
Citation: Han YF, Liu Z, Wang B, Zhu W, Li JZ, Qi YQ, Li XJ, Xu YY,
Dou XX, Mu GY. Semaphorin 7a
participants in pterygium by regulating vascular endothelial growth factor.
Int J Ophthalmol 2019;12(6):892-897
·Basic
Research·
Semaphorin 7a
participants in pterygium by regulating vascular endothelial growth factor
Yun-Fei Han1,2, Zhen
Liu3, Bang Wang4, Wei Zhu5, Jing-Zhen Li2,
Yue-Qin Qi2, Xiao-Jing Li2, Yan-Yun Xu1,
Xiao-Xiao Dou6, Guo-Ying Mu1
1Department
of Ophthalmology, Shandong Provincial Hospital Affiliated to Shandong
University, Jinan 250021, Shandong Province, China
2Aier Eye
Hospital Group, Hubin Aier Eye Hospital, Binzhou 256600, Shandong Province,
China
3Department
of Ophthalmology, the Second People’s Hospital of Liaocheng, Linqing 252600,
Shandong Province, China
4Neonatal
Intensive Care Unit, Binzhou Medical University Hospital, Binzhou 256600,
Shandong Province, China
5Department
of Ophthalmology, Jinan Central Hospital Affiliated to Shandong University,
Jinan 250013, Shandong Province, China
6Department
of Cardiovascular Medicine, the Second Affiliated Hospital of Zhejiang
University College of Medicine, Hangzhou 310009, Zhejiang Province, China
Correspondence to: Guo-Ying Mu. Department of Ophthalmology, Shandong Provincial Hospital
Affiliated to Shandong University, No.324, Jing 5 Wei 7 Road, Huaiyin District,
Jinan 250021, Shandong Province, China. mgyeyes@163.com
Received: 2018-10-23
Accepted: 2019-01-03
Abstract
AIM: To investigate the relationship
between semaphorin 7a
expression and cell proliferation and migration in pterygium fibroblasts.
METHODS: Twenty-six patients with
surgically diagnosed pterygium were enrolled, including 15 cases of primary
pterygium and 11 cases of recurrent pterygium. In addition, 12 cases of normal
conjunctival tissue were collected. The expression of semaphorin 7a in normal conjunctival tissue, primary
pterygium and recurrent pterygium was detected by real-time polymerase chain
reaction. Recurrent pterygium fibroblasts were isolated and cultured, and the
expression of semaphorin 7a was
silenced by small interfering RNA (siRNA) interference technique. Furthermore,
the effects of si-semaphorin 7a
interference on the mRNA and protein levels of β1-integrin, vascular endothelial
growth factor A (VEGFA) and vascular endothelial growth factor receptor
(VEGFR), and on fibroblast proliferation were analyzed. Transwell assay was
used to detect the effect of semaphorin 7a
interference on fibroblast migration.
RESULTS: Semaphorin 7a was highly expressed in the primary
pterygium and recurrent pterygium samples than that of the normal conjunctival
tissue. Compared with the primary pterygium, the expression of semaphoring 7a in the recurrent pterygium samples was
significantly increased (P<0.05). The mRNA and protein expression
levels of β1-integrin,
VEGFA and VEGFR were decreased after si-semaphorin 7a transfection, and as well as the cell proliferation
and migration.
CONCLUSION: Semaphorin 7a might play important roles in the pathogenesis
of pterygium by affecting the expression of β1-integrin, VEGFA and VEGFR.
KEYWORDS: semaphorin 7a; pterygium; β1-integrin; vascular
endothelial growth factor; fibroblast
DOI:10.18240/ijo.2019.06.02
Outline
INTRODUCTION.. 3
SUBJECTS
AND METHODS. 4
RESULTS. 7
DISCUSSION.. 10
ACKNOWLEDGEMENTS. 12
REFERENCES. 12
INTRODUCTION
Pterygium with a “pterygium belt” is characterized by a conjunctival
disease, in which the vascular tissue of the conjunctival fibrosis is invaded
by the cornea[1]. It is a common and
frequently-occurring disease in ophthalmology. The incidence of pterygium is
reported to be between 0.3% and 37.5% in the world[2].
