INTRODUCTION
S tudying the development of the human eye is essential to better understand complex visual pathophysiologic processes. There are many factors that affect the differentiation and maturation of the eye. Most important of all are genetic factors. Neural input, refractive error, and other generalized pathologic disorders can cause differentiation and maturation of ocular tissues in a non-physiologic manner[1-4]. One of the earliest signs of pathology in the eye is an alteration of normal axial length. Examples of such disorders include nanophthalmos, microphthalmos, and retinoblastoma, which classically have a decrease in axial length. In contrast,congenital glaucoma is usually associated to an increase in axial length.
Multiple studies have reported ocular axial length in pediatric subjects with intraocular pathology, mostly with congenital cataracts and glaucoma[1-11]. However, only a few small studies have been performed to evaluate the axial length in healthy pediatric patients without ocular disease in either eye[4,9,11-14]. The purpose of our study is to develop a growth curve for pediatric ocular axial lengths in healthy eyes.
SUBJECTS AND METHODS
Ethical Approval An Institutional Review Board-approved retrospective consecutive chart review was undertaken at the Bascom Palmer Eye Institute in 165 subjects that underwent examination under anesthesia due to a positive family history of retinoblastoma or other inherited disease, none of whom developed any disease by the end of the study.
Data regarding age of subjects at examination and ocular axial length was recorded. Axial length was determined using an immersion A-scan (Eye Cubed, Ellex, Adelaide, Australia)sonogram and a standardized A-scan probe directly over the cornea. Subjects with intraocular pathology in either eye were excluded from the study. Subjects were stratified to different age groups by 3, 6 or 12mo intervals, depending on the age of the patient.
Statistical Analysis Axial lengths were compiled and divided into those subjects 3 years of age and younger and those above the age of 3. Linear, logarithmic, quadratic, cubic, growth,exponential, and logistic regression models were then applied to each group of data to determine the best fit for the data of each group.
RESULTS
