
Citation: Jiang JQ, Li C, Cui CX, Ma YN,
Zhao GQ, Peng XD, Xu Q, Wang Q, Zhu GQ, Li CY. Inhibition of LOX-1 alleviates
the proinflammatory effects of high-mobility group box
·Basic Research·
Inhibition of LOX-1 alleviates the proinflammatory
effects of high-mobility group box
Jia-Qian Jiang, Cui Li, Cong-Xian
Cui, Yu-Na Ma, Gui-Qiu Zhao, Xu-Dong Peng, Qiang Xu, Qian Wang, Guo-Qiang Zhu,
Chen-Yu Li
Department of Ophthalmology, the Affiliated Hospital of Qingdao University,
Qingdao 266003, Shandong Province, China
Co-first authors: Jia-Qian Jiang and Cui Li
Correspondence to: Gui-Qiu Zhao. Department of Ophthalmology, the Affiliated Hospital of
Qingdao University, No.16 of Jiangsu Road, Shinan District, Qingdao 266003,
Shandong Province, China. Zhaoguiqiu_good@126.com
Received:
Abstract
AIM: To investigate the
inflammatory amplification effect of high-mobility group box 1 (HMGB1) in Aspergillus
fumigatus (A. fumigatus) keratitis
and the relationship between lectin-like oxidized low-density lipoprotein
receptor 1 (LOX-1) and HMGB
METHODS: Phosphate buffer saline (PBS),
and Boxb were injected into BALB/c mice subconjunctivally before the corneas
were infected with A. fumigatus. RAW264.7 macrophages and
neutrophils were pretreated with PBS and Boxb to determine the HMGB1
inflammatory amplification effects. Abdominal cavity extracted macrophages were
pretreated with Boxb and Poly (I) (a LOX-1 inhibitor) before A. fumigatus hyphae
stimulation to prove the the relationship between the two molecules. LOX-1,
interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), macrophage inflammatory
protein-2 (MIP-2) and IL-10 were assessed by polymerase chain reaction and
Western blot.
RESULTS: Pretreatment with Boxb
exacerbated corneal inflammation. In macrophages and neutrophils, A.
fumigatus induced LOX-1, IL-1β, TNF-α and MIP-2 expression in Boxb group was higher than
those in PBS group. Poly (I) treatments before infection alleviated the
proinflammatory effects of Boxb in abdominal cavity extracted macrophages.
Pretreatment with Boxb did not influence Dectin-1 mRNA levels in macrophages
and neutrophils.
CONCLUSION: In fungal keratitis, HMGB1 is
a proinflammatory factor in the first line of immune response. HMGB1 mainly
stimulates neutrophils and macrophages to produce inflammatory cytokines and
chemokines during the immune response. LOX-1 participates in HMGB1 induced
inflammatory exacerbation in A. fumigatus keratitis.
KEYWORDS: Aspergillus fumigatus keratitis; high-mobility group box 1; LOX-1
DOI:10.18240/ijo.2019.06.03
Citation: Jiang JQ, Li C, Cui CX, Ma YN, Zhao GQ, Peng XD, Xu Q,
Wang Q, Zhu GQ, Li CY. Inhibition of LOX-1 alleviates the proinflammatory
effects of high-mobility group box
Outline
Fungal keratitis is an infectious keratitis with a high rate of blindness
caused by pathogenic fungi[1]. Agricultural
trauma, contact lens abrasion, broad-spectrum antibiotics, glucocorticoids or
immunosuppressants for systemic or local long-term usage are increasing the
occurrence of fungal keratitis[2]. Fungal
keratitis, a serious infectious corneal disease, is not satisfactory for medical and
surgical treatment[3]. It is important to
investigate the pathogenesis of fungal keratitis.
High mobility group box1 (HMGB1), which was known as a proinflammatory cytokine,
is secreted by innate immune cells when the cells are stimulated with
pathogenic microorganisms, and it plays a central role in immunity[4-5]. When an appropriate external
signal stimulates neutrophils and macrophages, HMGB1 is released into the
extracellular milieu and recognized by TLR2, 4, and 9, thus promoting the
secretion of proinflammatory factors[1,6].
The structure of HMGB1 from the amino terminus to the carboxy terminus includes
an A box, a B box (Boxb) and a C-terminal domain that contains only glutamic
acids and aspartic acids[4]. As structure function
analysis showed that the B box of HMGB1 is a functional region that enhances
inflammation[6]. Lectin-like oxidized low-density
lipoprotein receptor 1 (LOX-1), a C-type lectin family member, is a key
receptor located in human corneal epithelial cells (HCECs), neutrophils and
macrophages[7]. Previous studies found that A.
fumigatus stimulation upregulates the expression of LOX
Our previous study found that HMGB1 participates in the immunity of fungal
keratitis and that TLR4/MyD88 is an important signaling pathway for HMGB1 to
induce inflammation[1]. The proinflammatory role
of LOX
Ethical Approval The study was conducted according to
the Declaration of Helsinki and approved by the Research Ethics Committee of
the Affiliated Hospital of Qingdao University. All mice were treated abided by
the RAVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Mice and Corneal Infection Specific pathogen-free (SPF) BALB/c
mice (8-week-old females) were purchased from Jinan Pengyue Laboratory Animal
Co., Ltd. (Jinan, China). Eight percent chloral hydrate was intraperitoneally
injected into mice for anesthesia. A stereoscopic microscope (×40
magnification) was used to amplify the eyes. The left eye of each mouse was
chosen as experimental eye, and scrapped the central corneal epithelium softly.