The cause of pterygium is generally considered to be a chronic inflammatory
disease caused by external stimuli. It has been reported that the prevalence in
people over 50 years old in rural areas of southern China is over 30%[3]. The pterygium affects not only the facial appearance,
but also the pterygium tissue may grow into the cornea to lead to corneal
astigmatism and vision loss. At present, surgical resection is the main method
for the treatment of pterygium in the clinic. However, the recurrence rate is
relatively high, up to 20%-40%[4]. Once the
pterygium relapses, it grows rapidly, and the treatment is difficult. Moreover,
recurrent pterygium can also cause serious complications such as limited eye
movement. Therefore, elucidating the molecular mechanism of recurrent
pterygium, and identifying the effective target genes is important for the
treatment of pterygium.
Previous studies suggested that neovascularization occurs in pterygium[5, 6]. The semaphorins protein family
containing a large number of transmembrane proteins and secretory proteins are
widely expressed in immune, cardiovascular, and respiratory systems, and play
important roles in vertebrate nerves and immune systems[7].
Semaphorins family proteins can be divided into 8 subfamilies based on sequence
similarity and structural features[8, 9].
Semaphorin 7a, also
known as CD108, is the only member of the seventh subfamily of the semaphorins
family[10]. Studies have shown that semaphorin 7a induces potent stimuli of monocytes by
proinflammatory cytokines[11], and it regulates T
cell immune responses via mediating T cell proliferation[12]. In addition, semaphorin 7a plays an important role in the migration of
osteoblasts and osteoclasts during bone remodeling, and in tumor angiogenesis[13, 14]. However, there has been no
related study of semaphorin 7a
in pterygium.
Previous studies have showed both hyperplasia and degeneration in the
epithelial layer and the superficial layer of pterygium epithelium, accompanied
by neovascularization and inflammatory cell infiltration[15].
Vascular endothelial growth factor (VEGF) and β1-integrin are important factors
involved in angiogenesis[16]. Recently, Boudria et
al[16] proposed that β1-integrin is required
for the invasive functions of vascular endothelial growth factor A (VEGFA), and
an invasive VEGFR/β1-integrin loop is required for proliferation and
invasiveness of cancers by VEGFA. In this study, the mRNA and protein
expression levels of semaphorin 7a
in primary pterygium and recurrent pterygium were examined. Primary culture of
fibroblasts from recurrent pterygium was performed; the expression of
semaphorin 7a was inhibited
by small interfering RNA (siRNA); and the expression levels of VEGFA, and
vascular endothelial growth factor receptor (VEGFR), and β1-integrin effected
by si-semaphorin 7a was
investigated. In addition, the proliferation, infiltration and migration of
fibroblasts were further studied. The results may indicate the role of
semaphorin 7a in
pterygium recurrence.
SUBJECTS AND METHODS
Ethical Approval The study protocol was approved by the
Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong
University and was conducted in accordance with the Declaration of Helsinki.
Patients were voluntarily signed informed consent document prior to entry into
the study.
Clinical Participants Twenty-six patients diagnosed with
pterygium and removed surgically in our hospital from March 2017 to February
2018 were enrolled. There were 15 cases of primary pterygium, including 7
females and 8 males with an average age of 45y (37-59y), and 11 cases of
recurrent pterygium including 6 males and 5 females with an average age of 43y
(39-56y). The pterygium head extending at least 2
mm into the cornea. The control group was conjunctival tissue
of conjunctival or post-traumatic eyeball removed from 12 accidental patients,
including 7 males and 5 females, with an average age of 49y (41-65y). All
patients had no other corneal or conjunctival disease, and all cases had not
received medical treatment before surgery.
mRNA Level of Semaphorin 7a
by Real-time Quantitative Polymerase Chain Reaction Total RNA from clinical samples was
extracted by TRIzol reagent (Invitrogen, USA), and determined for RNA purity
and concentration by OD260/OD280 ratio on spectrophotometer. RNA was reverse
transcribed into cDNA using the PrimeScript RT Master MIX (Perfect Real Time)
kit (TaKaRa) according to the manufacturer’s instructions. Fluorescence
real-time quantitative polymerase chain reaction (qPCR) was performed according
to the instructions of the SYBR Premix Ex Taq TM II (Perfect Real Time; Sigma,
USA). The reaction procedure was as follows: pre-reaction at 50℃ for 2min, 95℃ for 5min, denaturation at 95℃ for 15s, annealing at 60℃ for 60s, a total of 40
cycles; melting curve program was: 95℃,
15s, 60℃,
60s and 95℃,
15s. The real-time PCR primers are shown in Table 1. Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was the internal reference, and the relative expression
of each gene was calculated by 2-△△CT.
