INTRODUCTION
C entral retinal vein occlusion (CRVO) is a common sight-threatening retinal vascular disorder that can lead to severe visual impairment or even blindness[1]. Because it obstructs the central retinal venous outflow, CRVO induces fairly severe macular edema, which is the predominant cause of visual deterioration[2-3]. Nevertheless, timely treatment of macular edema significantly improves visual prognoses.Currently, intravitreal anti-vascular endothelial growth factor(VEGF) is a revolutionary treatment for macular edema secondary to CRVO. Conbercept, a novel and effective anti-VEGF agent, is a fusion protein that blocks all isoforms of VEGF-A, VEGF-B, VEGF-C, and placental growth factor(PIGF) and exhibits a substantially high blinding affinity for VEGF and a long half-life in the vitreous humor, and it has been broadly used in China[4]. Therefore, intravitreal injection with conbercept represents an excellent option with a satisfactory safety profile and efficacy when used to treat macular edema to improve visual acuity in CRVO[5-6]. Although many features of CRVO have been extensively evaluated using fundus photography, spectral domain optical coherence tomography (SD-OCT), and fluorescein angiography (FA),potential abnormalities in the retinal microvasculature cannot be comprehensively identified using these techniques.
Recently, optical coherence tomography angiography (OCTA)was introduced as a non-invasive, promising imaging technique that enables the detection of retinal and choroidal diseases and allows more detailed imaging of vascular microstructures without the use of exogenous dyes compared to FA[7]. More recently, the AngioAnalytics software was upgraded, and can now automatically detect the foveal avascular zone (FAZ)margin and quantify the latest FAZ parameters, including the foveal avascular zone perimeter (PERIM), the acircularity index (AI), and the vessel density within a 300-μm width ring surrounding the FAZ (FD-300).
Several previous clinical studies used OCTA to evaluate the retinal vascular changes that occur in CRVO, which include microaneurysms, telangiectasia, FAZ area and macular nonperfusion area (NPA)[8-10]. Recently, the short-term changes in the FAZ area and macular vessel density in eyes with macular edema due to diabetic retinopathy or CRVO after a single intravitreal injection of bevacizumab, aflibercept or ranibizumab were reported by Ghasemi Falavarjani et al[11]. However, no previous study has performed a followup analysis of patients with CRVO complicated by macular edema who were treated with the novel anti-VEGF drug conbercept and evaluated using OCTA. It also remains unclear what changes occur in these novel FAZ parameters quantified based on a retinal slab instead of the separated superficial retinal capillary plexus (SCP) and deep retinal capillary plexus(DCP) between before and after treatment and whether these changes affect visual function.
Therefore, in the current study, we aimed to use OCTA to quantitatively analyze changes in the retinal microvasculature in CRVO patients with macular edema who were treated with conbercept and evaluate correlations between these changes and best-corrected visual acuity (BCVA) and retinal thickness. In addition, we for the first time reported the latest FAZ parameters in CRVO eyes before and after anti-VEGF treatment.
SUBJECTS AND METHODS
Ethical Approval This was a retrospective case series of patients with macular edema caused by CRVO. Our study was formally reviewed and approved by the Ethics Committee of Zhongshan Ophthalmic Center (Guangzhou, China 2017KYPJ101) and conducted in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from each participant prior to enrollment in the study.
Subjects In this study, twenty-eight patients with unilateral CRVO complicated by macular edema diagnosed based on a history, ophthalmoscopy, and FA were enrolled at the Zhongshan Ophthalmic Center from November 2016 through May 2018. When the non-perfused area was larger than 10-disc areas on FA, CRVO was defined as an ischemic type. A group of 28 age- and sex-matched healthy subjects were included as a control group. All patients received a single intravitreal conbercept injection to treat macular edema at the initial visit and were followed up as the post-injection group at one month after treatment.
The main inclusion criterion was the presence of macular edema associated with CRVO that was treated by an intravitreal injection of conbercept. In addition, we required the central retina thickness to be greater than or equal to 320 μm based on the methods described by Sun et al[5]. The exclusion criteria were eyes with branch retinal vein occlusion,hemi-CRVO, retinal arterial occlusion, previous retinal surgery, ocular trauma or other coexisting ocular disorders,such as severe diabetic retinopathy, epiretinal membrane,retinitis pigmentosa, age-related macular degeneration (AMD),polypoidal choroidal vasculopathy (PCV), glaucoma, and pathologic myopia, that could affect the interpretation of OCTA quantitative parameters. Moreover, patients with poorquality OCTA images because of eye movements, significant media opacities or incorrect autosegmentation were also excluded from this study.
