INTRODUCTION
A fter Kelly and Wendel[1] first reported successful vitreous surgery for the macular hole (MH), Park et al[2] reported internal limiting membrane (ILM) peeling technology and then Michalewska et al[3] proposed inverted ILM flap technique.Liggett et al[4] first used autologous serum as an adjuvant to increase the MH closure rate, and serum was deeded that contains numerous growth factors, which can induce fibrocellular migration, adhesion, and proliferation[5]. Gas, usually C3F8 or SF6, as tamponade materials were widely used in MH surgery.Recently, several studies found that air filling is equivalent to long-effect air tamponade in MH surgery[6-8]. With the rapid development of MH surgery technique, the closure rate of MH surgery already reached more than 90%[9-10]. Since spectraldomain optical coherence tomography (SD-OCT) has been used to observe the macular microstructure, the fine changes in the process of MH reconstruction are being detected. Grewal et al[11] found the size of foveal lucency correlated with visual recovery and preoperative basal hole diameter was related to foveal lucency. Liu et al[12] reported that macular hole closure index (MHCI) was a new predictive method for anatomical outcomes after IMH surgery. Shpak et al[13] suggested preoperative central subfield retinal thickness could predict postoperative anatomical results of IMH. Kitao et al[14] have reported that the time until external limiting membrane (ELM)bridge and the glial cell disappearance were associated with postoperative best-corrected visual acuity (BCVA) at 3y,which indicates that ELM maybe a crucial early predictive factor to visual improvement. However, the predictive factors of visual improvement are still controversial and the relevant elements to the macular microstructure repair process are still unclear. The purpose of this study was to explore the macular microstructure changes after vitrectomy for idiopathic macular hole (IMH) and investigate the correlative predictive factors to visual improvement and macular microstructure repair for MH surgery.
SUBJECTS AND METHODS
Ethical Approval This was a retrospective study. The study was approved by the Second Xiangya Hospital Ethics Committee and conducted in accordance with the Declaration of Helsinki. All patients were informed about the risks and benefits of the surgery, and written informed consent was obtained in accordance with the guidelines for the Second Xiangya Hospital of Central South University.
The medical records of 19 eyes of 18 patients with an IMH who were underwent surgery in the Second Xiangya Hospital between October 2016 and April 2018 were reviewed. All patients included had been diagnosed with a stage 2, 3 or 4 IMH according to the Gass classification[15]. We excluded eyes with retinal detachment, glaucoma, diabetic retinopathy,high myopia, follow-up length less than 6mo postoperatively or any other secondary MHs. All patients had a standard ophthalmological examination before and 1wk, 1, 3, 6mo after surgery. Examinations included BCVA, binocular indirect ophthalmoscopy, slit-lamp bio-microscopy and SD-OCT. One surgeon (Zeng J) operated on all eyes under local anesthesia.
Standard 23-gauge pars plana vitrectomy was performed for all MHs. Before surgery, two to three milliliters blood from patients of MH were collected in a syringe and kept it in a vertical and static position for more than 30min. Triamcinolone acetonide (TA) was used to clarify the vitreous gel. Eighteen of 19 patients of MH underwent phacoemulsification. After core vitrectomy, posterior vitreous detachment was performed.Indocyanine green (ICGI; 0.125%) was used for ILM staining.
The ILM was peeled around vascular arch. Peeling cases then give gas-fluid exchange and intraocular lens implantation directly. For inverted ILM flap cases, the ILM around macular about one to two papillary diameter (PD) was inverted into MH so that it completely covered and filled the MHs. Next,serum was extracted gently from the post-static stratified blood and has been intravitreal injection about 0.05 mL. Gasfluid change was given slightly to prevent the flapped ILM shifting. Intraocular lens implantation was performed. All the patients were instructed to remain face-down position for 3 to 5d postoperatively. SD-OCT was used to confirm whether the MH closed and detect the macular microstructure changes.
The glial proliferation was defined as the presence of a foveal hyperreflective lesion on SD-OCT based on the previous reports of histopathologic studies of autopsy eyes and OCT images of surgically closed MHs[16-17].
For statistical analysis, logMAR BCVA and central retinal thickness (CRT) were analyzed using a one-way analysis of variance (ANOVA) when quantitative parameters were compared among baseline, postoperative 1wk, 1, 3 and 6mo. If the parameter was not normally distributed, the non-parametric Kruskal-Wallis one-way ANOVA on ranks was applied.Fisher’s exact test was performed to test the existent subretinal fluid (SRF), ELM defects, retinal never fiber layer (RNFL)defects, internal plexiform layer (IPL) defects and the closure of MH at postoperative 1wk, 1, 3 and 6mo. Correlations between preoperative parameters and postoperative visual acuity were analyzed with Spearman’s ranked correlation.Independent-samples t-test was used to analysis the influential factors between the continuous ELM and ELM defects. All analyses were conducted using SPSS (Version 19.0, USA), and P<0.05 was deemed to indicate statistical significance.