Protein Level of Semaphorin 7a
by Western Blot The pterygium samples were lysed,
and detected for semaphorin 7a
level by primary anti-semaphorin 7a
(1:1000, Cat. No.MA1-19203, ThermoFisher, USA) and secondary antibody IgG-HRP
(1:1000, sc-2392, BD, USA).
Isolation and Culture of Recurrent Pterygium Fibroblasts The pterygium tissue was removed
from the excised tissue, digested by collagenase at 37℃ incubator for 1h. The cells were
filtered, collected, resuspended in medium, and subcultured. The marker of
fibroblasts, vimentin, was verified by immunofluorescence staining using
antibody specific for vimentin (1:250, ab92547, Abcam, USA). Fibroblasts at
passages 2-5 were selected for subsequent experiments.
RNAi of Semaphorin 7a The semaphorin 7a gene sequence was searched from the NCBI
website, and the target sequence was designed using siRNA design software. A
total of three target sequences were designed: siRNA1, sense: 5’-GGACAAUCCTGACAAGAAU-3’; anti-sense: 5’-AUUCUUGUCAGGAUUGUCC-3’;
siRNA2, sense: 5’-GGGCAUGGGUUCUUGGAGA-3’; anti-sense: 5’-UCUCCAAGAACCCAUGCCC-3’;
siRNA3, sense: 5’-CUAAAUACCACUACCAGAA-3’; anti-sense: 5’-UUCUGGUAGUGGUAUUUAG-3’;
control sequence, sense: 5’-GUUCUCCGAACGUGUCACG-3’; anti-sense: 5’-CGUGACACGUUCGGAGAAC-3’.
The oligonucleotide sequences were synthesized and ligated to the vector. The
ligated DNA was transformed into E. coli strain DH5α. Positive clones were
picked and plasmids were extracted.
The pterygium fibroblasts (5×105 cells/well) were seeded in
6-well plates until the cell fusion degree was 70%-80%. si-semaphorin 7a and negative control si-negative were
transfected into fibroblasts according to the instructions of Lipofectamine
2000 Transfection Reagent (Invitrogen, USA), and the transfection efficiency
was detected by qPCR.
Effect of Semaphorin 7a
Interference on mRNA Expression of β1-integrin, VEGFA and VEGFR by Real-time
PCR After 24h of siRNA transfection,
fibroblasts were collected. The expression of β1-integrin, VEGFA and VEGFR in
each group was detected by real-time PCR. The method was mentioned above. The
primer sequences are shown in Table 1.
Table 1 Primers of semaphorin 7a,
β1-integrin, VEGFA, VEGFR and GAPDH
|
Gene
|
Upstream primer
|
Downstream primer
|
|
Semaphorin 7a
|
5’-TCATCAAAGCCACCATCG-3’
|
5’-AGCTCACATACAGCTTCCTCC-3’
|
|
β1-integrin
|
5’-CAAAGGAACAGCAGAGAAGC-3’
|
5’-GTGGAAAACACCAGCAGC-3’
|
|
VEGFA
|
5’-AGGGCAGAATCATCACGAAGT-3’
|
5’-AGGGTCTCGATTGGATGGCA-3’
|
|
VEGFR
|
5’-GGCCCAATAATCAGAGTGGCA-3’
|
5’-CCAGTGTCATTTCCGATCACTTT-3’
|
|
GAPDH
|
5’-AAATCCCATCACCATCTTCCAG-3’
|
5’-GAGTCCTTCCACGATACCAAAGTTG-3’
|
VEGFA: Vascular endothelial growth factor A; VEGFR: Vascular endothelial
growth factor receptor; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Effect of Semaphorin 7a
Interference on Protein Expression of β1-integrin, VEGFA and VEGFR by Western
Blot Analysis After 24h of siRNA transfection,
fibroblasts were collected, and the protein levels of β1-integrin, VEGFA and
VEGFR in each group was detected by Western blot. The antibodies were
anti-β1-integrin (1:2000, ab179471, Abcam, USA), anti-VEGFA (1:200, ab1316,
Abcam), anti-VEGFR (1:500, Ab11939, Abcam).
