Dear Editor,
W egener’s granulomatosis (WG) is a multisystem autoimmune disease characterized by granulomata of the respiratory tract and systemic necrotizing vasculitis[1]. As the early clinical manifestations of WG are often atypical, it is difficult to diagnosis, and treatment often be delayed, WG has been traditionally resulted in a multiple organ dysfunction or death[2]. Both simultaneous eye and ear involvement as the initial symptom is rare. Therefore, it is extremely difficult to diagnosis earlier, and WG could easily be misdiagnosed in these patients. We present the case of WG with simultaneous eye and ear involvement as the initial symptom, but who was misdiagnosed before.
The principles outlined in the Declaration of Helsinki were followed. Written informed consent on publishing the clinical and laboratory data was obtained from this patient. A 45-yearold Han Chinese woman was admitted to the hospital owing to redness and pain in the left eye for 2mo. The patient also had decreased vision, combined with suppuration in the right ear, hearing loss, tinnitus, and hoarseness without significant progress. At the local municipal hospital, she was diagnosed as peripheral ulcerative keratitis in the left eye and suppurative otitis media in the right ear. The eye was applied with tobramycin dexamethasone eye drop (tobramycin 3 mg/mL,dexamethasone 1 mg/mL) and tobramycin dexamethasone oculentum (tobramycin 0.3%, dexamethasone 0.1%), while the right ear was treated with irrigation of the external auditory canal and levofloxacin ear drops (3 mg/mL). However, the symptoms showed no improvement. The past medical record of the patient showed no previous medical or drug allergy history. The patient also had no infectious diseases or a history of inherited disease. As these symptoms becoming more serious, both the patient’s mood and sleep quality declined;fortunately, her appetite was unaffected, and bowel and bladder function were normal. Ophthalmologic examination showed acuity of 1.0 and 0.1 in the right and left eye, respectively;there was mixed hyperemia in the left eye, especially in the inferotemporal region; marginal corneal ulcers were present at the 1-7 o’clock position, and had a trough-like shape; there was deeper involvement at the 4-5 o’clock positions, with edema of the surrounding cornea, and dark red scleral tissue below the conjunctiva surface (Figure 1). The anterior chambers was clear,the pupils were round and responsive to light, with a diameter of 3 mm; the lenses was transparent, and there was no obvious abnormality in the posterior chamber, no abnormalities were found in fundus examination. There was no other significant abnormality in the right eye. Intraocular pressures were 12 mm Hg in the right eye and 14 mm Hg in the left. Bacterial and fungal cultures revealed no microbial growth in eye secretions.What’s more, inflammatory cells without obvious fungal hyphae were visible on corneal confocal microscopy. Ear examination revealed significant congestion and swelling in the right external auditory canal and eardrum, with a small amount of purulent secretion. The right ear had decreased acuity combined with tinnitus. Blood test completed after admission showed no obvious abnormalities in blood, urine, and liver and kidney function; chest and brain computed tomography yielded unremarkable findings. Erythrocyte sedimentation rate was 45 mm/h; C-reactive protein levels, 48.1 mg/L;antinuclear antibody titer, positive at 1:100. The CD3 count was 648 cells/µL; CD4 count, 336 cells/µL; CD8 count, within the physiological range. The toxoplasma IgG was 2.36 AU/L;rubella virus IgG, 15.34 AU/L; cytomegalovirus IgG, 10.37 AU/L; herpes simplex virus IgG, 24.83 AU/L. Autoimmune disease was considered on the basis of these findings. Primary diagnosis is a left peripheral ulcerative keratitis and right otitis media, but we don’t know the cause of peripheral ulcerative keratitis. After admission, the eye was treated with tacrolimus eyedrops (1 mg/mL) and tobramycin dexamethasone eye drop (tobramycin 3 mg/mL, dexamethasone 1 mg/mL), the ear was lavaged daily, levofloxacin ear drops (3 mg/mL)were administered, and systemic antibiotic treatment was also performed. However, symptoms were not relieved, hoarseness was aggravated, and headaches worsened. Inability to close the right eyelid developed 1wk later, and the left side of the mouth was angled (Figure 2); we found no specific abnormalities in head CT and MRI (Figure 3); the diagnosis was right facial nerve palsy. The Department of Otolaryngology recommends fibrous laryngoscopy, fiber laryngoscopy revealed a nodular neoplasm in the vocal cords and subglottic region (Figure 4).Further examination revealed anti-proteinase 3 (anti-PR3) and antineutrophil cytoplasmic antibody-C (c-ANCA) antibodies were detected in serum. Biopsy was performed during fiberoptic bronchoscopy; no cancer cells were observed, but chronic inflammation with focal necrosis was found (Figure 5).