RESULTS
The retrospective study was comprised of 19 eyes of 18 patients. Totally 19 of 19 the MHs closed successfully at postoperative 6mo. The 19 cases consisted of 17 (89%)women and 2 (11%) men ranging in age from 49 to 74y with an average age of 64.0±5.4y. The operated eye was the right eye in 11 patients (58%) and left eye in 8 patients (42%).All patients were phakic eyes before surgery, and 18 (95%)of them underwent phacoemulsification and foldable lens implantation during surgery. Preoperative mean logMAR BCVA was 1.3±0.48. The MHs were classified as stage 2 in 8 eyes (42%), stage 3 in 9 eyes (47%) and stage 4 in 2 eyes (11%)preoperatively. The mean axial length was 23±0.65 mm (range,22.06-24.61 mm). Mean preoperative minimum diameter of the holes was 430±169 μm (range, 177-780 μm). Mean preoperative basal diameter of the holes was 763±228 μm(range, 304-1165 μm). Symptom duration was 6.0±7.1mo(range, 0.5-24).
Changes in logMAR BCVA are shown in Table 1, which suggested that postoperative BCVA continuously increased over 6mo (P<0.001). Totally 19 operated eyes (100%)observed were anatomically closed detected by OCT at postoperative 1mo. It was observed that ELM reconstructed earlier compared to the reconstruction of the ellipsoid zone(EZ) and cone interdigitation zone (CIZ; Figure 1). At 1wk,1, 3, 6mo postoperatively, ELM defects were seen in 18 eyes (95%), 15 eyes (79%), 13 eyes (68%), 9 eyes (47%),respectively (P=0.011). EZ defects, CIZ defects were observed in all the eyes during all the follow-up period. During this process, the ELM was concomitantly reconstructed with the length of EZ defects and CIZ defects shorten showed in Figure 1.The area of the RNFL defects were observed in 11 eyes (63%)at 1wk, 15 eyes (84%) at 1mo, 18 eyes (95%) at 3mo and 18 eyes (95%) at 6mo postoperatively. The IPL defects were observed earliest at 1mo with 2 eyes (11%), and then 6 eyes(32%) at 3mo, 7 eyes (37%) at 6mo postoperatively.
Poorer postoperative logMAR BCVA correlated with larger MH minimum diameter (P<0.001), larger MH basal diameter(P=0.008), longer symptom duration (P=0.002) and poorer preoperative logMAR BCVA (P=0.010). More improvement in BCVA correlated only with poorer preoperative in logMAR BCVA (P=0.002). But improvement in BCVA has no correlation with symptom duration, MH minimum diameter and basal diameter. As for the change in age, it has no correlation with both postoperative logMAR BCVA and improvement in logMAR BCVA (Table 2).
In addition, it was found that cases with continued ELM at 6mo postoperatively had smaller MH basal diameter (P=0.022)and shorten symptom duration (P=0.008). But there were not differences in age, MH minimum diameter, preoperative in logMAR BCVA, postoperative logMAR BCVA and change in logMAR BCVA (Table 3).