MTT Assay for Cell Proliferation
The cells
were collected at 24, 48 and 72h after siRNA transfection, and 20 μL of MTT (5
mg/mL) was added to each well (96-well culture plate), and incubation was
continued at 37℃,
5% CO2 for 4h. The optical density (OD) value at 490 nm was
measured.
Cell Migration Test The effect of semaphorin 7a interference on the migration ability of
fibroblasts was examined using Transwell assay as described elsewhere. The number
of cell migration was quantified by randomly counting five independent fields
using microscope.
Statistical Analysis Statistical analysis was performed
using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Each experiment was repeated
three times. The t-test was used for comparing the two groups, and the
one-way analysis of variance (ANOVA) was used for comparing the multiple
groups. A P<0.05 was considered to be a significant difference.
RESULTS
Expression Level of Semaphorin 7a
Increased in Pterygium Samples Real-time PCR results showed that
the expression of semaphorin 7a
in the primary pterygium and recurrent pterygium samples was significantly
higher than that of the normal conjunctival tissue (all P<0.05).
Compared with the primary pterygium sample, the expression of semaphorin 7a was significantly increased in the
recurrent pterygium samples (P<0.05, Figure 1A). Western blot results showed that the protein band
of primary pterygium was significantly thicker than that of normal conjunctival
tissue, and the protein band of recurrent pterygium was significantly thicker
than that of primary pterygium (Figure 1B).
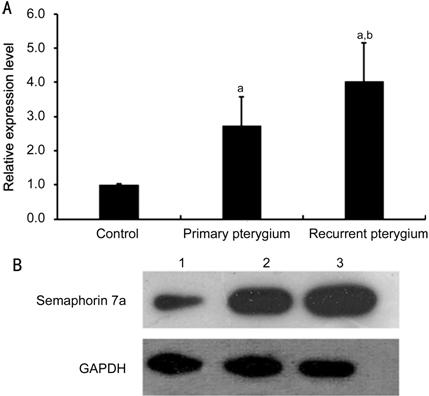
Figure 1 Real-time PCR (A) and Western blot (B) analysis of semaphorin 7a level in normal conjunctival tissue,
primary pterygium and recurrent pterygium aP<0.01 compared
with the control; bP<0.01 compared with primary pterygium.
Lane 1: Normal conjunctival tissue; Lane 2: Primary pterygium; Lane 3:
Recurrent pterygium.
mRNA Level of Semaphorin 7a
Decreased After si-semaphorin 7a
Transfection Fibroblasts of recurrent pterygium
were isolated. Vimentin is an essential marker of fibroblasts. To validate the
successful of isolation, vimentin was examined by immunofluorescence microscopy
(Figure 2). Real-time PCR results showed that the mRNA level of semaphorin 7a in fibroblasts was significantly decreased
after si-semaphorin 7a
transfection with an siRNA entrapment efficiency of 70%-80% (Figure 3A). Then siRNA1 was selected for further
experiments.
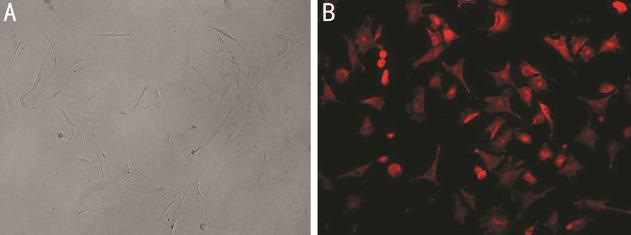
Figure 2 Isolation and characterization of fibroblasts A: Morphology of fibroblasts; B:
Immunofluorescence staining of vimentin.