A complete ophthalmic examination, including the assessment of BCVA using a Snellen chart, slit-lamp biomicroscopy,intraocular pressure, indirect fundus ophthalmoscopy and OCTA (Software Version 2017.1, RTVue XR Avanti,AngioVue; Optovue, Inc., Fremont, CA, USA) was performed before and at one month after intravitreal injection of conbercept in CRVO eyes, as well as in control eyes.
Optical Coherence Tomography Angiography Using default settings in the OCTA system, an SCP image was taken to encompass the areas from the internal limiting membrane(ILM) to -10 μm below the inner plexiform layer (IPL), a DCP image was taken from -10 μm below the IPL to 10 μm below the outer plexiform layer (OPL), the outer retina slab(for avascular retina) was imaged from 10 μm below the OPL to -10 μm below Bruch’s membrane (BRM); and the choriocapillaris (for the choroid capillaries) was imaged from-10 μm below the BRM to 30 μm below the BRM. In cases of severe macular edema, which can cause potential segmentation errors, specific manual correction was performed by two specialists.
Moreover, the AngioAnalytics software automatically overlays the Early Treatment Diabetic Retinopathy Study (ETDRS)grid which is comprised of 3 concentric rings, centered on an automatically detected foveal center, representing the foveal,parafoveal and perifoveal regions from inside to outside with diameters of 1, 3 and 5 mm, respectively. The outer rings are further divided into 4 sectors (temporal, superior, nasal and inferior) for quadrant analysis or 2 hemispheres (superior-hemi and inferior-hemi) defined by a horizontal line drawn through the foveal center.
Foveal Avascular Zone, Vessel Density, Flow Area and Retinal Thickness Measurements Based on an Angio Retina scan, FAZ parameters are automatically quantified on a retina slab, which was set to evaluate the region from the ILM to 10 μm below the OPL. The FAZ measurement values obtained included the FAZ area and PERIM, the AI and the FD-300. The AI was defined as the ratio between the measured perimeter and a perimeter with the same size circular area,with a perfectly circular FAZ having an AI equal to 1.
We quantitatively evaluated vessel density, which was defined as the percentage area occupied by OCTA-detected vasculature in the selected region, in the fovea and different sectors of parafovea at the superficial and deep en face slabs[12]. Furthermore, the vascular flow area was evaluated and automatically calculated as the area occupied by the vasculature in a 1-mm radius circle centered on the fovea in the choriocapillaris. In addition, the full retinal thickness was set to incorporate the region from the ILM to the retinal pigment epithelium (RPE). The software provided a retina map scan that was used to automatically quantify the full retinal thickness of the scanned area, which included the foveal region, parafoveal region and perifoveal region; this was then combined with a color-coded map to distinguish whether the retina had thickened or thinned.
In our study, we used OCTA built-in software to analyze OCTA parameters, including the flow area, FAZ and vessel density, in CRVO eyes with the aim of exploring differences between measurements acquired before and after treatment.We further analyzed associations between these parameters and BCVA and retinal thickness.
Statistical Analysis The BCVA data were converted to the logarithm of the minimum angle of resolution (logMAR)before statistical analysis. Quantitative data that were normally distributed were analyzed using the Student’s t-test. The relationships between OCTA parameters and logMAR BCVA as well as retinal thickness were analyzed using the Pearson correlation coefficient. Statistical analysis was performed using GraphPad Prism 6.0 (La Jolla, CA, USA) software. All collected data are presented as the mean±standard deviation(SD). Statistical significance was defined as P<0.05.
RESULTS
Demographic Data The medical files for 28 eyes (15 right eyes and 13 left eyes) of 28 patients with macular edema caused by CRVO (16 non-ischemic and 12 ischemic) were analyzed in this study. The mean age was 54.04±15.26 years old, and 12 patients (43%) were female. There were 8 patients with coexisting hypertension and 2 patients with concomitant diabetes mellitus. The mean duration time after onset of CRVO was 8.25± 5.45wk.
Best Corrected Visual Acuity The mean logMAR BCVA in eyes with CRVO was 0.92±0.45 (Snellen equivalent, 20/167).At the follow-up evaluation, the mean logMAR BCVA of eyes with intravitreally injected conbercept was 0.51±0.39(Snellen equivalent, 20/65). The difference between pre- and post-injection logMAR BCVA was statistically significant(P=0.0092).