Table 1 Postoperative time-dependent changes in logMAR BCVA and foveal microstructure n (%)
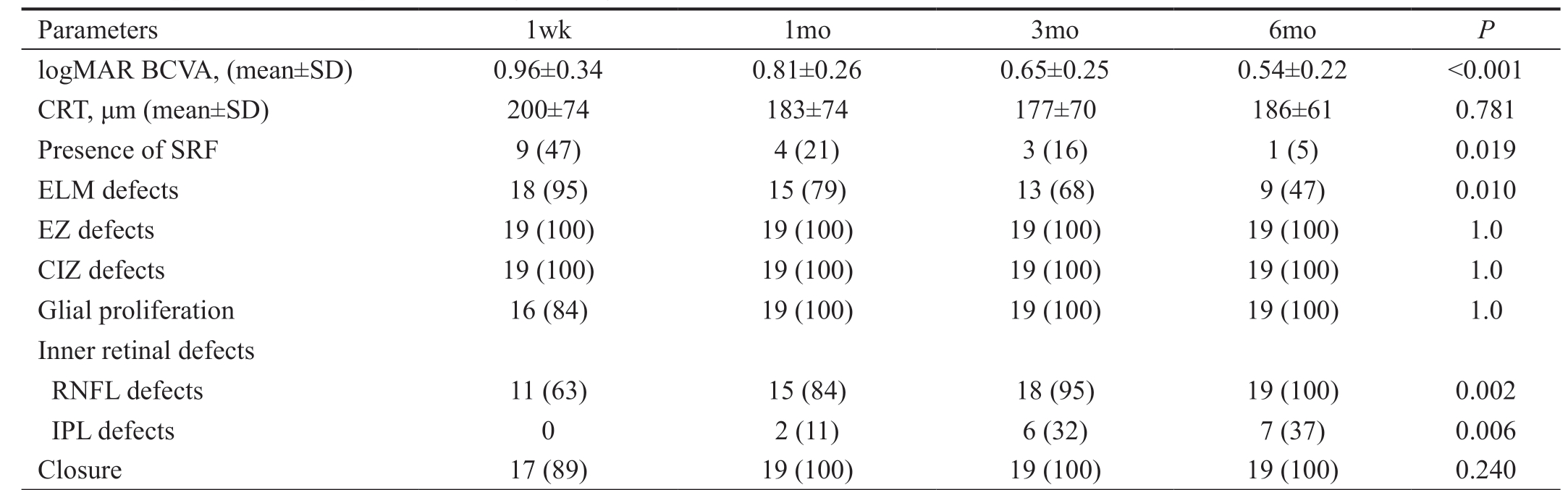
logMAR BCVA: Logarithm of the minimum angle of resolution best-corrected visual acuity; CRT: Central retinal thickness; SRF: Subretinal fluid;ELM: External limiting membrane; EZ: Ellipsoid zone; CIZ: Cone interdigitation zone; RNFL: Retinal never fiber layer; IPL: Internal plexiform layer.
Parameters 1wk 1mo 3mo 6mo P logMAR BCVA, (mean±SD) 0.96±0.34 0.81±0.26 0.65±0.25 0.54±0.22 <0.001 CRT, μm (mean±SD) 200±74 183±74 177±70 186±61 0.781 Presence of SRF 9 (47) 4 (21) 3 (16) 1 (5) 0.019 ELM defects 18 (95) 15 (79) 13 (68) 9 (47) 0.010 EZ defects 19 (100) 19 (100) 19 (100) 19 (100) 1.0 CIZ defects 19 (100) 19 (100) 19 (100) 19 (100) 1.0 Glial proliferation 16 (84) 19 (100) 19 (100) 19 (100) 1.0 Inner retinal defects RNFL defects 11 (63) 15 (84) 18 (95) 19 (100) 0.002 IPL defects 0 2 (11) 6 (32) 7 (37) 0.006 Closure 17 (89) 19 (100) 19 (100) 19 (100) 0.240
Table 2 Correlation between preoperative parameters and logMAR BCVA
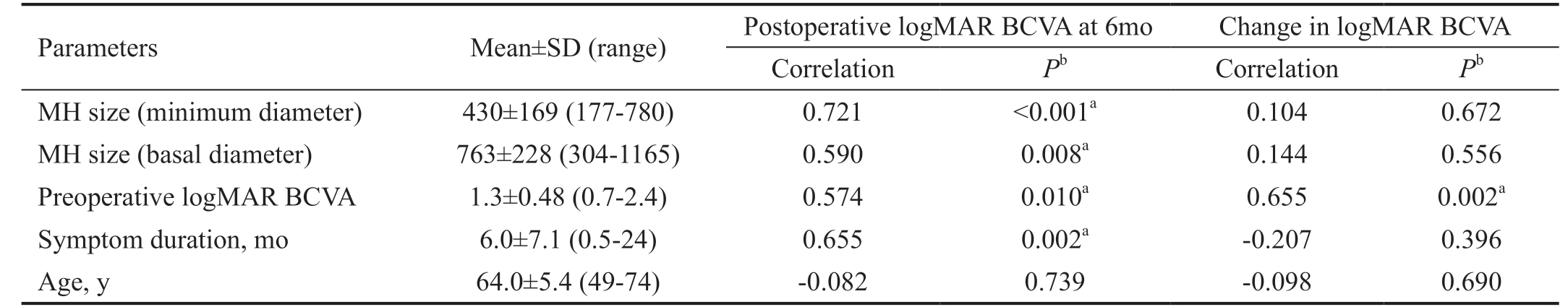
Parameters Mean±SD (range) Postoperative logMAR BCVA at 6mo Change in logMAR BCVA Correlation Pb Correlation Pb MH size (minimum diameter) 430±169 (177-780) 0.721 <0.001a 0.104 0.672 MH size (basal diameter) 763±228 (304-1165) 0.590 0.008a 0.144 0.556 Preoperative logMAR BCVA 1.3±0.48 (0.7-2.4) 0.574 0.010a 0.655 0.002a Symptom duration, mo 6.0±7.1 (0.5-24) 0.655 0.002a -0.207 0.396 Age, y 64.0±5.4 (49-74) -0.082 0.739 -0.098 0.690
logMAR BCVA: Logarithm of the minimum angle of resolution best-corrected visual acuity; MH: Macular hole. aStatistically significant correlation (P<0.05); bIndependent-samples t-test.