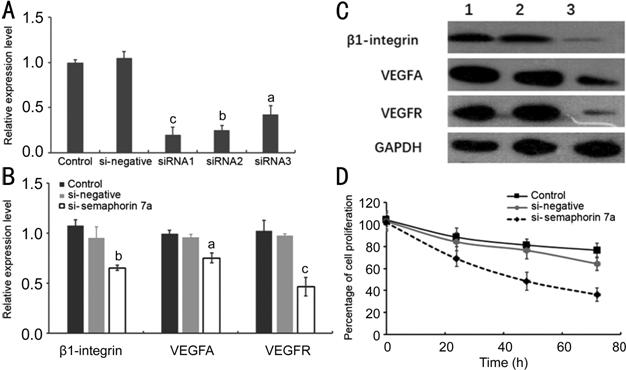
Figure 3 Effect of semaphorin 7a
interference on gene expression and cell function of fibroblasts A: si-semaphorin 7a silencing efficiency; B: The effects of
semaphorin 7a
interference on β1-integrin, VEGFA and VEGFR mRNA expression. aP<0.05,
bP<0.01, cP<0.001, respectively,
compared with the control group. C: The effect of semaphorin 7a interference on the protein levels of β1-integrin,
VEGFA, and VEGFR. Lane 1: Control group; Lane 2: si-negative group; Lane 3:
si-semaphorin 7a group. D:
The effect of semaphorin 7a
interference on fibroblast proliferation.
Expression Levels of β1-integrin, VEGFA and VEGFR Decreased After
si-semaphorin 7a
Transfection Treatment of fibroblasts with
si-semaphorin 7a for 24h
significantly inhibited the mRNA expression of β1-integrin, VEGFA and VEGFR (P<0.05,
Figure 3B).
Western blot results showed that the bands of β1-integrin, VEGFA and VEGFR
were significantly thinner in si-semaphorin 7a treated group (Figure 3C). The results suggested that semaphorin 7a interference can significantly affect the
expression of β1-integrin, VEGFA and VEGFR.
Cell Proliferation Inhibited After si-semaphorin 7a Transfection
Compared
with the control group, the cell proliferation ability of si-semaphorin 7a treated group was significantly weaker in
a time-dependent manner (P<0.05, Figure 3D).
Cell Migration Ability Impaired After si-semaphorin 7a Transfection Transwell results showed that the
migration ability of fibroblasts in si-semaphorin 7a transfected cells was significantly lower than that
in si-negative treated and control cells (P<0.05, Figure 4).
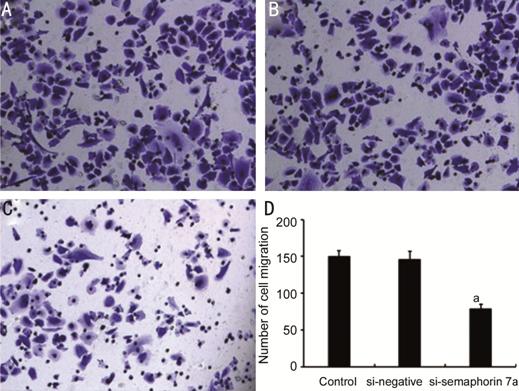
Figure 4 Effect of semaphorin 7a
interference on fibroblast migration ability A: Control group; B: si-negative group;
C: si-semaphorin 7a group; D:
The number of migratory cells in each group. aP<0.01
compared with the control group.
DISCUSSION
Pterygium is a chronic proliferative eye disease mainly with abnormally
proliferating epithelial cells, fibroblasts and neovascularization in pterygium
tissue[17]. Semaphorin 7a has been reported to be associated with a variety
of cancers, such as breast cancer[18], and oral
squamous cell carcinoma[19]. Thus, this study
aims to identify the relationship between semaphorin 7a and pterygium.
Real-time PCR and Western blot showed that the expression of semaphorin 7a in primary pterygium and recurrent
pterygium were higher than that in normal conjunctival tissue, and that in
recurrent pterygium samples was highest. Semaphorin 7a showed pro-angiogenic properties is associated with
angiogenesis in vascularized corneas[13]. It is
also involved in corneal nerve regeneration and inflammation in the cornea[20]. This suggested that semaphorin 7a overexpression may be associated with the
recurrence of pterygium, and cell proliferation and migration in pterygium
tissues.
In addition, and the expression levels of VEGFA and VEGFR affected by
si-semaphorin 7a were also
decreased. VEGF as one of the most potent angiogenic factors was suggested to
be involved in the pathogenesis of pterygium by triggering angiogenesis in
neovascularization[21]. Previous studies
indicated that VEGF was highly expressed in the development of pterygium[21, 22]. Overexpressed VEGFR in
primary pterygia and recurrent pterygia was recognized[23].