Retinal Thickness The full retinal thickness of the fovea and that of different sectors of the parafoveal and perifoveal regions were significantly higher in CRVO patients than in healthy subjects. After treatment, there was a notable reduction in retinal thickness in the foveal, parafoveal, and perifoveal regions. In addition, the comparisons between the post-injection group and normal group in retinal thickness in the foveal, parafoveal, and perifoveal regions were also statistically significant, indicating that macular edema was significantly reduced but the mean retinal thickness was not completely reduced to normal in the early stage of treatment.The results for full retinal thickness are shown in Table 1.
Optical Coherence Tomography Angiography Findings
The results of OCTA parameters evaluated in control eyes,pre-injection eyes and post-injection eyes are shown in Table 2. First, in terms of the latest FAZ parameters, the AI was markedly higher in CRVO eyes than in healthy eyes,suggesting that the pathological FAZ border was more tortuous in the CRVO group. After treatment, the mean AI was decreased but the difference was not significant. Moreover,the FD-300 was notably lower in pre-treatment CRVO eyes than in healthy eyes, and no significant changes were observed after treatment. There were also no statistically significant differences in the FAZ area and PERIM among the three groups.Second, the flow area in the choriocapillaris was dramatically lower in CRVO eyes than in the controls. After conbercept therapy, the flow area of the choriocapillaris was significantly improved in CRVO eyes.
Finally, for vascular densities in the whole images and different sectors of parafoveal regions in the SCP and DCP, they were all significantly lower in the CRVO group than in the control group. In contrast, the foveal vessel densities of the SCP and DCP were slightly higher in CRVO eyes than in healthy eyes, but this difference was not statistically significant. After treatment, there was a slight but non-significant increase in vessel density in the whole images and parafoveal regions of SCP and DCP in CRVO eyes. In contrast, there was a slight reduction in post-treatment foveal vascular density in the SCP and DCP.Representative images demonstrating the changes observed in vessel density and flow area between pre- and post-treatment eyes when measured on OCTA are shown in Figure 1.
Table 1 Full retinal thickness evaluated in control eyes, CRVO eyes and post-injection eyes mean±SD
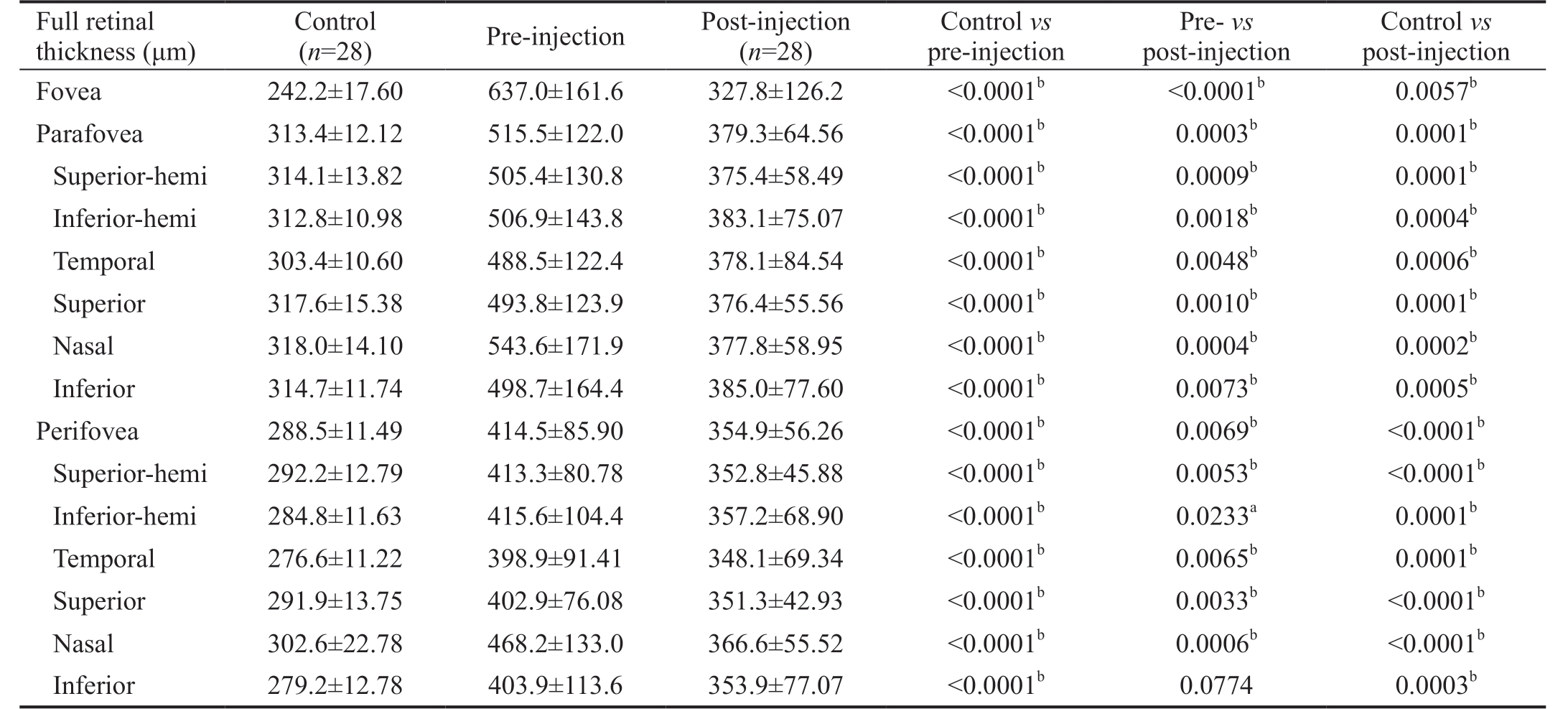
CRVO: Central retinal vein occlusion. aP<0.05, bP<0.01.