The corneal surface was covered with a 5-μL aliquot [1×108 colony
forming units (CFU)/mL] of A. fumigatus (strain3.0072, China General
Microbiological Culture Collection Center) and a sterile contact lens, then
gently sutured the eyelids. Mouse corneas were collected at 1, 3 and 5d after
infection.
Macrophages and Neutrophils Extraction
For
macrophage extraction, 1 mL of 3% thioglycollate medium was intraperitoneally
injected into mice. After 7d of stimulation, the mice were sacrificed. For
neutrophil extraction, 1 mL 9% casein (Sigma, Shanghai, China) was
intraperitoneally injected into mice. After 24h, the mice were given a similar
intraperitoneal injection, and 3h after injection, the mice were sacrificed.
After being wiped with 75% alcohol, 10 mL Dulbecco’s modified Eagle’s medium
(DMEM) (Gibco, San Diego, CA, USA) was injected into the abdominal cavity to
collect cells. After centrifugation, purification and suspension, the cells
were cultivated in culture plates.
Cell Culture and Stimulation RAW264.7
macrophages were obtained from the Shanghai Chinese Academy of Sciences
(Shanghai, China), and the cells were grown in DMEM with 10% fetal bovine serum
(FBS; Gibco), then, they were cultured at
Boxb Treatment of BALB/c Mice One day before infection, Boxb (0.5
µg/5µL) or control PBS was administered to the experimental eyes (n=6/group)
of BALB/c mice by subconjunctival injection. An additional 0.5 µg/100 µL Boxb
or control PBS was injected intraperitoneally 1 and 3d after infection.
Real-Time Reverse Transcriptase Polymerase Chain Reaction RNAiso plus reagent (TaKaRa, Japan)
was used to separate cornea and cell total RNA, which was rapidly quantified by
spectrophotometry. Complementary DNA was obtained through reverse transcription
of 1 µg RNA. Next 2 µL cDNA was diluted in 23 µL diethylpyrocarbonate-treated
water. Reverse transcriptase polymerase chain reaction (RT-PCR, 20 µL reaction
volume) was performed using a 2-µL cDNA aliquot and SYBRgreen. β-actin was used
as control. The oligonucleotide primers in this study are shown in Table 1.
Table 1 Nucleotide sequences of mouse primers for RT-PCR
|
Gene |
GenBank No. |
Primer sequence ( |
|
β-actin |
NM_007393.3 |
F: GAT TAC TGC TCT GGC TCC TAG C |
|
|
|
R: GAC TCA TCG TAC TCC TGC TTG C |
|
LOX-1 |
NM_138648.2 |
F: AGG TCC TTG TCC ACA AGA CTG G |
|
|
|
R: ACG CCC CTG GTC TTA AAG AAT TG |
|
Dectin-1 |
NM_020008.3 |
F: GAC CCA AGC TAC TTC CTC |
|
|
|
R: GCA GCA CCT TTG TCA TAC T |
|
IL-1β |
NM_008361.3 |
F: CGC AGC AGC ACA TCA ACA AGA GC |
|
|
|
R: TGT CCT CAT CCT GGA AGG TCC ACG |
|
TNF-α |
NM_013693.2 |
F: ACC CTC ACA CTC AGA TCA TCT T |
|
|
|
R: GGT TGT CTT TGA GAT CCA TGC |
|
MIP-2 |
NM_009140.2 |
F: TGT CAA TGC CTG AAG ACC CTG CC |
|
|
|
R: AAC TTT TTG ACC GCC CTT GAG AGT
GG |
|
IL-10 |
NM_010548.2 |
F: TGC TAA CCG ACT CCT TAA TGC AGG
AC |
|
|
|
R: CCT TGA TTT CTG GGC CAT GCT TCT
C |
RT-PCR: Reverse transcriptase polymerase chain reaction; IL: Interleukin;
TNF: Tumor necrosis factor; MIP: Macrophage inflammatory protein.
Western Blot Analysis Cells were collected after 24h of
infection. For protein extraction, the cells were lysed in
radioimmunoprecipitation assay (RIPA; Solarbio) lysis buffer with PMSF
(Solarbio) (100:1) for 2h. Twelve percent polyacrylamide SDS-PAGE was used to
separate the total protein, and the separated proteins were transferred onto
PVDF membranes (Solarbio). Five percent BSA (Beyotime, China) was used to block
the membranes at
Statistical Analysis One-way ANOVA was used to analyze
statistical significance with GraphPad 5.0 software. P<0.05 was
considered to indicate significantly differencest between comparisons. To
ensure reproducibility, all experiments were repeated at least three times.