In addition, Feng et al[24] indicated that
β1-integrin related to adhesion and migration of conjunctiva cells participants
in the occurrence and recurrence of pterygium. β1-integrin recruitment and
VEGFR clustering was found associated with DNA synthesis and cell migration[25]. The β1-integrin/EGFR/VEGFA/VEGFR-1 signaling axis is
needed for cancer invasion and metastasis[26].
Semaphorin 7a containing
an RGD motif binds to β1-integrin to decreases integrin-mediated cell
attachment[27]. Although the indirect
VEGF-semaphorin interactions, the expression levels of VEGF- and
semaphorin-related genes were highly correlated in breast cancer[28]. Thus after semaphorin 7a interference, the highly expressed VEGFA, VEGFR and
β1-integrin could increase the blood supply of pterygium, and thus participated
in neovascularization of pterygium, which contribute to the progression and
recurrence of pterygium[21, 29, 30]. Additionally, the proliferation and migration
ability of fibroblasts was also decreased significantly after inhibition of
semaphorin 7a
expression. These results suggested that semaphorin 7a may be a potential target for the treatment of
primary or recurrent pterygium.
In conclusion, the expression of semaphorin 7a might be closely associated with the malignant
nature of pterygium growth and recurrence. Inhibiting semaphorin 7a in fibroblasts can significantly inhibit
the expression of VEGFA and VEGFR, and also reduce the proliferation and
migration of pterygium cells significantly.
ACKNOWLEDGEMENTS
Conflicts of Interest: Han YF, None; Liu Z, None; Wang B,
None; Zhu W, None; Li JZ, None; Qi YQ, None; Li XJ,
None; Xu YY, None; Dou XX, None; Mu GY, None.
REFERENCES
|
1 Detorakis ET, Spandidos DA. Pathogenetic mechanisms and treatment
options for ophthalmic pterygium: trends and perspectives (Review). Int J Mol
Med 2009;23(4):439-447.
https://doi.org/10.3892/ijmm_00000149
PMid:19288018
|
|
|
|
2 Singh H, Laad S, Pattebahadur RS, Saluja P, Ramnani P. A comparative
study of postoperative outcome after pterygium excision using autologous
blood and sutures. J Evid Based Med 2017;4(95):6022-6027.
https://doi.org/10.18410/jebmh/2017/1213
PMid:28596718
|
|
|
|
|
3 Wu KL, He MG, Xu JJ, Li SZ. Pterygium in aged population in Doumen
county, China. Yan Ke Xue Bao 2002;18(3):181-184.
|
|
|
|
|
4 Fukushima S, Inoue T, Inoue T, Ozeki S. Postoperative irradiation of
pterygium with 90Sr eye applicator. Int J Radiat Oncol Biol Phys
1999;43(3):597-600.
https://doi.org/10.1016/S0360-3016(98)00431-3
|
|
|
|
|
5 Liu DW, Peng C, Jiang ZX, Tao LM. Relationship between expression of
cyclooxygenase 2 and neovascularization in human pterygia. Oncotarget
2017;8(62):105630-105636.
https://doi.org/10.18632/oncotarget.22351
|
|
|
|
|
6 Mak RK, Chan TC, Marcet MM, Choy BN, Shum JW, Shih KC, Wong IY, Ng AL.
Use of anti-vascular endothelial growth factor in the management of
pterygium. Acta Ophthalmol 2017;95(1):20-27.
https://doi.org/10.1111/aos.13178
PMid:27473792
|
|
|
|
|
7 Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and
disease. Curr Opin Neurobiol 2009;19(3):263-274.
https://doi.org/10.1016/j.conb.2009.06.001
PMid:19541473 PMCid:PMC2730419
|
|
|
|
|
8 Worzfeld T, Offermanns S. Semaphorins and plexins as therapeutic
targets. Nat Rev Drug Discov 2014;13(8):603-621.
https://doi.org/10.1038/nrd4337
PMid:25082288
|
|
|
|
|
9 Nogi T, Yasui N, Mihara E, Matsunaga Y, Noda M, Yamashita N, Toyofuku
T, Uchiyama S, Goshima Y, Kumanogoh A, Takagi J. Structural basis for
semaphorin signalling through the plexin receptor. Nature
2010;467(7319):1123-1127.