Control vs post-injection Fovea 242.2±17.60 637.0±161.6 327.8±126.2 <0.0001b <0.0001b 0.0057b Parafovea 313.4±12.12 515.5±122.0 379.3±64.56 <0.0001b 0.0003b 0.0001b Superior-hemi 314.1±13.82 505.4±130.8 375.4±58.49 <0.0001b 0.0009b 0.0001b Inferior-hemi 312.8±10.98 506.9±143.8 383.1±75.07 <0.0001b 0.0018b 0.0004b Temporal 303.4±10.60 488.5±122.4 378.1±84.54 <0.0001b 0.0048b 0.0006b Superior 317.6±15.38 493.8±123.9 376.4±55.56 <0.0001b 0.0010b 0.0001b Nasal 318.0±14.10 543.6±171.9 377.8±58.95 <0.0001b 0.0004b 0.0002b Inferior 314.7±11.74 498.7±164.4 385.0±77.60 <0.0001b 0.0073b 0.0005b Perifovea 288.5±11.49 414.5±85.90 354.9±56.26 <0.0001b 0.0069b <0.0001b Superior-hemi 292.2±12.79 413.3±80.78 352.8±45.88 <0.0001b 0.0053b <0.0001b Inferior-hemi 284.8±11.63 415.6±104.4 357.2±68.90 <0.0001b 0.0233a 0.0001b Temporal 276.6±11.22 398.9±91.41 348.1±69.34 <0.0001b 0.0065b 0.0001b Superior 291.9±13.75 402.9±76.08 351.3±42.93 <0.0001b 0.0033b <0.0001b Nasal 302.6±22.78 468.2±133.0 366.6±55.52 <0.0001b 0.0006b <0.0001b Inferior 279.2±12.78 403.9±113.6 353.9±77.07 <0.0001b 0.0774 0.0003b Full retinal thickness (μm)Control(n=28) Pre-injection Post-injection (n=28)Control vs pre-injection Pre- vs post-injection
Table 2 OCTA parameters evaluated in control eyes, CRVO eyes and post-injection eyes mean±SD
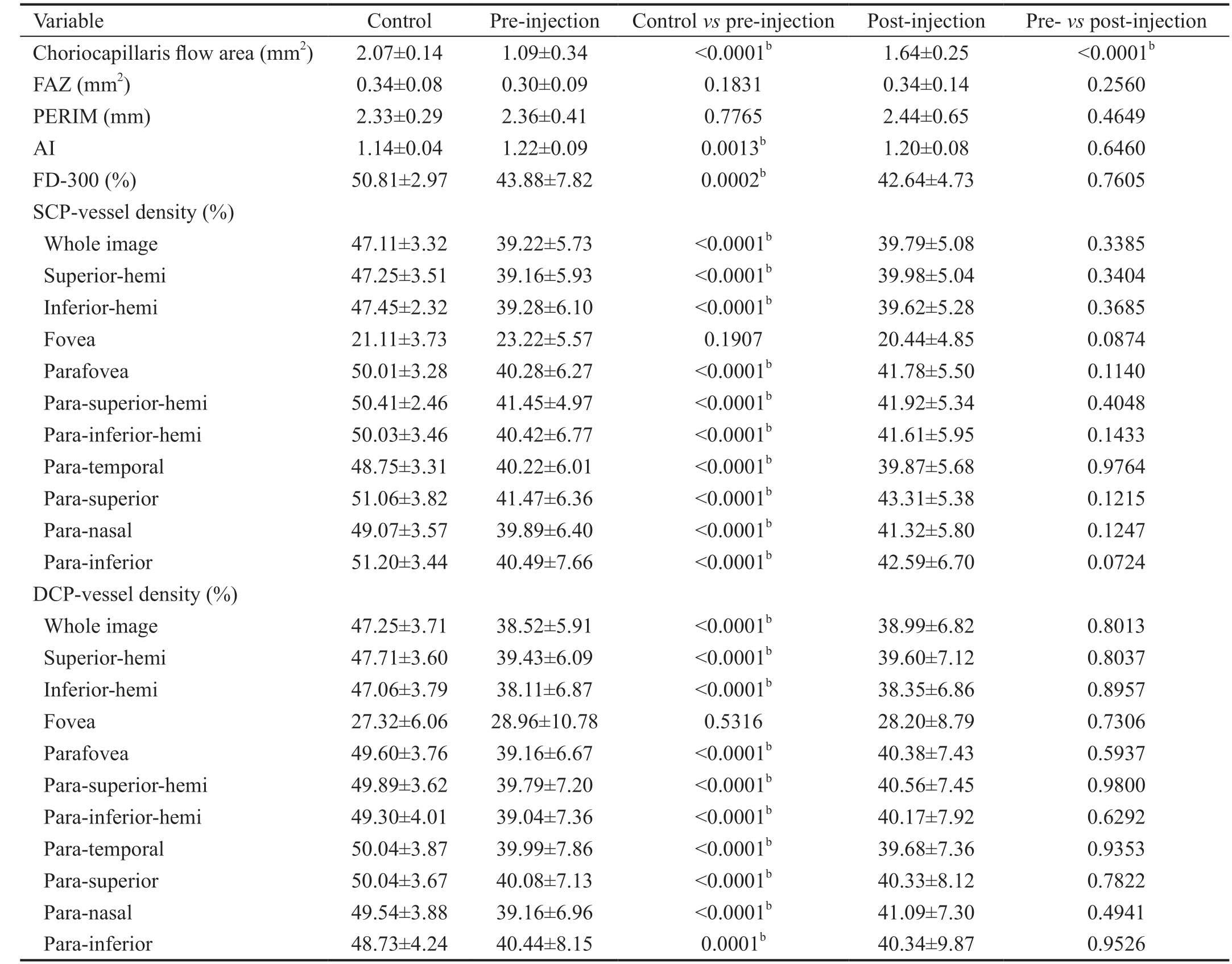
OCTA: Optical coherence tomography angiography; CRVO: Central retinal vein occlusion; FAZ: Foveal avascular zone; SCP: Superficial capillary plexus; DCP: Deep capillary plexus; FD-300: Vessel density within a 300 μm wide ring surrounding the FAZ; AI: Acircularity index;PERIM: Foveal avascular zone perimeter. bP<0.01.
Variable Control Pre-injection Control vs pre-injection Post-injection Pre- vs post-injection Choriocapillaris flow area (mm2) 2.07±0.14 1.09±0.34 <0.0001b 1.64±0.25 <0.0001b FAZ (mm2) 0.34±0.08 0.30±0.09 0.1831 0.34±0.14 0.2560 PERIM (mm) 2.33±0.29 2.36±0.41 0.7765 2.44±0.65 0.4649 AI 1.14±0.04 1.22±0.09 0.0013b 1.20±0.08 0.6460 FD-300 (%) 50.81±2.97 43.88±7.82 0.0002b 42.64±4.73 0.7605 SCP-vessel density (%)Whole image 47.11±3.32 39.22±5.73 <0.0001b 39.79±5.08 0.3385 Superior-hemi 47.25±3.51 39.16±5.93 <0.0001b 39.98±5.04 0.3404 Inferior-hemi 47.45±2.32 39.28±6.10 <0.0001b 