A total of 165 subjects (330 eyes) were evaluated under anesthesia. The mean age at the time of examination was 30.62(SD 18.04)mo with a mean axial length of 21.37 (SD 1.03) mm.The subjects were stratified based on age (Table 1; Figure 1).A one-way ANOVA was performed to calculate the mean difference of axial length among the age groups. One-way ANOVA showed a statistically significant difference in the mean axial lengths between each of the age groups (P<0.001).The t-test analysis failed to show a statistical difference between the right and left eye of subjects (P=0.25, 95%CI:-0.01 to 0.04). Multiple regression models were then applied across all ages (Table 2; Figure 2). One eye was chosen at random from each subject. The line of best fit was calculated:eye length=0.966×loge(age)+18.270 (r2=0.479, r=0.692,P≤0.001; Figure 2).

Figure 1 Stratification of patients by age.
Table 1 Axial lengths based on age group
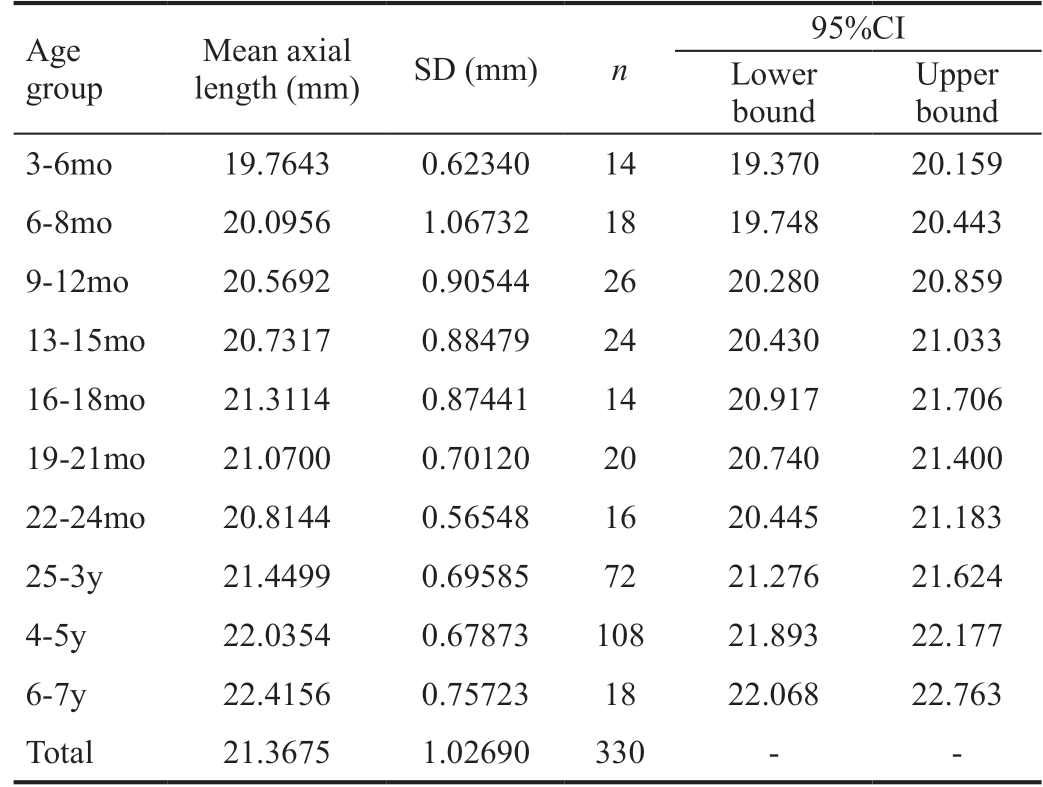
One way ANOVA was performed to evaluate the mean difference in eye length among the age groups. A statistically significant difference was present between each of the age groups (P<0.001).
Age group Mean axial length (mm) SD (mm) n 95%CI Lower bound Upper bound 3-6mo 19.7643 0.62340 14 19.370 20.159 6-8mo 20.0956 1.06732 18 19.748 20.443 9-12mo 20.5692 0.90544 26 20.280 20.859 13-15mo 20.7317 0.88479 24 20.430 21.033 16-18mo 21.3114 0.87441 14 20.917 21.706 19-21mo 21.0700 0.70120 20 20.740 21.400 22-24mo 20.8144 0.56548 16 20.445 21.183 25-3y 21.4499 0.69585 72 21.276 21.624 4-5y 22.0354 0.67873 108 21.893 22.177 6-7y 22.4156 0.75723 18 22.068 22.763 Total 21.3675 1.02690 330 - -
No statistical difference was noted between both eyes. Nonlinearity across age groups was found (P=0.411) showing faster axial length growth during the first 10mo with a decline in the rate of growth afterwards.
DISCUSSION
as well as retinal surface changes[19] in children. Limitations in these studies include small cohorts and contralateral eye pathology[10-14]. Based on our large series of healthy pediatric subjects, we have derived a logarithmic regression model for axial length growth of pediatric eyes with a graph template for clinical use (Figure 3). This model shows that both eyes grow at similar rates. Although it is well known that axial length increases significantly during the first few years of life[20-21],normative data from young children less than 2 years of age is scarce because healthy children do not generally undergo routine ultrasonography.

Figure 2 Logarithmic regression model of axial lengths Mean(solid line) and 95% standard deviation (dotted lines) are plotted.
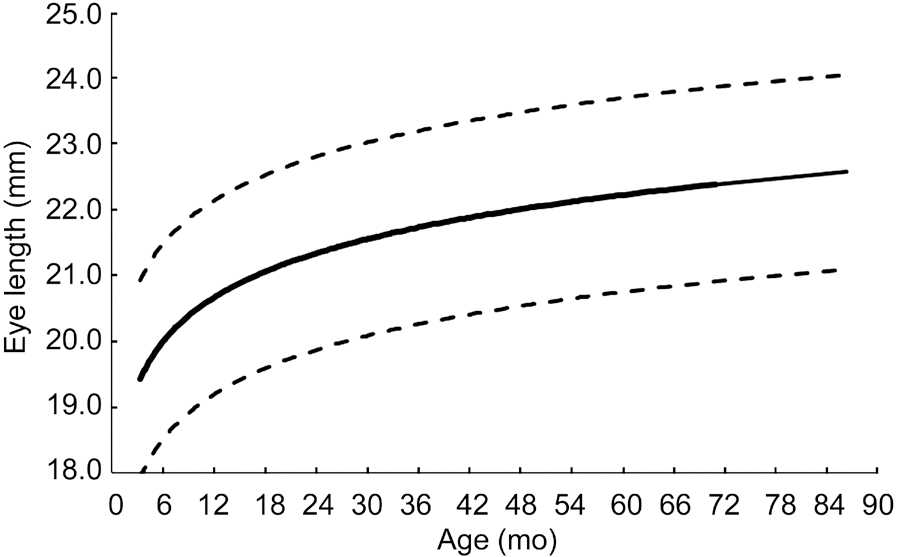
Figure 3 Template for plotting axial lengths based on age with mean (solid line) and 95%CI (dotted lines) curve.
