus, among these challenges is the question of how to best evaluate HIV positive patients who are identified as “glaucoma suspects,” usually because of clinician concern for enlarged cup to disc ratio.
The Studies of Ocular Complications of AIDS (SOCA) research group demonstrated as part of the landmark Longitudinal Study of Ocular Complications of AIDS (LSOCA) multicenter clinical observational study that nearly 40% of eyes of HIV positive patients have some visual field abnormalities.Although the LSOCA participants had lower CD4 cell counts and more comorbidities and opportunistic infections than patients in our study, their findings suggest that a large percentage of patients living with HIV infection in the United States have visual field loss[7]. They also observed an association between CD4 cell count and contrast sensitivity with mean contrast sensitivity decreasing with declining CD4 cell count, adding support to the hypothesis that virus control may be related to visual dysfunction[7].
The association between HIV infection and visual field abnormalities[7,17-19] coupled with increasing reliance on OCT to detect early glaucomatous RNFL thinning[23] emphasizes the importance of research investigating how to interpret RNFL abnormalities in otherwise healthy patients with HIV infection and enlarged cups.
The literature varies regarding the reported associations between RNFL thickness and HIV infection. In a prospective study using the Heidelberg Retinal Tomograph confocal scanning laser ophthalmoscope, Plummer and associates imaged the eyes of HIV positive patients with and without cytomegalovirus retinitis and HIV negative control patients and found thinner peripapillary RNFL in both HIV positive groups compared with HIV negative controls[24]. Using scanning laser polarimetry, Kozak et al[10] divided HIV positive patients into low-nadir and high-nadir CD4 cell count groups (using <100 cells/mm3 for at least 6mo as their cutoff) and detected significantly lower RNFL index scores for the low nadir group compared with the high nadir group and no significant difference between the high nadir and HIV negative patients. Following the introduction of OCT, Kozak et al[9] then performed a similar study using time domain OCT and again observed that eyes of HIV positive patients with low nadir CD4 cell counts had thinner average RNFL and thinner temporal, superior, and inferior quadrant RNFL compared with eyes of patients with high nadir CD4 cell count and HIV negative control eyes. In contrast, Demirkaya et al[20] and Pathai et al[22] did not find significant differences in peripapillary RNFL thickness between HIV positive patients and HIV negative controls.
Our finding that HIV positive patients with a lower nadir CD4 cell count had thicker RNFL in two quadrants compared with higher nadir CD4 cell count patients is thus somewhat surprising. The finding was reproducible at both time points.We investigated other parameters including classes of ART,CD4 cell count at the time of OCT measurements, time since HIV diagnosis, and viral load data without finding associations.RNFL thickness may thus be falsely reassuring in some HIVpositive patients and may mask glaucomatous RNFL thinning.As yet, the etiology of this finding remains unclear, and possible explanations include inflammation, microvascular disease, or a factor unique to the virus or ART. Kalyani et al[25]reported a subgroup of HIV-positive patients with average RNFL thickness greater than normal. In this group, duration of HIV disease was shorter compared with the group whose RNFL thickness was less than normal. Although the difference was not statistically significant, the authors hypothesized that axon compromise resulted in an initial phase of RNFL swelling before becoming atrophic. This association was not observed in our data.
There is increasing interest in the effects of immune responses,particularly cellular immunity, on RGC damage and protection in an effort to better understand glaucoma pathogenesis.Given the emerging role of T cells in our understanding of the etiology of glaucoma[26], one possibility is that a period of low T cells is actually protective against ganglion cell death and RNFL loss.
We limited our study to patients who received their HIV specialized care at the Center for Special Studies (CSS) at NewYork-Presbyterian Hospital/WCMC. CSS has been a leader in HIV care since 1988 and was one of the first programs in New York to be recognized as a Designated AIDS Center by the Department of Health. Although our study is limited by its retrospective nature, we feel that the Center’s outstanding electronic medical records are strength of our study and allowed for detailed chart review and CD4 data since HIV diagnosis in most of the patients in our series.