Table 3 BCVA (logMAR) and ELM reconstruction at 6mo after surgery mean±SD
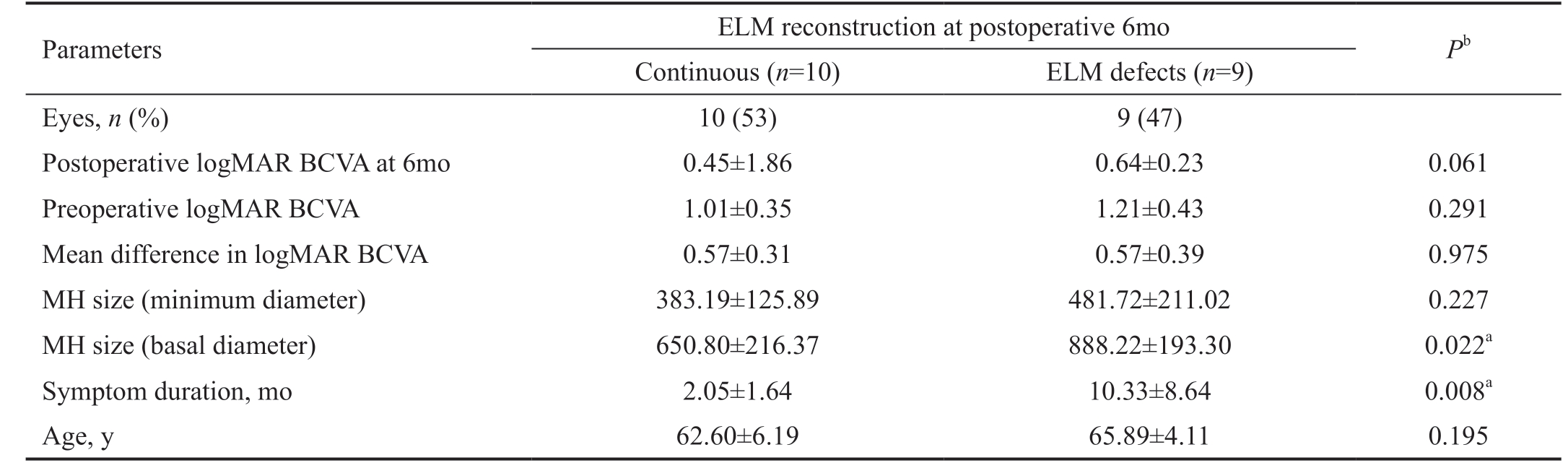
logMAR BCVA: Logarithm of the minimum angle of resolution best-corrected visual acuity; ELM: External limiting membrane; MH: Macular hole; aStatistically significant correlation (P<0.05); bIndependent-samples t-test.
Parameters ELM reconstruction at postoperative 6mo Pb Continuous (n=10) ELM defects (n=9)Eyes, n (%) 10 (53) 9 (47)Postoperative logMAR BCVA at 6mo 0.45±1.86 0.64±0.23 0.061 Preoperative logMAR BCVA 1.01±0.35 1.21±0.43 0.291 Mean difference in logMAR BCVA 0.57±0.31 0.57±0.39 0.975 MH size (minimum diameter) 383.19±125.89 481.72±211.02 0.227 MH size (basal diameter) 650.80±216.37 888.22±193.30 0.022a Symptom duration, mo 2.05±1.64 10.33±8.64 0.008a Age, y 62.60±6.19 65.89±4.11 0.195
DISCUSSION
In this study, we aimed to investigated OCT-measured microstructure characteristics in fovea post MH surgery and relevant predictive factors to visual improvement and macular microstructure features.