This study shows an increase in axial length over time. The most significant increases in axial length occur during the first 10mo of age, at which point axial length continues to increase,though at a decreased rate. The logarithmic regression model(Figure 2) shows this trend.
Axial length is of essential value when calculating the power of intraocular lenses (IOLs). IOL power continues to be a topic of debate among pediatric ophthalmologists partly because of the scarcity of reliable model of axial length in the pediatric population. The main challenge when deciding IOL power is the future change in refraction due to growth associated changes in axial length, anterior segment, and corneal power[7,15,22]. During childhood there is a decrease in corneal
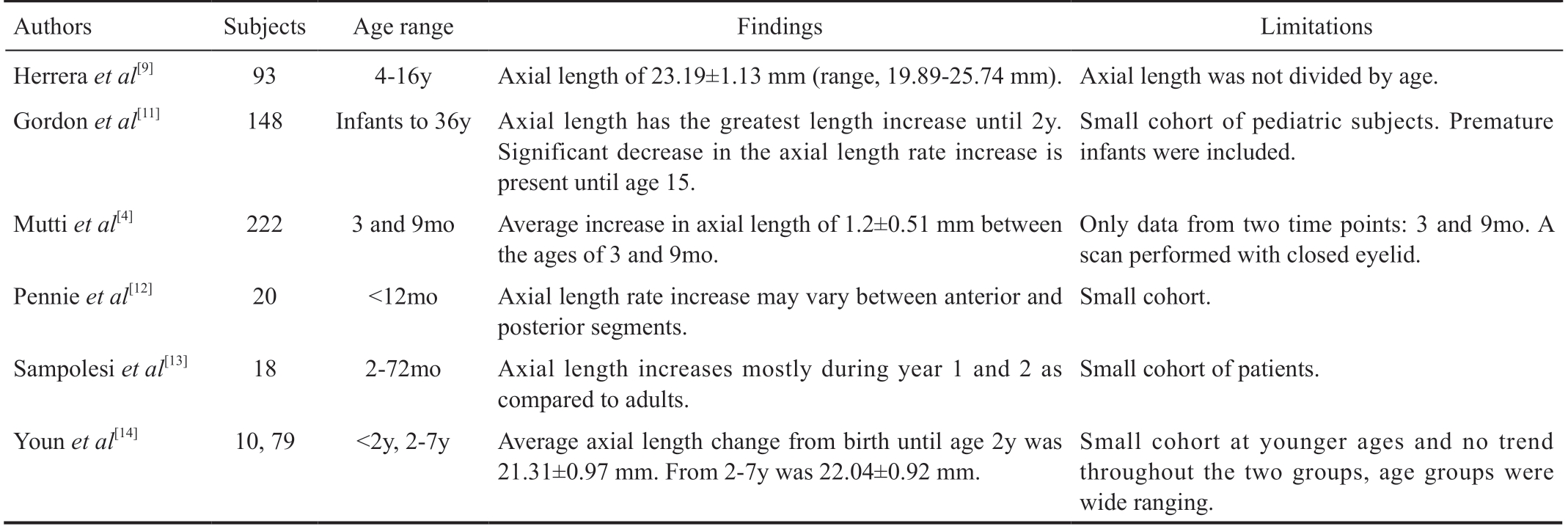
Currently, no large study has examined the ocular axial length of healthy pediatric subjects without intraocular pathology in either eye up to the age of 7y. Only six studies have measured axial length in healthy children. Table 3 lists the studies,their findings, and the limitations to each of the studies when compared to this study. The authors have attempted to reach out to all aforementioned authors to make a collaborative data set, though access to these data sets were not available.Previous studies have also evaluated changes in corneal curvature[6,15], endothelial cell density[16-17], lens changes[18],curvature and increase in axial length. Furthermore, the IOL’effective position may affect the long-term refractive outcome.No study to date has evaluated if IOL axial location changes as the eye grows. One study has shown that there is a likelihood of IOL to dislocate in pediatric eyes due to the relatively small size of the capsule when compared to adult eyes[23].Another factor that significantly impacts IOL calculations in children is the decrease in corneal curvature that occurs early in life[7,15,22]. Currently, IOL calculations are performed using later generation algorithms such as the SRK-T, Hoffer-Q, and Holladay II equations. These formulas have historically been based on adult normative data and may lead to refractive errors when applied to the pediatric population due to assumptions made in the formulas like that of the corneal curvature. The current series provides the largest data set to date on axial length growth in the normal pediatric eyes and may help pediatric cataract surgeons during preoperative evaluation in children in the amblyopic age range.