39.62±5.28 0.3685 Fovea 21.11±3.73 23.22±5.57 0.1907 20.44±4.85 0.0874 Parafovea 50.01±3.28 40.28±6.27 <0.0001b 41.78±5.50 0.1140 Para-superior-hemi 50.41±2.46 41.45±4.97 <0.0001b 41.92±5.34 0.4048 Para-inferior-hemi 50.03±3.46 40.42±6.77 <0.0001b 41.61±5.95 0.1433 Para-temporal 48.75±3.31 40.22±6.01 <0.0001b 39.87±5.68 0.9764 Para-superior 51.06±3.82 41.47±6.36 <0.0001b 43.31±5.38 0.1215 Para-nasal 49.07±3.57 39.89±6.40 <0.0001b 41.32±5.80 0.1247 Para-inferior 51.20±3.44 40.49±7.66 <0.0001b 42.59±6.70 0.0724 DCP-vessel density (%)Whole image 47.25±3.71 38.52±5.91 <0.0001b 38.99±6.82 0.8013 Superior-hemi 47.71±3.60 39.43±6.09 <0.0001b 39.60±7.12 0.8037 Inferior-hemi 47.06±3.79 38.11±6.87 <0.0001b 38.35±6.86 0.8957 Fovea 27.32±6.06 28.96±10.78 0.5316 28.20±8.79 0.7306 Parafovea 49.60±3.76 39.16±6.67 <0.0001b 40.38±7.43 0.5937 Para-superior-hemi 49.89±3.62 39.79±7.20 <0.0001b 40.56±7.45 0.9800 Para-inferior-hemi 49.30±4.01 39.04±7.36 <0.0001b 40.17±7.92 0.6292 Para-temporal 50.04±3.87 39.99±7.86 <0.0001b 39.68±7.36 0.9353 Para-superior 50.04±3.67 40.08±7.13 <0.0001b 40.33±8.12 0.7822 Para-nasal 49.54±3.88 39.16±6.96 <0.0001b 41.09±7.30 0.4941 Para-inferior 48.73±4.24 40.44±8.15 0.0001b 40.34±9.87 0.9526

Figure 1 Changes in vessel density and flow area before and after intravitreal conbercept injection in eyes with central retinal vein occlusion measured on OCTA The vessel density in the superficial capillary plexus before (A) and after treatment (D). The vessel density in the deep capillary plexus before (B) and after treatment (E). The flow area in the choriocapillaris before (C) and after treatment (F).
Correlation Analysis of Different Parameters In the current study, we also analyzed the relationship between OCTA parameters and logMAR BCVA as well as retinal thickness. Overall, there was no correlation between logMAR BCVA and any OCTA parameters either before or after treatment. Morphological parameters, including foveal and parafoveal retinal thickness, were negatively correlated with the flow area of the choriocapillaris at baseline (r=-0.5645,P=0.0033; r=-0.4645, P=0.0168, respectively). There was no correlation between OCTA parameters and foveal and parafoveal retinal thickness after treatment. Moreover, we