SRF was a common appearance after successful MH surgery[18].But the exact effects of SRF on surgical outcomes of MH were still controversial. Zambarakji et al[19] had reported that cases with SRF had bad effects on visual or IS/OS outcomes after successful MH surgery. On the contrary, Tranos et al[18]observed that the presence of SRF was not related to worse outcomes. In our study, we found that postoperative SRF absorbed with the follow-up time. It should be noted that our study observed that the main SRF disappearance period was in the first 3mo after surgery. The closure of MHs was observed on OCT within postoperative 1wk in most successful cases.In present study, CRT was 200±74 μm at postoperative 1wk.
Then it has a decreasing period and a gentle increase, and develop into 186±61 μm at postoperative 6mo. Takamura et al[20] suggested that postoperative CRT was associated with visual outcomes. But it is difficult to ensure the precise measurement to CRT considering all those values were measured manually. The reconstruction of ELM, EZ and CIZ represents the integrity of the photoreceptor layer. In our study,ELM reconstruction completed earliest compared to EZ and CIZ and the reconstruction of EZ and CIZ have not completed at postoperative 6mo but with the length of EZ defects and CIZ defects shortened (Figure 1). It suggested that macular microstructure repair was step by step and mostly more than 6mo.After MH surgery, there were some arcuate striaes observed in the field of ILM peeling, which was named dissociated optic nerve fiber layer (DONFL)[21]. The DONFL appearance was found correlate with RNFL defects and IPL defects,which maybe caused by the irreversible damage to the part of the Müller cell during the process of peeling ILM[21]. It was deemed that the appearance of those defects had no effects on visual improvement[22], but Sabry et al[23] found that concentric macular dark spots (CMDS) was correlated to RNFL defects.In present study, the RNFL defects and IPL defects were also seen and which became evidently with the follow-up time and the appearance of the IPL defects later than the RNFL defects. Meanwhile, the RNFL defects appeared slightly at the beginning, and then became deeper and wider with the followup time. Similar performance also showed in study observed by Runkle et al[24]. The rate of RNFL defects was evident and observed in all eyes postoperatively in Sabry et al’s[23] study.
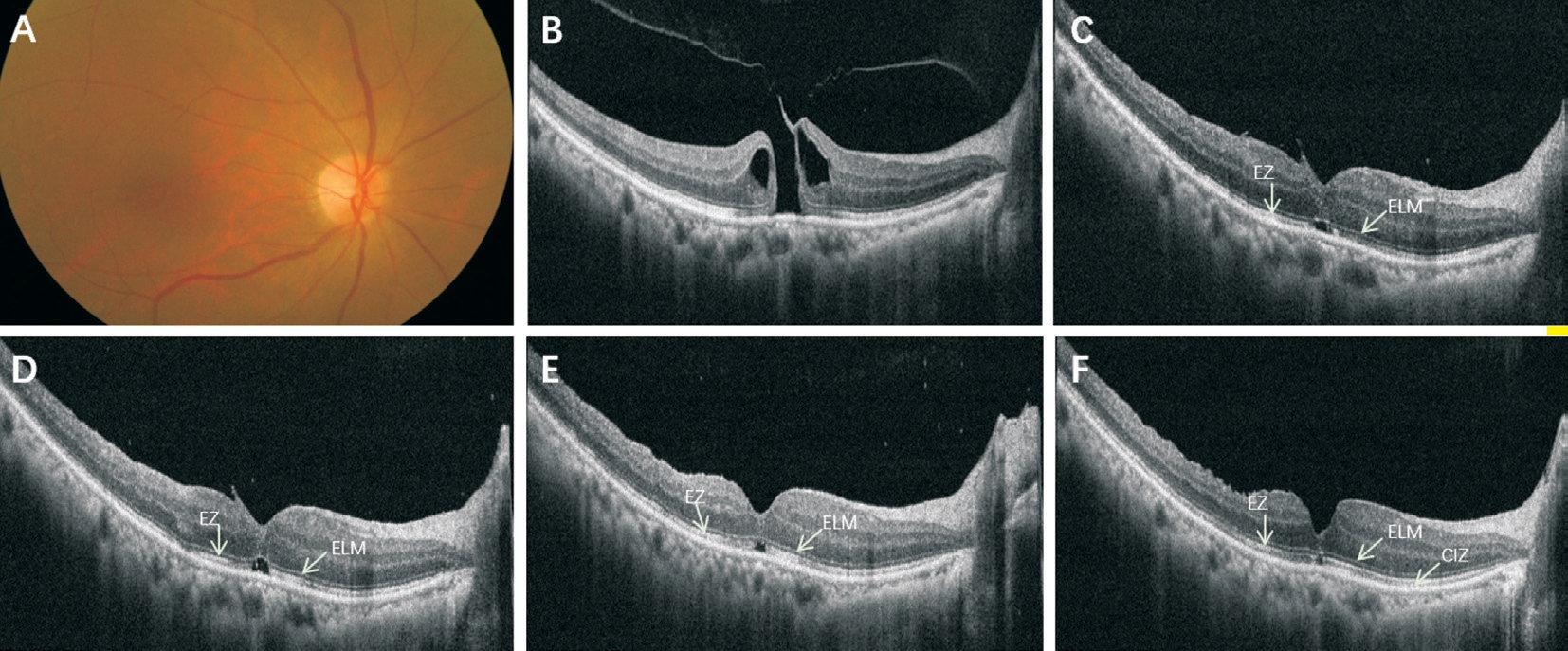
Figure 1 A 63 years old female with 1mo symptom duration A: Preoperative color fundus photo image of the MH; B: The SD-OCT showed a full-thickness stage 2 MH (398 μm). The logMAR BCVA was 0.92. C: At postoperative 1wk, the ELM appeared to be continuous with moderately reflective dots. There was a disruption of the EZ and CIZ. The SRF in the fovea looked like a cystic space was also presented. The logMAR BCVA was 0.6. D-F: At postoperative 1mo (D), 3mo (E), and 6mo (F), the morphology of the fovea improved persistently with the absorption of SRF, the shorten of CIZ and EZ defects, the reduction of glial proliferation (the hyperreflective dots in fovea). All these changes were accompanied by visual improvements to 0.6, 0.4, and 0.22 respectively.