After comprehensive analysis of clinical symptoms and results the diagnosis was considered to be WG after consultation in Rheumatology Department. Thus, intravenous methylprednisolone was administered twice at a dose of 40 mg,cyclophosphamide, at 150 mg/d. The patient received supplemental nerve protection and drugs to improve the microcirculation. Symptoms of eye and ear were significantly improved, and the headache also remitted quickly. After 1wk,steroids were changed to oral prednisolone at 75 mg/d. Visual acuity in the left eye improved to 0.2, temporal congestion was relieved, the sclera was reddish-brown, most of the peripheral corneal ulcers were healed, and ulcers in the 4-5 o’clock positions were faded and had a crack-like appearance(Figure 6). There was no purulent secretion, and tinnitus was improved. At 1-month follow-up, eye congestion has alleviated, ulcers were almost healed, brownish pigment could be seen through the sclera (Figure 7), tinnitus decreased, and hoarseness improved, but facial nerve palsy had not recovered.
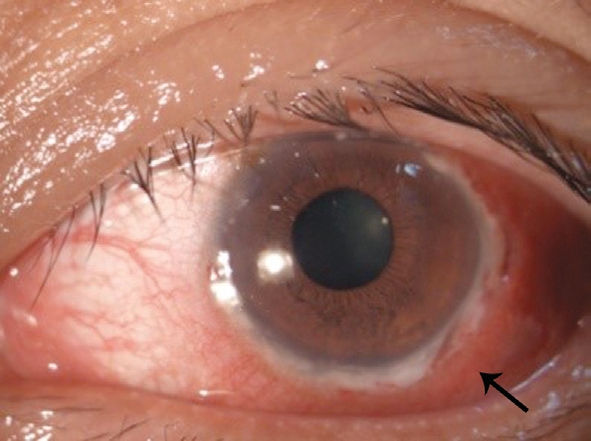
Figure 1 Before treatment, circular ulcer of the corneal limbus in the 1-7 o’clock position.
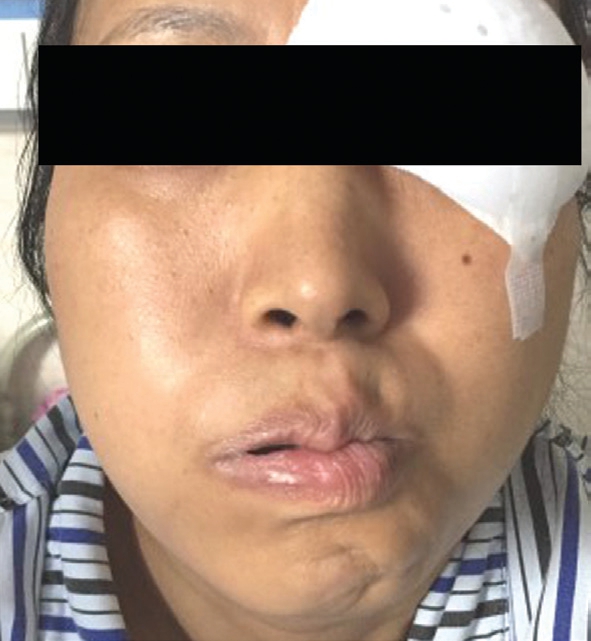
Figure 2 One week after admission, facial nerve palsy occurred on the right, the left mouth was skewed, the right nasolabial fold was blunted, and whistling was disabled.
WG is a rare, chronic multisystem disease, with an annual incidence of ten cases per million population[3]. The average age of diagnosis is usually between 20y and 40y, and men are more prone to this disease than women (1.5:1.0)[4]. Typical WG mainly involves the upper and lower respiratory tracts and kidney, while limited WG is confined to an anatomical site without involving multiple systems[5]. Most patients have limited WG that is initially diagnosed in the Departments of Ophthalmology and Otolaryngology. Permanent loss of vision is due to delayed diagnosis and treatment.Furthermore, as lethal WG can decrease the survival rate,it is of important clinical significance to understand the early ocular manifestations of WG. The manifestations of WG in the eye are limbal infiltration in one or both eyes,which gradually develops into an ulcer, and spreads to the central cornea, resulting in perforation in the end. The lesion develops a trough-like shape, similar to that in Mooren’s corneal ulcer. Scleritis is also a common ocular sign of WG;necrotizing granuloma can also be considered a characteristic of WG sometimes[6]. However, due to the severe conjunctiva hyperemia of this patient, the scleral necrosis lesion under the sclera was concealed, so necrotizing sclera with necrotizing sclera was not detected in time. WG of the eyes can present as keratitis, scleritis, uveitis, retinopathy, and orbital involvement.