Table 2 Comparisons of regression models for all patients n=330 eyes
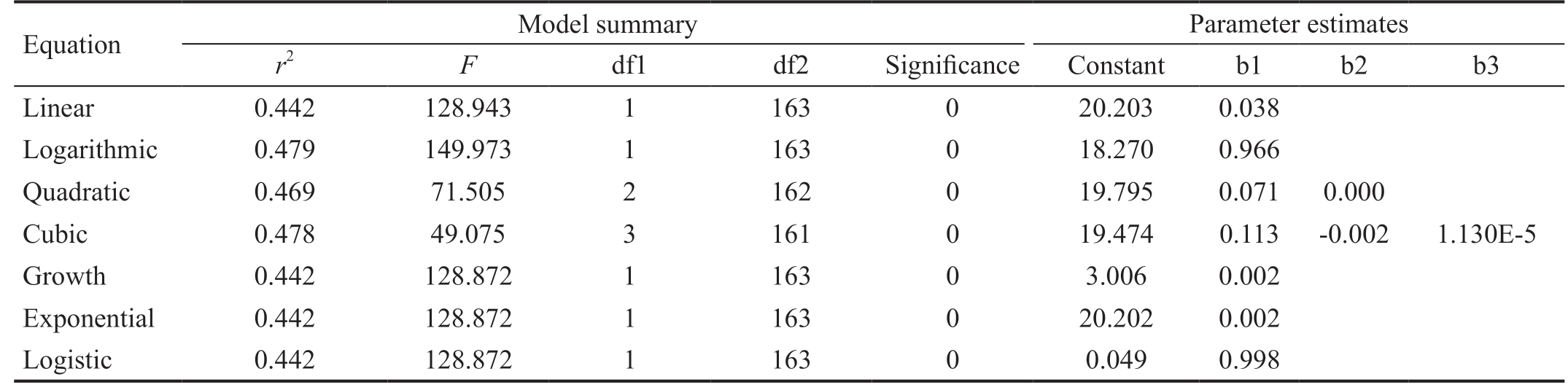
Equation Model summary Parameter estimates r2 F df1 df2 Significance Constant b1 b2 b3 Linear 0.442 128.943 1 163 0 20.203 0.038 Logarithmic 0.479 149.973 1 163 0 18.270 0.966 Quadratic 0.469 71.505 2 162 0 19.795 0.071 0.000 Cubic 0.478 49.075 3 161 0 19.474 0.113 -0.002 1.130E-5 Growth 0.442 128.872 1 163 0 3.006 0.002 Exponential 0.442 128.872 1 163 0 20.202 0.002 Logistic 0.442 128.872 1 163 0 0.049 0.998
Table 3 Summary of prior studies measuring pediatric axial lengths in normal children
?
The most common long-term refractive error following pediatric cataract surgery has been shown to be due to a myopic shift due to axial length increase[24-26]. These studies have shown that the patients who underwent cataract surgery at a younger age had a greater average myopic shift in postoperative refraction of approximately 6.00 diopters when compared to those who had cataract surgery at an older age[27].This model may help determine proper lens power based on predicted axial growth and may assist in the prevention of amblyopia post-operatively[28].
There have been many new advances in understanding different disease states that affect eyes of pediatric patients.With a global focus on diagnosing and preventing progressive axial myopia[29-31] and changes in choroidal thickness[9,32],having a normative model for pediatric axial lengths may enhance the ophthalmologists’ ability to diagnose and monitor such diseases.