Our findings are limited by the size of our sample, which may have limited our ability to detect associations, for example the influence of medications on RNFL. Future prospective studies of larger cohorts are needed to better understand the relationship between nadir CD4 count, other markers of HIV control, and RNFL and GCIPL measurements. Our inclusion of only two OCT studies is an additional limitation. Most of the included patients were seen in follow up only two to three times over the study period. Our department commenced use of Cirrus HD-OCT in 2008, and we included only patients who underwent testing on the Cirrus HD-OCT. Although there is some consensus that patients with CD4 cell counts <50 mm3 should undergo regular dilated funduscopic examinations every 6-12mo to evaluate for HIV-related complications such as infectious retinitis[27], there are no firm guidelines for eye exams in other HIV positive patients. This coupled with barriers to care in this patient population may have contributed to infrequent follow up.
Finally, we observed slightly thinner mean CCT in the nadir CD4 cell count <200 cells/mm3 group, although this finding did not reach statistical significance. Given the known association between thinner CCT and glaucoma risk, one might have expected the low nadir group with thinner corneas to have thinner RNFL as well. In fact, the observation of two quadrants of thicker RNFL in the group with thinner corneas (and low nadir CD4 cell count) may add weight to our findings.
In our series, HIV positive patients under evaluation for suspicion of glaucoma who had a nadir CD4 cell count<200 cells/mm3 had thicker RNFL in two quadrants compared with higher-nadir patients. This finding leaves open the possibility that RNFL thickness may be falsely reassuring in some HIV-positive patients and may mask glaucomatous RNFL thinning. Prospective studies and further evaluation of ganglion cell data are needed to corroborate these findings.As the life expectancy of patients living with HIV continues to rise, greater numbers of HIV patients will be at risk for diseases for aging, requiring specialized attention to the role of HIV in glaucoma surveillance and treatment.
ACKNOWLEDGEMENTS
Conflicts of Interest: Van Tassel SH, None; Petrakos P,
None; Marlow E, None; Mauer E, None; Singh HK, None;
Demetriades AM, None.
1 Sommer A, Katz J, Quigley HA, Miller NR, Robin AL, Richter RC,Witt KA. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol 1991;109(1):77-83.
2 Estimated New HIV Infections, U.S. Statistics. HIV.gov. Available at: https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics.Accessed on June 24, 2017.
3 Jabs DA. AIDS and ophthalmology. Arch Ophthalmol 2008;126(8):1143-1146.
4 Vellozzi C, Brooks JT, Bush TJ, Conley LJ, Henry K, Carpenter CC,Overton ET, Hammer J, Wood K, Holmberg SD, SUN Study Investigators.The study to understand the natural history of HIV and AIDS in the era of effective therapy (SUN Study). Am J Epidemiol 2009;169(5):642-652.
5 Egger M, May M, Chêne G, Phillips AN, Ledergerber B, Dabis F,Costagliola D, D’Arminio Monforte A, de Wolf F, Reiss P, Lundgren JD, Justice AC, Staszewski S, Leport C, Hogg RS, Sabin CA, Gill MJ, Salzberger B, Sterne JA, ART Cohort Collaboration. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 2002;360(9327):119-129.
6 Mellors JW, Muñoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR Jr.Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997;126(12):946-954.
7 Freeman WR, Van Natta ML, Jabs D, Sample PA, Sadun AA, Thorne J, Shah KH, Holland GN, SOCA Research Group. Vision function in HIV-infected individuals without retinitis: report of the Studies of Ocular Complications of AIDS Research Group. Am J Ophthalmol 2008;145(3):453-462.
8 Thorne JE, Van Natta ML, Jabs DA, Duncan JL, Srivastava SK, Studies of Ocular Complications of AIDS Research Group. Visual field loss in patients with cytomegalovirus retinitis. Ophthalmology 2011;118(5):895-901.
9 Kozak I, Bartsch DU, Cheng LY, Kosobucki BR, Freeman WR.Objective analysis of retinal damage in HIV-positive patients in the HAART era using OCT. Am J Ophthalmol 2005;139(2):295-301.
10 Kozak I, Bartsch DU, Cheng LY, McCutchan A, Weinreb RN, Freeman WR. Scanning laser polarimetry demonstration of retinal nerve fiber layer damage in human immunodeficiency virus-positive patients without infectious retinitis. Retina 2007;27(9):1267-1273.