https://doi.org/10.1038/nature09473
PMid:20881961
|
|
|
|
|
10 Czopik AK, Bynoe MS, Palm N, Raine CS, Medzhitov R. Semaphorin 7A is a
negative regulator of T cell responses. Immunity 2006;24(5):591-600.
https://doi.org/10.1016/j.immuni.2006.03.013
PMid:16713976
|
|
|
|
|
11 Holmes S, Downs AM, Fosberry A, Hayes PD, Michalovich D, Murdoch P,
Moores K, Fox J, Deen K, Pettman G, Wattam T, Lewis C. Sema7A is a potent
monocyte stimulator. Scand J Immunol 2002;56(3):270-275.
https://doi.org/10.1046/j.1365-3083.2002.01129.x
|
|
|
|
|
12 Kang SJ, Okuno T, Takegahara N, Takamatsu H, Nojima S, Kimura T,
Yoshida Y, Ito D, Ohmae S, You DJ, Toyofuku T, Jang MH, Kumanogoh A.
Intestinal epithelial cell-derived semaphorin 7A negatively regulates
development of colitis via αvβ1 integrin. J Immunol 2012;188(3):1108-1116.
https://doi.org/10.4049/jimmunol.1102084
PMid:22198947
|
|
|
|
|
13 Ghanem RC, Han KY, Rojas J, Ozturk O, Kim DJ, Jain S, Chang JH, Azar
DT. Semaphorin 7A promotes angiogenesis in an experimental corneal
neovascularization model. Curr Eye Res 2011;36(11):989-996.
https://doi.org/10.3109/02713683.2011.593730
PMid:21999225 PMCid:PMC3709026
|
|
|
|
|
14 Garcia-Areas R, Libreros S, Amat S, Keating P, Carrio R, Robinson P,
Blieden C, Iragavarapu-Charyulu V. Semaphorin7A promotes tumor growth and
exerts a pro-angiogenic effect in macrophages of mammary tumor-bearing mice.
Front Physiol 2014;5:17.
https://doi.org/10.3389/fphys.2014.00017
PMid:24550834 PMCid:PMC3914020
|
|
|
|
|
15 Jiménez B, Volpert OV. Mechanistic insights on the inhibition of tumor
angiogenesis. J Mol Med 2001;78(12):663-672.
https://doi.org/10.1007/s001090000178
PMid:11434719
|
|
|
|
|
16 Boudria A, Abou Faycal C, Jia T, Gout S, Keramidas M, Didier C,
Lemaître N, Manet S, Coll JL, Toffart AC, Moro-Sibilot D, Albiges-Rizo C,
Josserand V, Faurobert E, Brambilla C, Brambilla E, Gazzeri S, Eymin B.
VEGF165b, a splice variant of VEGF-A, promotes lung tumor progression and
escape from anti-angiogenic therapies through a β1 integrin/VEGFR autocrine loop.
Oncogene 2019;38(7):1050-1066.
https://doi.org/10.1038/s41388-018-0486-7
PMid:30194450
|
|
|
|
|
17 Cárdenas-Cantú E, Zavala J, Valenzuela J, Valdez-García JE. Molecular
basis of pterygium development. Seminars in Ophthalmology 2014:1-17.
https://doi.org/10.3109/08820538.2014.971822
PMid:25415268
|
|
|
|
|
18 Black SA, Nelson AC, Gurule NJ, Futscher BW, Lyons TR. Semaphorin 7a
exerts pleiotropic effects to promote breast tumor progression. Oncogene
2016;35(39):5170-5178.
https://doi.org/10.1038/onc.2016.49
PMid:27065336 PMCid:PMC5720143
|
|
|
|
|
19 Liu TJ, Guo JL, Wang HK, Xu X. Semaphorin-7A contributes to growth,
migration and invasion of oral tongue squamous cell carcinoma through
TGF-β-mediated EMT signaling pathway. Eur Rev Med Pharmacol Sci
2018;22(4):1035-1043.
|
|
|
|
|
20 Namavari A, Chaudhary S, Ozturk O, Chang JH, Yco L, Sonawane S, Katam
N, Khanolkar V, Hallak J, Sarkar J, Jain S. Semaphorin 7a links nerve
regeneration and inflammation in the cornea. Invest Ophthalmol Vis Sci
2012;53(8):4575-4585.
https://doi.org/10.1167/iovs.12-9760
PMid:22700709 PMCid:PMC3394693
|
|
|
|
|
21 Khalfaoui T, Mkannez G, Colin D, Imen A, Zbiba W, Errais K, Anane R,
Beltaief O, Zhioua R, Ben Hamida J, Lizard G, Ouertani-Meddeb A.