However, at different times of the disease, the symptoms of not all organs will appear at the same time. Only about 15 percent of the patients are affected by the eyes, and about 25 percent of the patients are first manifested by ear lesions[7]. Subglottic neoplasms and stenosis are the main manifestations of WG in the larynx and trachea, with an incidence of 16%-23%[8], and can be accompanied by lesions in other areas. These can also be the initial or only symptoms, and 2% of patients present with subglottic stenosis as the initial symptom[9]. The incidence of WG combined with unilateral or bilateral facial nerve palsy is 8%-10%[10]. The case with simultaneous involvement of the eyes and ears may also present with vocal cord, subglottic neoplasms and facial nerve paralysis is rare. Although the patient was initially complicated with ear symptoms, we did not consider the diagnosis of WG in the early phase.
It is the gradual appearance of vocal cord and facial nerve abnormalities that is considered systemic disease. Usually, the disease can be diagnosed when any of the organs show typical WG findings, with typical histological changes, and positive c-ANCA. Pathological diagnosis is essential for WG. However,for subglottic neoplasms, tissue biopsy cannot play a role in diagnosis. Biopsy specimens often show chronic, nonspecific inflammation, which rarely aids in diagnosis. The positive diagnostic rate is only 5.0%-15.4%[11]. The pathological diagnosis is more difficult when a subglottic neoplasm