evaluated the correlations between the duration time after onset of CRVO and OCTA parameters as well as logMAR BCVA. Noteworthily, the duration time after onset of CRVO is positively correlated with the PERIM (r=0.4121, P=0.0405),which suggests that the longer the duration time after onset of CRVO, the more irregular the FAZ boundaries at baseline.
DISCUSSION
Macular edema can occur during the course of CRVO and is a major complication that can lead to severe impairment of central vision. Increased production of VEGF plays a pivotal role in the development of macular edema caused by retinal vein occlusion (RVO). Hemodynamic changes occurring early in RVO reduce retinal perfusion, leading to an increase in the release of VEGF. This increase in the level of VEGF is a crucial contributor to the progression of retinal non-perfusion and worsened retinal ischemia and edema, which cause visual impairment[13-14].
Hence, numerous studies have shown that inducing a timely and aggressive blockade against VEGF with anti-VEGF therapy provides a marked visual benefit and substantially reduces retinal thickening in eyes with RVO-related macular edema[2,5,15-16]; this result was further confirmed in our study.In the current study, the mean logMAR BCVA and the full retinal thickness obtained on OCTA in the foveal, parafoveal,and perifoveal regions were all significantly decreased after conbercept therapy.
The impact of intravitreal conbercept injection on vascular density has not previously been investigated utilizing OCTA in eyes with CRVO complicated by macular edema. Overall,we found that the vessel density of the whole image and all different sectors of parafovea both in the SCP and DCP was significantly lower in the CRVO group than in the control group, in accordance with previous studies[9,12,17]. After a single intravitreal conbercept injection, the vessel density of whole image and of all quadrants of parafovea was slightly increased in the SCP and DCP, but these difference were not significant,consistent with other studies[11-12,17]. Moreover, several studies have demonstrated that timely treatment with anti-VEGF agents helps to prevent the progression of retinal non-perfusion and promotes reperfusion in RVO eyes[14,18].
