INTRODUCTION
I t is known that ocular refraction is statistically correlated with not only axial length but also, to a lesser extent,corneal power and lens power[1-5]. However, the association between refractive errors and ocular biometrics such as corneal power, central corneal thickness, anterior chamber depth (ACD), and lens thickness are inconclusive[6-13]. The recent study of Hashemi et al[14] for individuals aged 40-64y,showed that corneal power and axial length make the greatest contribution to spherical equivalent in high hyperopia and high myopia. Moreover, anterior segment biometric components show stronger correlation in hyperopia than in myopia.
They also reported that lower levels of refractive errors are attributed to an imbalance among the components of ocular biometrics, whereas at higher levels, ACD plays a greater role.Due to the variation and correlations of biometric components during growth, the refractive error of the eye is governed by the multifactorial condition involving corneal and lens power, ACD and vitreous chamber depth (VCD) of the eye.Ametropia develops often due to the abnormal increase in axial length. However, in some instances, axial length is not the sole component for the development of refractive error. A refractive imbalance among the individual components is the mechanism that produces refractive error, i.e. refractive error is the result of collective imbalance among ocular components.
In 2006, Lin[15] first introduced a new standard for emmetropic state governed by a relative axial length of (L-L*), rather than its absolute axial length (L), where the referenced axial length given by L*=24.0+0.36(43.1-P1) +0.23(22.3-P2) +0.5(S0-3.3)+0.35(T-4.0), with P1 and P2 are the cornea and lens power;S0 is ACD and T is the lens thickness. A large L* may be due to flat cornea or lens, or deep ACD or VCD, or thick lens(T). The commonly accepted concept of long axial length resulting myopia is only statistically true. The refractive state of a specific subject depends on its L* defined by the above new criterion. For example, a subject with L=26 mm will have about 2.7 D myopia when L*=25 mm, whereas it becomes 1.4 D of hyperopia, when L*=27 mm.
The previously published refractive regression formulas were given by the empirical analysis based on the statistic mean values of ocular components. The regression of individual biometric parameters was theoretically presented based on a refractive state theory and the rate function of ocular components[15]. Empirical regression formulas are often limited to the corneal power, ACD and VCD, whereas lens power are calculated based on the known ocular parameters.Consistent with the empirical data, we will present theoretical formulas for the correlation of spherical equivalent (SE) and ocular components are highest for SE with axial length, ACD and VCD and weakest for corneal power and lens power. Our previous model[15-16] is modified to include the nonlinear terms counting for large changing amount and effects of ametropia.Our revised regression formulas will show the asymmetric feature between hyperopia and myopia in terms of correlation of SE with axial length, ACD and VCD, where stronger correlation in hyperopia than in myopia.
MATERIALS AND METHODS
We have reported the refractive state theory[15] which related the spherical refractive error (Re) with the ocular components including corneal and lens power (P1 and P2), the ACD and VCD (S and X), lens thickness (T) and the axial length (L) as follows[15]:
where z=1-SP1/1336, with S=ACD+0.6T, being the effective ACD; P3=1336/X, with X being the effective VCD; axial length L=S+X+0.046T, with T being the lens thickness. Eq.(1)shows that the refractive error (with Re<0 for myopia and Re>0 for hyperopia) is resulted from the mismatching of the vitreous power (P3) and the summation of the corneal and lens power. The emmetropic state (E-state, with Re=0) may be defined by multiple matching values of the ocular components.
The changing rate of refractive errors (dRe) per mm (or unit)change of the ocular components (dQj) are given by taking the derivative of Re, in Eq.(1), with respect to the ocular components (Qj), with the rate functions given by mj=dRe/dQj, reported by Lin et al[15-16] as follows:

Relating the corneal focal length f=1336/P1 and X=1336/P3,we may also express m1 and m2 as follows: m1=-(P12/1336)
The above rate functions defined previously[15-16] have been modified to include the second-order terms (1-0.04dS) and (1-dX/X) counting for the nonlinear feature of the rate function when dS and/or dX>1.0 mm. Furthermore, we also included 1+0.06Re, 1+0.04Re and 1+0.01Re, counting for effects of ametropic states (with Re>0 or Re<0). We will show later that the above nonlinear regression formulas predict much better the empirical data than the conventional linear regressions which are only valid for a best linear fit to the mean data for low Re<0.5 D.Substituting dX=dL-dS in Eq.(2) such that the first two terms will be given by dL and dS1 only. In addition, the cornea and lens power will be represented by their surface radius; P1=330/r, P2=243/R, with r and R are, respectively, the anterior surface radius of the cornea and lens. dP1=-m5dr, with m5=(330/r2)(1-dr/r)(1+0.01Re); dP2=-m6dR, with m6=(230/R2)(1-dR/R)(1+0.01Re); Eq.(2) becomes:
which shows that dS has positive negative regression (with m1-m2>0), where as others have negative regressions, since m1<0,m2<0, m5<0, m6<0.