As for the study of the predictive factors, there were still controversial. Goker et al[25] reported that duration of hole symptom, Gass stage and preoperative BCVA did not find to affect visual outcome. Kim et al[26] found that choroidal thickness and MH basal diameter were significant predictors of visual improvement, but there were not significant differences between final visual acuity and preoperative visual acuity,hole size, sex or age. Table 2 showed correlations between preoperative parameters and logMAR BCVA. In present study,it suggested that postoperative logMAR BCVA at 6mo had significantly positive correlation with MH minimum diameter(P<0.001) and symptom duration (P=0.002). Also, there were positive correlations between postoperative logMAR BCVA and preoperative logMAR BCVA (P=0.01), postoperative logMAR BCVA and basal diameter (P=0.008). Contrary to Kim et al’s[26] study, there was a positive correlation between postoperative logMAR BCVA and MH minimum diameter.In former studies, Kazmierczak et al[27] reported that IS/OS junction defects correlated with the diameter of the MH and the presence of ELM defects were the reason of inferior BCVA. In our study, we found that more improvement in BCVA correlated with poorer preoperative in logMAR BCVA(P=0.002), but have negatives results in symptom duration,MH minimum diameter and basal diameter. It suggested that MH minimum diameter and symptom duration are good predictive factors to longtime visual improvement.
Moreover, it was found that cases with continued ELM at 6mo postoperatively had smaller MH basal diameter (P=0.022)and shorten symptom duration (P=0.008; Table 3, Figure 2).
As we all known, the integrity of outer retina is key to visual outcomes. ELM restored earliest than IS/OS, that means that only after ELM completed its reconstruction, can IS/OS become continuous. As for MH basal diameter, it is the base of the disruption of hole, also the beginning of outer retina restoration. Kitao et al[14] had reported that there was significant correlation between the time until ELM reconstruction and postoperative BCVA at 3y. Thus, it suggested that basal diameter of MH maybe a crucial factor to final visual acuity.Apart from that, it was also found that cases with continued ELM at 6mo postoperatively had shorten symptom duration.It indicated that symptom duration played an important role in visual outcome. Anatomically, shorten symptom duration was benefit to the early restoration of macular microstructure,which also contributed to visual improvement.