Pathologic axial myopia may lead to visually threatening complications. These may include: amblyopia, retinal detachment, retinal schisis, staphyloma, tilted optic nerve head, and subretinal neovascularization. An axial length growth model may allow earlier diagnosis and treatment.Atropine therapy and other investigational therapies may be tailored based on the growth curve. Other applications for the new model may include the diagnosis and management of patients with congenital glaucoma. This algorithm and chart will be of indispensable use for monitoring pediatric glaucoma. As glaucoma damage in the pediatric population results in elongation in axial length, monitoring change in axial length when compared to normative values can give the ophthalmologist another tool in their decision making and treatment algorithm for this blinding disease.
Limitations to our study include its retrospective nature and a lack of other data points that are currently used to calculate I OL powers. These include keratometry, anterior chamber depth, lens thickness, and white-to-white distance.
The current study presents the largest series of bilateral ocular axial lengths without intraocular pathology. This data may aid in the diagnosis and management of multiple diseases including IOL power calculation in the pediatric population,pathologic myopia, and congenital glaucoma. Further studies are needed to evaluate refractive outcomes after pediatric cataract extraction and IOL implantation using this model.
ACKNOWLEDGEMENTS
Joyce Schiffman for assistance with statistical analysis. Fiona J Ehlies and Bernadette Ayres for echography.
Foundation: Supported by NIH Center Core Grant(P30EY014801), Research to Prevent Blindness Unrestricted Grant, Department of Defense at the Bascom Palmer Eye Institute.
Conflicts of Interest: Bach A, None; Villegas VM, None;Gold AS, None; Shi W, None; Murray TG, None.
1 Tadros D, Trivedi RH, Wilson ME, Davidson JD. Ocular axial growth in pseudophakic eyes of patients operated for monocular infantile cataract:a comparison of operated and fellow eyes measured at surgery and 5 or more years later. J AAPOS 2016;20(3):210-213.
2 Shen L, You QS, Xu XL, Gao F, Zhang ZB, Li B, Jonas JB. Scleral and choroidal volume in relation to axial length in infants with retinoblastoma versus adults with malignant melanomas or end-stage glaucoma. Graefes Arch Clin Exp Ophthalmol 2016;254(9):1779-1786.
3 Lambert SR. Changes in ocular growth after pediatric cataract surgery.Dev Ophthalmol 2016;57:29-39.
4 Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK,Moeschberger ML, Zadnik K. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci 2005;46(9):3074-3080.
5 Hu YY, Wu JF, Lu TL, Wu H, Sun W, Guo DD, Jiang WJ, Wang XR, Jonas JB, Bi HS. Prevalence and associations of anisometropia in children. Invest Ophthalmol Vis Sci 2016;57(3):979-988.
6 Jonas JB, Xu L, Wang YX, Bi HS, Wu JF, Jiang WJ, Nangia V, Sinha A,Zhu D, Tao Y, Guo Y, You QS, Wu LJ, Tao LX, Guo XH, Ohno-Matsui K, Panda-Jonas S. Education-related parameters in high myopia: adults versus school children. PLoS One 2016;11(5):e0154554.
7 Matsuda K, Yokoyama T, Matsumoto H, Yamashita R, Kohno T, Shiraki K. Relationship between birth month and corneal radius or axial length.Nihon Ganka Gakkai Zasshi 2013;117(2):102-109.
8 Tideman JW, Polling JR, Voortman T, Jaddoe VW, Uitterlinden AG,Hofman A, Vingerling JR, Franco OH, Klaver CC. Low serum vitamin D is associated with axial length and risk of myopia in young children. Eur J Epidemiol 2016;31(5):491-499.
9 Herrera L, Perez-Navarro I, Sanchez-Cano A, Perez-Garcia D, Remon L, Almenara C, Caramello C, Cristóbal JA, Pinilla I. Choroidal thickness and volume in a healthy pediatric population and its relationship with age,axial length, ametropia, and sex. Retina 2015;35(12):2574-2583.
10 Hussain RN, Shahid F, Woodruff G. Axial length in apparently normal pediatric eyes. Eur J Ophthalmol 2014;24(1):120-123.
11 Gordon RA, Donzis PB. Refractive development of the human eye.Arch Ophthalmol 1985;103(6):785-789.
12 Pennie FC, Wood IC, Olsen C, White S, Charman WN. A longitudinal study of the biometric and refractive changes in full-term infants during the first year of life. Vision Res 2001;41(21):2799-2810.