RESULTS
For typical ocular values of P1=43 D (or f=31 mm), P2=25 D,L=43, X=17.2, S=5.6 mm, r=7.8 mm, R=11.5 mm, z=0.82 mm, the rate functions are: m1=-1.32 (D/mm) (at Re=0) and(-1.62, -1.02), for Re=(+5.0, -5.0) D; m2=-2.93(at Re=0)and (-3.13, -2.73) at Re=(+5.0, -5.0) D; m6=4.73, m6=1.58;m3=-1.0, (at Re=0). These values are within 2% deviation comparing to the exact solutions of Eq.(1).
Defining the change of refractive error by a emmetropic state power (R0), dRe=Re-R0, Eq.(2) becomes: Re=R0+m2L+m12S+m3P1+m4P2, with m12=1.55(+0.02Re); R0=123.2 given by the typical values for emmetropic state: (L, S, P1, P2)=(23 mm,5.6 mm, 43 D, 25 D). The above formula may be compared to the empirical regression formula of Olsen et al[5]: Re=110-2.3L-0.89P1-0.62P2, which is comparable to our nonlinear formula when Re<0.5 D, with nonlinear terms neglected, Eq.(6)becomes: Re=123.2-2.93L-1.0P1-0.67P2+1.55dS+4.73dr-0.79dT. Another linear regression formula was reported by Zhang et al[17]: dRe=-2.3dL+-1.58dS+5.72dr-1.79dT.
The nonlinear terms of our regression formulas, 1+0.06Re,1+0.04Re and 1+0.01Re, show the asymmetric feature that axial length, ACD and VCD are more strongly correlated with refractive errors in eyes with hyperopia than in myopia,particularly for severe hyperopia, which has higher negative regression coefficient. We should note that the regression coefficients of VCD (or X) is about 2 times of ACD (or S),typical values are m2=-2.93 versus m1=-1.32 (D/mm).Eq.(2) and our regression formulas may be used to analyze and compare with the empirical regression equations reported by Hashemi et al[14] as follows.
The empirical regression coefficients (ERC) shown by Hashemi et al[14] are based on their empirical fit to a linear regression equation. In their third model, ACD+LT was correlated with SE only in eyes with moderate and severe hyperopia but not in moderate and severe myopia. Their ERC have the same trend as our theoretical values that hyperopia has higher value than myopia due to the factor of 1+0.04De.Moreover, they have the similar proportional features, i.e.regression of VCD is about 2 times stronger than ACD.