11 Sommerhalder J, Baglivo E, Barbey C, Hirschel B, Roth A, Pelizzone M. Colour vision in AIDS patients without HIV retinopathy. Vision Res 1998;38(21):3441-3446.
12 Shah KH, Holland GN, Yu F, Van Natta M, Nusinowitz S, Studies of Ocular Complications of AIDS (SOCA) Research Group. Contrast sensitivity and color vision in HIV-infected individuals without infectious retinopathy. Am J Ophthalmol 2006;142(2):284-292.
13 Moschos MM, Mostrou G, Psimenidou E, Spoulou V, Theodoridou M. Objective analysis of retinal function in HIV-positive children without retinitis using optical coherence tomography. Ocul Immunol Inflamm 2007;15(4):319-323.
14 Faria E Arantes TE, Garcia CR, Mello PA, Muccioli C. Structural and functional assessment in HIV-infected patients using optical coherence tomography and frequency-doubling technology perimetry. Am J Ophthalmol 2010;149(4):571-576.e2.
15 Cetin EN, Sayin Kutlu S, Parca O, Kutlu M, Pekel G. The thicknesses of choroid, macular segments, peripapillary retinal nerve fiber layer, and retinal vascular caliber in HIV-1-infected patients without infectious retinitis. Retina 2018.
16 Bartsch DU, Kozak I, Grant I, Knudsen VL, Weinreb RN, Lee BR, Freeman WR. Retinal nerve fiber and optic disc morphology in patients with human immunodeficiency virus using the heidelberg retina tomography 3. PLoS One 2015;10(8):e0133144.
17 Sample PA, Plummer DJ, Mueller AJ, Matsubara KI, Sadun A, Grant I, Freeman WR. Pattern of early visual field loss in HIV-infected patients.Arch Ophthalmol 1999;117(6):755-760.
18 Goldbaum MH, Kozak I, Hao JC, Sample PA, Lee T, Grant I, Freeman WR. Pattern recognition can detect subtle field defects in eyes of HIV individuals without retinitis under HAART. Graefes Arch Clin Exp Ophthalmol 2011;249(4):491-498.
19 Kozak I, Ahuja A, Gangaputra S, Van Natta ML, Thorne JE, Freeman WR. Optic nerve head morphology and visual field function in patients with AIDS and without infectious retinitis. Ocul Immunol Inflamm 2012;20(5):342-348.
20 Demirkaya N, Wit FW, van Den Berg TJ, Kooij KW, Prins M,Schlingemann RO, Abramoff MD, Reiss P, Verbraak FD, AGEhIV Cohort Study Group. HIV-associated neuroretinal disorder in patients with wellsuppressed HIV-infection: a comparative cohort study. Invest Ophthalmol Vis Sci 2016;57(3):1388-1397.
21 Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017;4(8):e349-e356.
22 Pathai S, Lawn SD, Weiss HA, Cook C, Bekker LG, Gilbert CE.Retinal nerve fibre layer thickness and contrast sensitivity in HIVinfected individuals in South Africa: a case-control study. PLoS One 2013;8(9):e73694.
23 Stein JD, Talwar N, Laverne AM, Nan B, Lichter PR. Trends in use of ancillary glaucoma tests for patients with open-angle glaucoma from 2001 to 2009. Ophthalmology 2012;119(4):748-758.
24 Plummer DJ, Bartsch DU, Azen SP, Max S, Sadun AA, Freeman WR.Retinal nerve fiber layer evaluation in human immunodeficiency viruspositive patients. Am J Ophthalmol 2001;131(2):216-222.
25 Kalyani PS, Holland GN, Fawzi AA, Arantes TE, Yu F, Sadun AA, Studies of the Ocular Complications of AIDS Research Group.Association between retinal nerve fiber layer thickness and abnormalities of vision in people with human immunodeficiency virus infection. Am J Ophthalmol 2012;153(4):734-742,742.e1.
26 Bell K, Holz A, Ludwig K, Pfeiffer N, Grus FH. Elevated regulatory T cell levels in glaucoma patients in comparison to healthy controls. Curr Eye Res 2017;42(4):562-567.
27 Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS,Horberg MA, Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014;58(1):1-10.