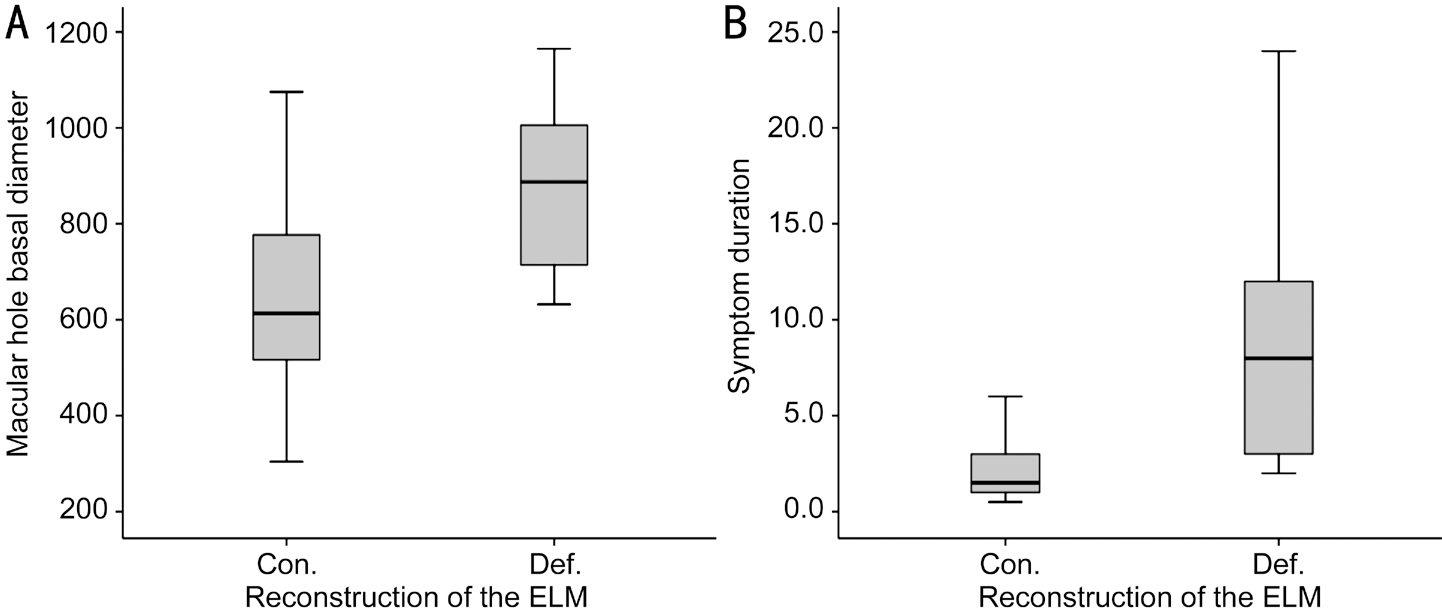
Figure 2 Boxplot diagram showed that the MH basal diameter in cases with continuous ELM (Con.) bigger than cases with ELM defects(Def.) at postoperative 6mo (A; P=0.022) and the symptom duration of cases with continuous ELM longer than cases with ELM defects at postoperative 6mo (B; P=0.008).
One of the limitations of the present study was small number of patients. Further studies are needed to confirm our results.In addition, we only applied one examination to detect the repair of MHs. It needs more examinations including microperimetry, electroretinogram or OCT angiography to has a more comprehensive exploration about the repair process and predictive factors of MHs.
In conclusion, ELM reconstruction completed earliest compared to EZ and CIZ in the process of macular microstructure repair after successful MH surgery. Preoperative BCVA, symptom duration, minimum diameter and basal diameter of MH were important predictive factors for visual improvement. The MH basal diameter and symptom duration were key factors in early reconstruction of ELM.
ACKNOWLEDGEMENTS
Conflicts of Interest: Zou JL, None; Zeng J, None.
1 Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes.Results of a pilot study. Arch Ophthalmol 1991;109(5):654-659.
2 Park DW, Sipperley JO, Sneed SR, Dugel PU, Jacobsen J. Macular hole surgery with internal-limiting membrane peeling and intravitreous air.Ophthalmology 1999;106(7):1392-1398.
3 Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes.Ophthalmology 2010;117(10):2018-2025.
4 Liggett PE, Skolik DS, Horio B, Saito Y, Alfaro V, Mieler W. Human autologous serum for the treatment of full-thickness macular holes. A preliminary study. Ophthalmology 1995;102(7):1071-1076.
5 Saito Y, Tano Y. Intraoperative adjunctive agents in vitrectomy: serum,cytokines, and glue. Semin Ophthalmol 2000;15(1):36-43.
6 Hasegawa Y, Hata Y, Mochizuki Y, Arita R, Kawahara S, Kita T,Noda Y, Ishibashi T. Equivalent tamponade by room air as compared with SF6 after macular hole surgery. Graefes Arch Clin Exp Ophthalmol 2009;247(11):1455-1459.
7 Usui H, Yasukawa T, Hirano Y, Morita H, Yoshida M, Ogura Y.Comparative study of the effects of room air and sulfur hexafluoride gas tamponade on functional and morphological recovery after macular hole surgery: a retrospective study. Ophthalmic Res 2013;50(4):227-230.