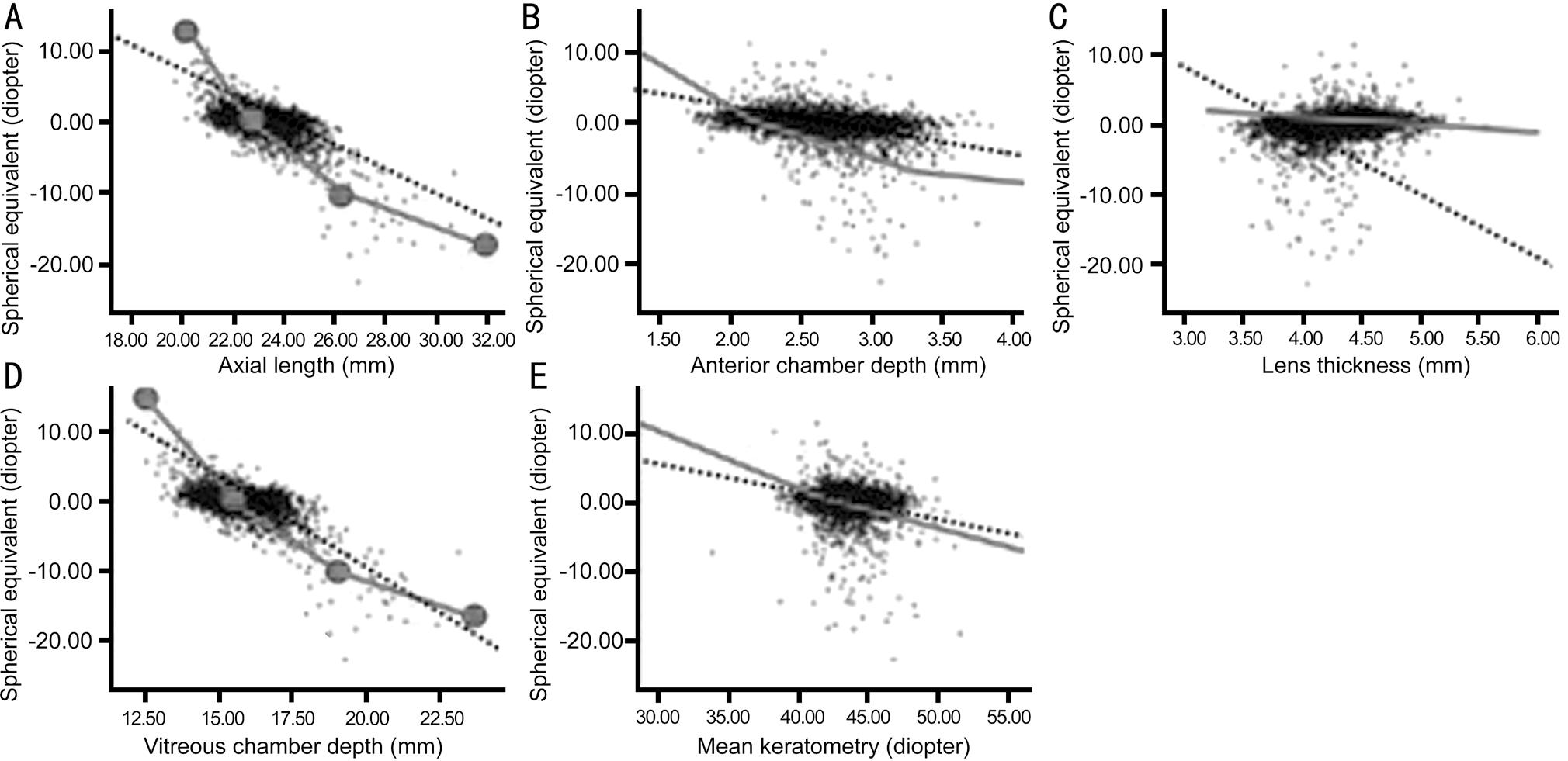
Figure 1 Comparisons of empirical fit regression curves (in black dashed) based on data of Hashemi et al[14] and the theoretical curves(in solid gray line) for various ocular components: axial length (A), anterior chamber depth (B), lens thickness (C), vitreous chamber depth (D),and mean keratometry (E).
As shown by Figure 1 of Hashemi et al[14], the Pearson correlation coefficients were highest for spherical equivalent(SE) with axial length (L) and VCD, and weakest for corneal power (CP). The coefficients for correlations between SE and L, VCD, ACD, lens thickness (LT), CP, and ACD+LT were:-0.610, -0.594, -0.301, 0.168, -0.146, and -0.173, respectively;noting that all of them have a negative correlation, except dT is positive. This feature is consistent with our regression formulas, except dS, which has positive correlation, also shown by the empirical data of Zhang et al[17]. Moreover, the strength of Pearson correlation is related to our rate functions(mj), which have the similar features as that of Hashemi et al[14].Hashemi et al[14] reported that, for eyes with age of 40 to 64y, VCD and corneal power (CP) correlated more strongly with spherical equivalent (SE) in eyes with myopia than hyperopia, while lens thickness (LT) and ACD correlated more strongly with SE in hyperopias than myopias. However, our nonlinear theory shows that AL, CP, ACD and VCD are all more strongly correlated with SE in eyes with hyperopia than myopia, particularly for severe hyperopia. Our theory based on individual biometric parameters are consistent with the partial correlation testing (PCT) of Hashemi et al[14] that VCD,LT, and CP had the strongest correlations with SE in high and moderate hyperopia (SE>4.0 D).