is present without other lesions; thus, laboratory testing is also necessary. c-ANCA has greater value for the early diagnosis of WG, and the sensitivity and specificity when combined with anti-PR3 testing are more than 90% and 98%[12], respectively.Thereafter, c-ANCA combined with anti-PR3 can be used as the main indicator for the diagnosis and monitoring of WG. Increase of serum c-ANCA titer suggests that the disease is active or relapsed, while an obvious decrease or conversion to a negative titer indicates that the condition has improved or stabilized. This case also suggests that local glucocorticoid therapy alone cannot control the eye condition.The main treatment for WG is glucocorticoids combined with immunosuppressive agents. The prognosis of untreated WG is very poor, and 90% of patients may die within 2y, usually of respiratory or renal failure. Booth et al[13] reported that the 5-year survival rate was 76%.
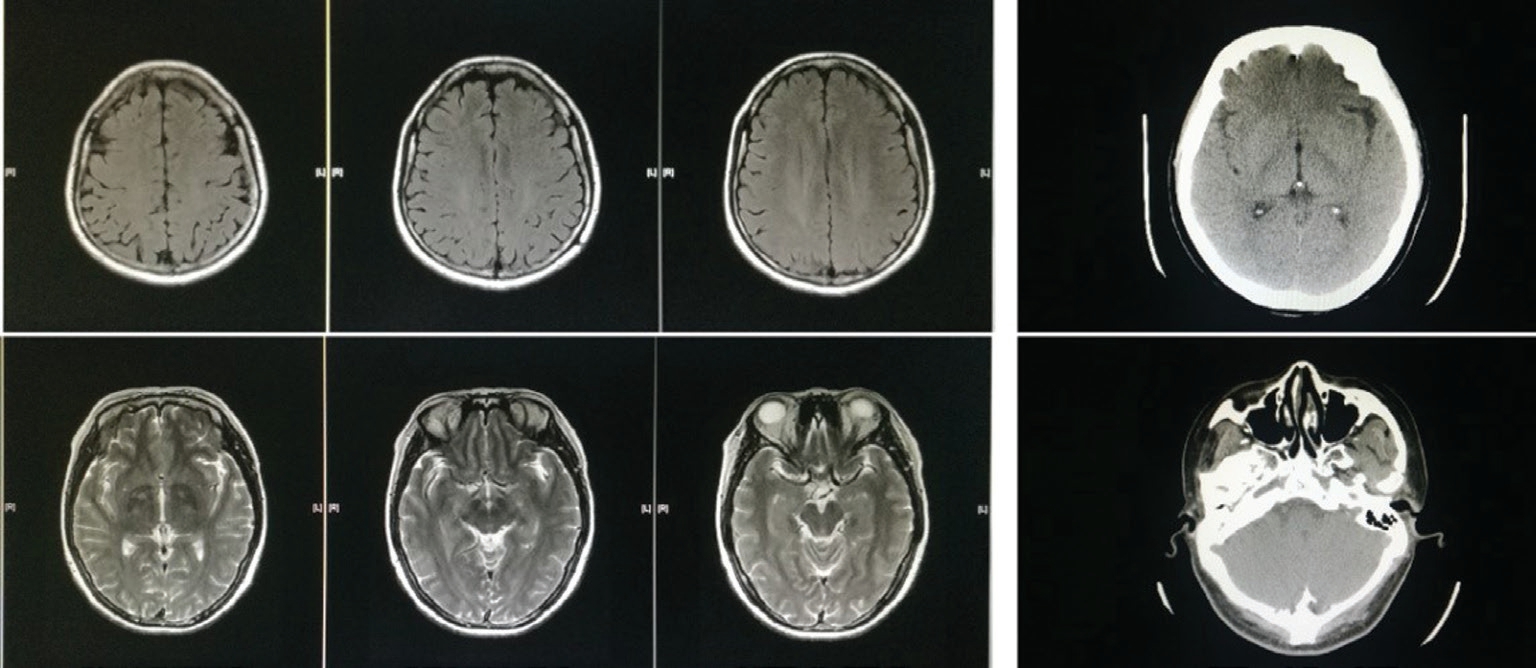
Figure 3 No abnormalities were found in head CT and MRI.
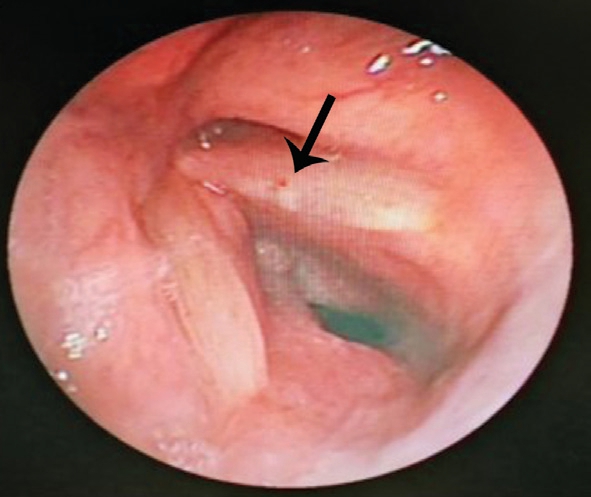
Figure 4 Nodular-like neoplasm in the left vocal cord.
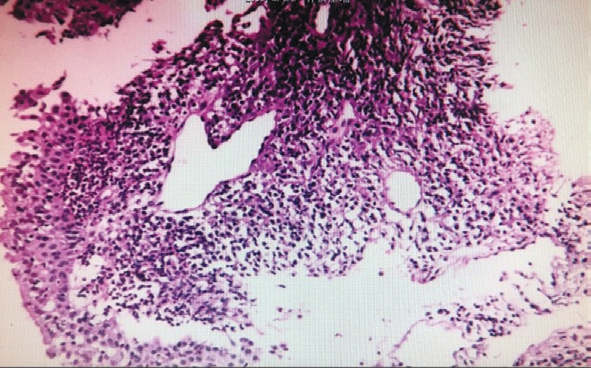
Figure 5 Biopsy under bronchoscopy showed no cancer cells, and chronic inflammation with focal necrosis was found in bronchial mucosal tissue.
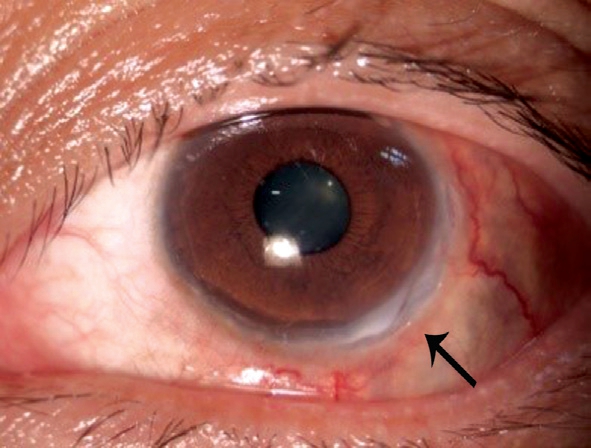
Figure 6 One week after systemic medication, mixed hyperemia had almost disappeared, and corneal ulcers at the 4-5 o’clock position had a crack-like appearance.