13 Sampaolesi R, Caruso R. Ocular echometry in the diagnosis of congenital glaucoma. Arch Ophthalmol 1982;100(4):574-577.
14 Youn DH, Yu YS, Park IW. Intraocular pressure and axial length in children. Korean J Ophthalmol 1990;4(1):26-29.
15 Inagaki Y. The rapid change of corneal curvature in the neonatal period and infancy. Arch Ophthalmol 1986;104(7):1026-1027.
16 Nucci P, Brancato R, Mets MB, Shevell SK. Normal endothelial cell density range in childhood. Arch Ophthalmol 1990;108(2):247-248.
17 Møller-Pedersen T, Møller HJ. Viability of human corneal keratocytes during organ culture. Acta Ophthalmol Scand 1996;74(5):449-455.
18 Wood IC, Mutti DO, Zadnik K. Crystalline lens parameters in infancy.Ophthalmic Physiol Opt 1996;16(4):310-317.
19 Robb RM. Increase in retinal surface area during infancy and childhood. J Pediatr Ophthalmol Strabismus 1982;19(4):16-20.
20 Larsen JS. The sagittal growth of the eye. IV. Ultrasonic measurement of the axial length of the eye from birth to puberty. Acta Ophthalmol(Copenh) 1971;49(6):873-886.
21 Fledelius HC, Christensen AC. Reappraisal of the human ocular growth curve in fetal life, infancy, and early childhood. Br J Ophthalmol 1996;80(10):918-921.
22 Tucker SM, Enzenauer RW, Levin AV, Morin JD, Hellmann J. Corneal diameter, axial length, and intraocular pressure in premature infants.Ophthalmology 1992;99(8):1296-1300.
23 Long T, Xu YN, Wu XM, Zhao J, Li Y, Xie LX. IOL position[corrected] in pediatric eyes. Ophthalmology 2013;120(1):212,212.e1-e3.
24 McClatchey SK, Dahan E, Maselli E, Gimbel HV, Wilson ME,Lambert SR, Buckley EG, Freedman SF, Plager DA, Parks MM. A comparison of the rate of refractive growth in pediatric aphakic and pseudophakic eyes. Ophthalmology 2000;107(1):118-122.
25 Crouch ER, Crouch ER Jr, Pressman SH. Prospective analysis of pediatric pseudophakia: myopic shift and postoperative outcomes. J AAPOS 2002;6(5):277-282.
26 Plager DA, Kipfer H, Sprunger DT, Sondhi N, Neely DE. Refractive change in pediatric pseudophakia: 6-year follow-up. J Cataract Refract Surg 2002;28(5):810-815.
27 Lambert SR, Cotsonis G, DuBois L, Wilson ME, Plager DA, Buckley EG, McClatchey SK; Infant Aphakia Treatment Study Group. Comparison of the rate of refractive growth in aphakic eyes versus pseudophakic eyes in the Infant Aphakia Treatment Study. J Cataract Refract Surg 2016;42(12):1768-1773.
28 Lam CS, Tang WC, Tse DY, Tang YY, To CH. Defocus Incorporated Soft Contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: a 2-year randomised clinical trial. Br J Ophthalmol 2014;98(1):40-45.
29 La Spina C, Corvi F, Bandello F, Querques G. Static characteristics and dynamic functionality of retinal vessels in longer eyes with or without pathologic myopia. Graefes Arch Clin Exp Ophthalmol 2016;254(5):827-834.
30 Zhao ZN, Zhou XT, Jiang CH, Sun XH. Effects of myopia on different areas and layers of the macula: a Fourier-domain optical coherence tomography study of a Chinese cohort. BMC Ophthalmol 2015;15:90.
31 Li SM, Li H, Li SY, Liu LR, Kang MT, Wang YP, Zhang FJ, Zhan SY, Gopinath B, Mitchell P, Wang NL; Anyang Childhood Eye Study Group. Time outdoors and myopia progression over 2 years in Chinese children: the Anyang childhood eye study. Invest Ophthalmol Vis Sci 2015;56(8):4734-4740.
32 Tenlik A, Gürağaç FB, Güler E, Dervişoğulları MS, Totan Y. Choroidal thickness measurement in healthy pediatric population using Cirrus HD optical coherence tomography. Arq Bras Oftalmol 2015;78(1):23-26.