Hashemi et al[14] also reported that the axial length range (dL)from emmetropic to severe hyperopic cases has a relatively narrower range than that in severe myopias, from 23.13 to 26.35 mm (or dL=+3.22 mm). This feature may be quantized by our calculations as follows. For a given diopter change of±5.0 D, dL=Re/m2=+5/3.13=1.6 mm (for hyperopia) which is narrow than dL=5/2.73=1.83 mm (for myopia), as shown by our nonlinear formula m2=(1336/F2)(1+0.033Re), where m2 in hyperopia (-3.13) is smaller than myopia (-2.73).
DISCUSSION
Above analysis are for the old eyes (age of 40 to 64y), where most ocular parameters are stable, except the reduction of lens power due to the volume refractive index decrease. The lack of lens information in the study of Hashemi et al[14] may result to the deviation of their empirical data, even the partial correlation testing to our regression data based on individual biometry.
Zhu et al[18] reported that, for young eyes (age 20-40y), genes responsible for the growth of the ACD are different from those of the VCD, or the age-related impact of environmental factors may affect the posterior segment to a greater extent. Our theory shows that the growth of VCD has a refractive power change rate (-2.93 D/mm) about twice of ACD (-1.38 D/mm). The empirical data based on averaged values may be significantly different from their individual values, which have a wide range due to difference in biometric parameters. In contrast, our theoretical values based on individual biometric parameters provides more accurate prediction and match much better than that of empirical regression. As shown in Figure 1, we compare the regression curves of theoretical predicted (in solid gray line) and the empirical curves of Hashemi et al[14] (in black dashed). It should be emphasized that the theoretical regression curves based on individual biometric parameters are nonlinear and well fit the widely scattered empirical data, which have a poor fit to the linear, averaged empirical regression curves.
We note that refractive errors may be also related to the ocular components by the ratio between the axial length (L) and the anterior cornea radius (r), where L/r=3.0 related to an emmetropic eye; and L/r>3.0 for myopic eye[15,19-20]. The recent work of Li et al[21-22] showing the components of young Chinese eyes concluded that myopic eyes have lower lens power and longer anterior segment length, that partially compensate their longer axial length. These features are consistent with our theory that axial length is not the sole component for the development of refractive error. A refractive imbalance between the individual components is the mechanism that produces refractive error. Other studies have found differences between the sexes, with boys having longer axial lengths than girls at 6 to 9y and older children having more myopia and longer axial lengths than younger children[23-24]. Therefore, our next study is to extend our effective eye model to include the age-dependent lens and cornea power and their imbalance with the dynamic growth of axial length for the development of refractive error.
ACKNOWLEDGEMENTS
Foundation: Supported by an Internal Research of New Vision Inc. and Nobel Eye Institute.
Conflicts of Interest: Chang CK, None; Lin JT, the CEO of
New Vision Inc. and has financial interest; Zhang Y, None.
1 Pedrotti LS , Pedrotti F. Optics and Vision Liper Saddle River, USA:Prentice Hall,1998.
2 Atchison DA, Smith G. Optics of the Human Eye Woburn, USA:Butterworth Heinemann, 2000.
3 Resnikoff S, Pascolini D, Mariotti SP, Pokharel GP. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull World Health Organ 2008;86(1):63-70.
4 Warrier S, Wu HM, Newland HS, Muecke J, Selva D, Aung T, Casson RJ. Ocular biometry and determinants of refractive error in rural Myanmar: the Meiktila Eye Study. Br J Ophthalmol 2008;92(12):1591-1594.
5 Olsen T, Arnarsson A, Sasaki H, Sasaki K, Jonasson F. On the ocular refractive components: the Reykjavik Eye Study. Acta Ophthalmol Scand 2007;85(4):361-366.
6 Mallen EA, Gammoh Y, Al-Bdour M, Sayegh FN. Refractive error and ocular biometry in Jordanian adults. Ophthalmic Physiol Opt 2005;25(4):302-309.