Kang et al[9] found that ischemic changes did not significantly affect foveal vessel density because the FAZ area occupies a large portion of the fovea. Consistent with this finding, in our study, we found that the foveal vessel density was only slightly higher in the SCP and DCP in CRVO eyes than in control eyes and slightly reduced after treatment. Although we had adjusted the significant segmentation errors as much as possible by manually altering the segmentation lines, macular edema may still cause small segmentation errors in the foveal region,which could account for the differences in the foveal vessel density before and after treatment.
No previous study has analyzed the blood flow area in the choriocapillaris in eyes with CRVO. We therefore evaluated this parameter in our study. We found that the flow area in the choriocapillaris was significantly smaller in CRVO eyes than in healthy eyes. We speculated that the shadowing effect of fluid accumulation due to macular edema may cause the attenuated Optical Coherence Tomography signal, thereby leading to potential overestimation of the degree of decreased vascular perfusion in the choriocapillaris[19]. After conbercept therapy,the flow area of the choriocapillaris markedly increased,which may also result from the reduction of shadowing effect caused by the attenuation of macular edema. Furthermore,we found that the baseline flow area of the choriocapillaris was negatively correlated with foveal and parafoveal retinal thickness, indicating that more severe macular edema is associated with lower perfusion in the choriocapillaris. Despite the frequency of this disease, the nature of the retinal and choroidal hemodynamic changes that are caused by CRVO is not yet clear and remains speculative.
In addition, we performed the first analysis of the latest FAZrelevant parameters, including the FAZ area, the PERIM, the AI and the FD-300, on a retina slab in CRVO eyes both before and after anti-VEGF treatment. There have been conflicting regarding the changes in FAZ area between pre- and posttreatment because the size of the FAZ is highly variable in the general population, which complicates how these data can be interpreted. Our findings are in line with those of Winegarner et al[20], who reported that the FAZ area was statistically unchanged during the course of 12mo of anti-VEGF therapy in RVO patients. In addition, the AI was markedly higher in CRVO eyes than in healthy eyes, and this may contribute to distinguish between the control and CRVO patient groups.
Several limitations of our study included the small sample,the short-term follow-up with only one injection and latent segmentation errors in the presence of macular edema.
In conclusion, OCTA, a non-invasive and promising technique,can be used to obtain quantitative data and more detailed information regarding the vascular network of the SCP, DCP,outer retina and choriocapillaris in eyes with CRVO. Our study results demonstrate that retinal vessel density was lower in CRVO eyes with macular edema than in healthy subjects and was not significantly altered at one month after intravitreal conbercept treatment. The changes observed in a retinal slab in the latest FAZ-relevant parameters including the FAZ area,the PERIM, the AI and the FD-300 as well as the flow area in choriocapillaris, should be further investigated in a study with a larger sample size, a longer follow-up period, and more injections to confirm our findings.
ACKNOWLEDGEMENTS
Authors’ contributions: Chi W, Deng Y, Zhang AQ, Zhong QW and Su LS designed the study. Deng Y, Cai XJ, Jin CJ,Zhang SC, Su LS, Chen H, Lin Y, Sun LM, Chen GD, Lu MZ and Zhong LT collected and analyzed the data. Deng Y, Zhang AQ and Zhong QW drafted the manuscript. Chi W, Deng Y,Zhang AQ and Zhong QW contributed to the interpretation of the results and critical revision of the manuscript. All authors read and approved the manuscript.
Foundation: Supported by National Natural Science Foundation of China (No.81570830).
Conflicts of Interest: Deng Y, None; Zhong QW, None;Zhang AQ, None; Cai XJ, None; Lu MZ, None; Zhang SC,None; Su LS, None; Chen H, None; Lin Y, None; Sun LM,None; Chen GD, None; Zhong LT None; Jin CJ, None; Chi W, obtained a grant from Chengdu Kanghong Biotechnology,Inc. The funding organization had no role in the design or conduct of this research.
1 McAllister IL. Central retinal vein occlusion: a review. Clin Exp Ophthalmol 2012;40(1):48-58.
2 Rhoades W, Dickson D, Nguyen QD, Do DV. Management of macular edema due to central retinal vein occlusion-The role of aflibercept. Taiwan J Ophthalmol 2017;7(2):70-76.
3 Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual
outcome in central retinal vein occlusion. Ophthalmology 2011;118(1):119-133.e1-2.
4 de Oliveira Dias JR, de Andrade GC, Novais EA, Farah ME, Rodrigues EB. Fusion proteins for treatment of retinal diseases: aflibercept, zivaflibercept, and conbercept. Int J Retina Vitreous 2016;2:3.