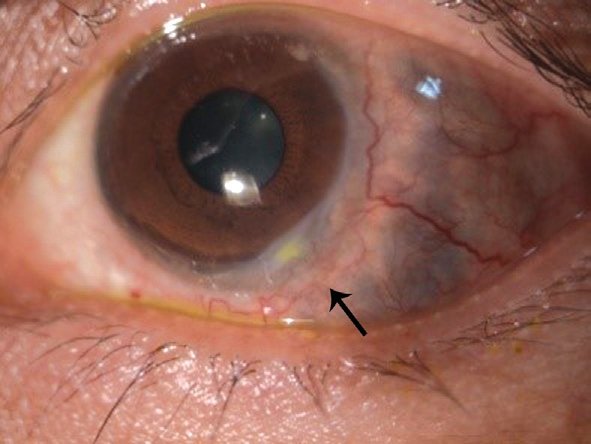
Figure 7 One month after treatment, brownish pigment can be seen through the sclera.
WG is a lethal disease. Early diagnosis and treatment are essential to prevent deterioration of the disease, and can be salvaging. The possibility of WG should be considered in patients who develop refractory peripheral ulcerative keratitis with scleral necrosis, intractable otitis media, progressive sensorineural hearing loss, vocal cord and subglottic neoplasms, and facial nerve palsy.
ACKNOWLEDGEMENTS
Conflicts of Interest: Liang J, None; Fan W, None; Yuan RD, None.
1 Tarabishy AB, Schulte M, Papaliodis GN, Hoffman GS. Wegener’s granulomatosis: clinical manifestations, differential diagnosis, and management of ocular and systemic disease. Surv Ophthalmol 2010;55(5):429-444.
2 Littlejohn GO, Ryan PJ, Holdsworth SR. Wegener’s granulomatosis:clinical features and outcome in seventeen patients. Aust N Z J Med 1985;15(2):241-245.
3 Khan AM, Elahi F, Hashmi SR, Mahida KH, Ingrams DR. Wegener’s granulomatosis: a rare, chronic and multisystem disease. Surgeon 2006;4(1):45-52.
4 Rezende CEB, Rodrigues REC, Yoshimura R, Uvo IP, Rapoport PB.Granulomatose de Wegener: relato de caso. Rev Bras Otorrinolaringol 2003;69(2):261-265.
5 Takwoingi YM, Dempster JH. Wegener’s granulomatosis: an analysis of 33 patients seen over a 10-year period. Clin Otolaryngol Allied Sci 2003;28(3):187-194.
6 Gu JJ, Zhou S, Ding RX, Aizezi W, Jiang AX, Chen JQ. Necrotizing scleritis and peripheral ulcerative keratitis associated with Wegener’s granulomatosis. Ophthalmol Ther 2013;2(2):99-111.
7 Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, Rottem M, Fauci AS. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;116(6):488-498.
8 Gluth MB, Shinners PA, Kasperbauer JL. Subglottic stenosis associated with Wegener’s granulomatosis. Laryngoscope 2003;113(8):1304-1307.
9 Herridge MS, Pearson FG, Downey GP. Subglottic stenosis complicating Wegener’s granulomatosis: surgical repair as a viable treatment option. J Thorac Cardiovasc Surg 1996;111(5):961-966.
10 Takagi D, Nakamaru Y, Maguchi S, Furuta Y, Fukuda S. Otologic manifestations of Wegener’s granulomatosis. Laryngoscope 2002;112(9):1684-1690.
11 Gluth MB, Shinners PA, Kasperbauer JL. Subglottic stenosis associated with Wegener’s granulomatosis. Laryngoscope 2003;113(8):1304-1307.
12 Gubbels SP, Barkhuizen A, Hwang PH. Head and neck manifestations of Wegener’s granulomatosis. Otolaryngol Clin North Am 2003;36(4):685-705.
13 Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH,Plaisance M, Pusey CD, Jayne DR; Pan-Thames Renal Research Group.Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003;41(4):776-784.