Immunohistochemical analysis of vascular endothelial growth factor (VEGF) and
p53 expression in pterygium from Tunisian patients. Pathol Biol
2011;59(3):137-141.
https://doi.org/10.1016/j.patbio.2009.04.006
PMid:19481369
|
|
|
|
|
22 Lu CW, Hao JL, Yao L, Li HJ, Zhou DD. Efficacy of curcumin in inducing
apoptosis and inhibiting the expression of VEGF in human pterygium
fibroblasts. Int J Mol Med 2017;39(5):1149-1154.
https://doi.org/10.3892/ijmm.2017.2944
PMid:28393179 PMCid:PMC5403353
|
|
|
|
|
23 Wang YC, Lin JY, Chen LX, Wang LM, Hao P, Han RF, Ying M, Li X.
Expression of peroxiredoxin 2 and vascular endothelial growth factor receptor
2 in pterygium. Cornea 2017;36(7):841-844.
https://doi.org/10.1097/ICO.0000000000001213
PMid:28489720
|
|
|
|
|
24 Feng QY, Hu ZX, Song XL, Pan HW. Aberrant expression of genes and
proteins in pterygium and their implications in the pathogenesis. Int J
Ophthalmol 2017;10(6):973-981.
|
|
|
|
|
25 Chen TT, Luque A, Lee S, Anderson SM, Segura T, Iruela-Arispe ML. Anchorage
of VEGF to the extracellular matrix conveys differential signaling responses
to endothelial cells. J Cell Biol 2010;188(4): 595-609.
https://doi.org/10.1083/jcb.200906044
PMid:20176926 PMCid:PMC2828913
|
|
|
|
|
26 Jeong BY, Cho KH, Jeong KJ, Park YY, Kim JM, Rha SY, Park CG, Mills
GB, Cheong JH, Lee HY. Rab25 augments cancer cell invasiveness through a β1
integrin/EGFR/VEGF-A/Snail signaling axis and expression of fascin. Exp Mol
Med 2018;50(1):e435.
https://doi.org/10.1038/emm.2017.248
PMid:29371698 PMCid:PMC5799805
|
|
|
|
|
27 Scott GA, McClelland LA, Fricke AF. Semaphorin 7a promotes spreading
and dendricity in human melanocytes through beta1-integrins. J Invest
Dermatol 2008;128(1):151-161.
https://doi.org/10.1038/sj.jid.5700974
PMid:17671519
|
|
|
|
|
28 Bender RJ, Mac Gabhann F. Expression of VEGF and semaphorin genes
define subgroups of triple negative breast cancer. PLoS One 2013;8(5):e61788.
https://doi.org/10.1371/journal.pone.0061788
PMid:23667446 PMCid:PMC3648524
|
|
|
|
|
29 Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT. Corneal
neovascularization: an anti-VEGF therapy review. Surv Ophthalmol
2012;57(5):415-429.
https://doi.org/10.1016/j.survophthal.2012.01.007
PMid:22898649 PMCid:PMC3709023
|
|
|
|
|
30 Marcovich AL, Morad Y, Sandbank J, Huszar M, Rosner M, Pollack A,
Herbert M, Bar-Dayan Y. Angiogenesis in pterygium: morphometric and
immunohistochemical study. Curr Eye Res 2002;25(1):17-22.
https://doi.org/10.1076/ceyr.25.1.17.9959
|
|
Citation: Han YF, Liu Z, Wang B, Zhu W, Li JZ, Qi YQ, Li XJ, Xu YY,
Dou XX, Mu GY. Semaphorin 7a
participants in pterygium by regulating vascular endothelial growth factor.
Int J Ophthalmol 2019;12(6):892-897