8 Chen X, Yao Y, Hao XL, Liu XC, Liu TC. A comparative study of vitrectomy combined with internal limiting membrane peeling for the treatment of idiopathic macular hole with air or C3F8 intraocular tamponade. J Ophthalmol 2018;2018:1672501.
9 Ota H, Kunikata H, Aizawa N, Nakazawa T. Surgical results of internal limiting membrane flap inversion and internal limiting membrane peeling for macular hole. PLoS One 2018;13(9):e0203789.
10 Chow DR, Chaudhary KM. Optical coherence tomography-based positioning regimen for macular hole surgery. Retina 2015;35(5):899-907.
11 Grewal DS, Reddy V, Mahmoud TH. Assessment of foveal microstructure and foveal lucencies using optical coherence tomography radial scans following macular hole surgery. Am J Ophthalmol 2015;160(5):990-999.e1.
12 Liu PP, Sun YY, Dong CY, Song D, Jiang YR, Liang JH, Yin H,Li XX, Zhao MW. A new method to predict anatomical outcome after idiopathic macular hole surgery. Graefes Arch Clin Exp Ophthalmol 2016;254(4):683-688.
13 Shpak AA, Shkvorchenko DO, Sharafetdinov IKh, Yukhanova OA.Predicting anatomical results of surgical treatment of idiopathic macular hole. Int J Ophthalmol 2016;9(2):253-257.
14 Kitao M, Wakabayashi T, Nishida K, Sakaguchi H, Nishida K.Long-term reconstruction of foveal microstructure and visual acuity after idiopathic macular hole repair: three-year follow-up study. Br J Ophthalmol 2019;103(2):238-244.
15 Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol 1988;106(5):629-639.
16 Rosa RH Jr, Glaser BM, de la Cruz Z, Green WR. Clinicopathologic correlation of an untreated macular hole and a macular hole treated by vitrectomy, transforming growth factor-beta 2, and gas tamponade. Am J Ophthalmol 1996;122(6):853-863.
17 Ko TH. Ultrahigh-resolution optical coherence tomography of surgically closed macular holes. Arch Ophthalmol 2006;124(6):827.
18 Tranos PG, Stavrakas P, Vakalis AN, Asteriadis S, Lokovitis E, Konstas AG. Persistent subretinal fluid after successful full-thickness macular hole surgery: prognostic factors, morphological features and implications on functional recovery. Adv Ther 2015;32(7):705-714.
19 Zambarakji HJ, Schlottmann P, Tanner V, Assi A, Gregor ZJ.Macular microholes: pathogenesis and natural history. Br J Ophthalmol 2005;89(2):189-193.
20 Takamura Y, Tomomatsu T, Matsumura T, Arimura S, Gozawa M,Takihara Y, Inatani M. Correlation between central retinal thickness after successful macular hole surgery and visual outcome. Jpn J Ophthalmol 2015;59(6):394-400.
21 Tadayoni R, Paques M, Massin P, Mouki-Benani S, Mikol J,Gaudric A. Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology 2001;108(12):2279-2283.
22 Demirel S, Abdullayev A, Yanık Ö, Batıoğlu F, Özmert E. Evaluation of ganglion cell-inner plexiform layer thickness after vitreoretinal surgery with internal limiting membrane peeling in cases with idiopathic macular hole. Turk J Ophthalmol 2017;47(3):138-143.
23 Sabry D, El-Kannishy A, Kamel R, Abou Samra W. Correlation between en face optical coherence tomography defects of the inner retinal layers and ganglion cell inner plexiform layer analysis after internal limiting membrane peeling for idiopathic full-thickness macular hole.Invest Ophthalmol Vis Sci 2016;57(9):OCT444-OCT450.
24 Runkle AP, Srivastava SK, Yuan A, Kaiser PK, Singh RP, Reese JL,Ehlers JP. Factors associated with development of dissociated optic nerve fiber layer appearance in the pioneer intraoperative optical coherence tomography study. Retina 2018;38(Suppl 1):S103-S109.
25 Goker YS, Koc M, Yuksel K, Yazici AT, Demir A, Gunes H, Ozpinar Y. Relationship between peeled internal limiting membrane area and anatomic outcomes following macular hole surgery: a quantitative analysis. J Ophthalmol 2016;2016:5641273.
26 Kim SH, Kim HK, Yang JY, Lee SC, Kim SS. Visual recovery after macular hole surgery and related prognostic factors. Korean J Ophthalmol 2018;32(2):140-146.
27 Kaźmierczak K, Stafiej J, Stachura J, Żuchowski P, Malukiewicz G.Long-term anatomic and functional outcomes after macular hole surgery.J Ophthalmol 2018;2018:3082194.