5 Sun ZH, Zhou HY, Lin B, Jiao X, Luo YD, Zhang F, Tao SS, Wu Q, Ke ZH, Liu XL. Efficacy and safety of intravitreal conbercept injections in macular edema secondary to retinal vein occlusion. Retina
2017;37(9):1723-1730.
6 Li FJ, Sun M, Guo JL, Ma AH, Zhao BJ. Comparison of conbercept with ranibizumab for the treatment of macular edema secondary to branch retinal vein occlusion. Curr Eye Res 2017;42(8):1174-1178.
7 Wylęgała A, Teper S, Dobrowolski D, Wylęgała E. Optical coherence angiography: a review. Medicine (Baltimore) 2016;95(41):e4907.
8 Coscas F, Glacet-Bernard A, Miere A, Caillaux V, Uzzan J, Lupidi M,Coscas G, Souied EH. Optical coherence tomography angiography in retinal vein occlusion: evaluation of superficial and deep capillary plexa.Am J Ophthalmol 2016;161:160-171.e1-2.
9 Kang JW, Yoo R, Jo YH, Kim HC. Correlation of microvascular structures on optical coherence tomography angiography with visual acuity in retinal vein occlusion. Retina 2017;37(9):1700-1709.
10 Suzuki N, Hirano Y, Tomiyasu T, Esaki Y, Uemura A, Yasukawa T,Yoshida M, Ogura Y. Retinal hemodynamics seen on optical coherence tomography angiography before and after treatment of retinal vein occlusion. Invest Ophthalmol Vis Sci 2016;57(13):5681-5687.
11 Ghasemi Falavarjani K, Iafe NA, Hubschman JP, Tsui I, Sadda SR,Sarraf D. Optical coherence tomography angiography analysis of the foveal avascular zone and macular vessel density after anti-VEGF therapy in eyes with diabetic macular edema and retinal vein occlusion. Invest Ophthalmol Vis Sci 2017;58(1):30-34.
12 Sellam A, Glacet-Bernard A, Coscas F, Miere A, Coscas G, Souied EH. Qualitative and quantitative follow-up using optical coherence tomography angiography of retinal vein occlusion treated with anti-VEGF: optical coherence tomography angiography follow-up of retinal vein occlusion. Retina 2017;37(6):1176-1184.
13 Campochiaro PA, Hafiz G, Shah SM, Nguyen QD, Ying H, Do DV,Quinlan E, Zimmer-Galler I, Haller JA, Solomon SD, Sung JU, Hadi Y, Janjua KA, Jawed N, Choy DF, Arron JR. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther 2008;16(4):791-799.
14 Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology 2013;120(4):795-802.
15 Heier JS, Clark WL, Boyer DS, Brown DM, Vitti R, Berliner AJ, Kazmi H, Ma Y, Stemper B, Zeitz O, Sandbrink R, Haller JA.Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study.Ophthalmology 2014;121(7):1414-1420.e1.
16 Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, Murahashi WY, Rubio RG, BRAVO Investigators. Ranibizumab for macular edema following branch retinal vein occlusion: sixmonth primary end point results of a phase III study. Ophthalmology 2010;117(6):1102-1112.e1.
17 Glacet-Bernard A, Sellam A, Coscas F, Coscas G, Souied EH. Optical coherence tomography angiography in retinal vein occlusion treated with dexamethasone implant: a new test for follow-up evaluation. Eur J Ophthalmol 2016;26(5):460-468.
18 Mir TA, Kherani S, Hafiz G, Scott AW, Zimmer-Galler I, Wenick AS, Solomon S, Han I, Poon D, He LM, Shah SM, Brady CJ, Meyerle C, Sodhi A, Linz MO, Sophie R, Campochiaro PA. Changes in retinal nonperfusion associated with suppression of vascular endothelial growth factor in retinal vein occlusion. Ophthalmology 2016;123(3):625-634.e1.
19 Novais EA, Waheed NK. Optical coherence tomography angiography of retinal vein occlusion. Dev Ophthalmol 2016;56:132-138.
20 Winegarner A, Wakabayashi T, Fukushima Y, Sato T, Hara-Ueno C,Busch C, Nishiyama I, Shiraki N, Sayanagi K, Nishida K, Sakaguchi H,Nishida K. Changes in retinal microvasculature and visual acuity after antivascular endothelial growth factor therapy in retinal vein occlusion.Invest Ophthalmol Vis Sci 2018;59(7):2708-2716.