7 Garner LF, Stewart AW, Kinnear RF, Frith MJ. The Nepal longitudinal study: predicting myopia from the rate of increase in vitreous chamber depth. Optom Vis Sci 2004;81(1):44-48.
8 Jiang BC, Woessner WM. Vitreous chamber elongation is responsible for myopia development in a young adult. Optom Vis Sci 1996;73(4):231-234.
9 Shufelt C, Fraser-Bell S, Mei YL, Torres M, Varma R, Los Angeles Latino Eye Study Group. Refractive error, ocular biometry, and lens opalescence in an adult population: the Los Angeles Latino Eye Study.Invest Ophthalmol Vis Sci 2005;46(12):4450-4460.
10 Yekta A, Fotouhi A, Hashemi H, et al. Relationship between refractive errors and ocular biometry components in carpet weavers. Iranian Journal of Ophthalmology 2010;22(2):45-54.
11 Fotouhi A, Hashemi H, Shariati M, et al. Cohort profile: Shahroud Eye Cohort Study. Int J Epidemiol 2013;42(5):1300-1308.
12 O’Donnell C, Hartwig A, Radhakrishnan H. Correlations between refractive error and biometric parameters in human eyes using the LenStar 900. Cont Lens Anterior Eye 2011;34(1):26-31.
13 Park SH, Park KH, Kim JM, Choi CY. Relation between axial length and ocular parameters. Ophthalmologica 2010;224(3):188-193.
14 Hashemi H, Khabazkhoob M, Emamian MH, Shariati M, Miraftab M,Yekta A, Ostadimoghaddam H, Fotouhi A. Association between refractive errors and ocular biometry in iranian adults. J Ophthalmic Vis Res 2015;10(3):214-220.
15 Lin JT. Analysis of refractive state ratios and the onset of myopia.Ophthalmic Physiol Opt 2006;26(1):97-105.
16 Chang CK, Lin JT, Zhang Y. Human eye ocular component analysis for refractive state and refractive surgery. Int J Ophthalmol 2017;10(7):1076-1080.
17 Zhang H, Jin N, Shi X, et al. Prevalence of refractive errors and ocular biometry distribution in medical university students in Tianjin, China.
The 18th International Congress of Ophthalmology and Optometry China(COOC) Shanghai, March 22-25, 2018.
18 Zhu D, Wang Y, Yang XR, Yang DY, Guo K, Guo YY, Jing XX, Pan CW. Pre- and postcycloplegic refractions in children and adolescents.PLoS One 2016;11(12):e0167628.
19 Grosvenor T, Scott R. Role of the axial length/corneal radius ratio in determining the refractive state of the eye. Optom Vis Sci 1994;71(9):573-579.20 Hong R. The relationship between optical axial length and corneal curvature radius. Yan Shi Guang Xue Za Zhi 2003;5(1):37-38.
21 Li SM, Iribarren R, Kang MT, Li H, Li SY, Liu LR, Sun YY, Meng B,Zhan SY, Rozema JJ, Wang NL. Corneal power, anterior segment length and lens power in 14-year-old Chinese children: the Anyang childhood eye study. Sci Rep 2016;6:20243.
22 Li SM, Li SY, Kang MT, Zhou YH, Li H, Liu LR, Yang XY, Wang YP, Yang Z, Zhan SY, Gopinath B, Mitchell P, Atchison DA, Wang NL.Distribution of ocular biometry in 7- and 14-year-old Chinese children.Optom Vis Sci 2015;92(5):566-572.
23 Ojaimi E, Rose KA, Morgan IG, Smith W, Martin FJ, Kifley A, Robaei D, Mitchell P. Distribution of ocular biometric parameters and refraction in a population-based study of Australian children. Invest Ophthalmol Vis Sci 2005;46(8):2748-2754.
24 Zadnik K, Manny RE, Yu JA, Mitchell GL, Cotter SA, Quiralte JC, Shipp M, Friedman NE, Kleinstein R, Walker TW, Jones LA,Moeschberger ML, Mutti DO; Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study Group. Ocular component data in schoolchildren as a function of age and gender. Optom Vis Sci 2003;80(3):226-236.