Boxb Exacerbated Inflammation and Elevated the Clinical Score in A.
fumigatus Keratitis in BALB/c Mice
To
investigate the effects of HMGB
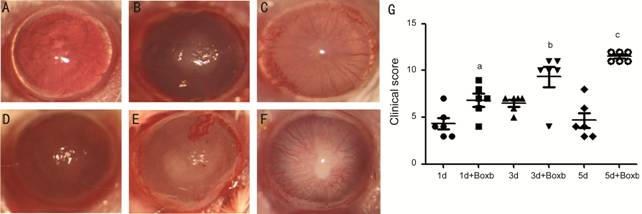
Figure 1 Boxb exacerbated inflammation and elevated the clinical score in A.
fumigatus keratitis of BALB/c mice Slit lamps were used to photograph PBS
(A, B, C) and Boxb (D, E, F) pretreated corneas. The clinical score (G) at 1,
3, 5d were also illustrated the disease severity. aP<0.05 vs
1d A. fumigatus group; bP<0.05 vs 3d A.
fumigatus group; cP<0.01 vs 5d A. fumigatus
group.
Boxb Up-regulated LOX-1 Expression in the Corneas of BALB/c Mice To investigate the effects of Boxb
in A. fumigatus keratitis, the corneas were treated with Boxb (0.5 µg/5
µL) or PBS 1d before infection. The results demonstrated increased LOX-1 mRNA
levels in the Boxb pretreated groups compared with those in the PBS control
groups (P<0.01; Figure 2).
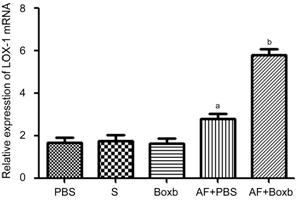
Figure 2 Effects of Boxb treatment on LOX
Boxb Up-regulated LOX-1, IL-1β, TNF-α, MIP-2 and IL-10 Expression in
RAW264.7 Macrophages To investigate the effects of Boxb
on RAW264.7 macrophages, cells were preconditioning with Boxb or PBS for 2h,
and stimulated with A. fumigatus for 12 or 24h. RT-PCR showed that
compare with PBS groups, the mRNA levels of LOX-1 (P<0.05; Figure
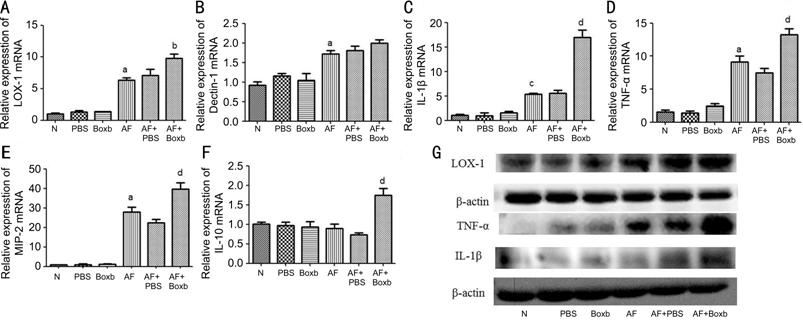
Figure 3 Effects of Boxb treatment on LOX-1, Dectin-1, IL-1β, TNF-α, MIP-2
and IL
Boxb Upregulated LOX-1, IL-1β, TNF-α, MIP-2 and IL-10 Expression in
Neutrophils from BALB/c Mice We next sought to investigate
whether Boxb has the same effect on neutrophils from mice. The cells were
pretreated with Boxb or PBS for 2h, and stimulated with A. fumigatus for
12h. The results showed that the mRNA levels of LOX-1 (P<0.01; Figure
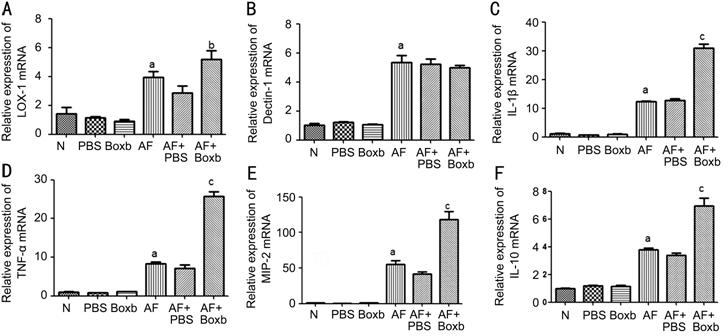
Figure 4 Effects of Boxb treatment on LOX-1, Dectin-1, IL-1β, TNF-α, MIP-2
and IL
Inhibition of LOX-1 Alleviated the Proinflammatory Effect of Boxb in
Macrophages from BALB/c Mice To investigate the influence of LOX-1
on HMGB1 proinflammatory effect, macrophages were preconditioning with Boxb and
Poly (I), and stimulated with A. fumigatus for 12 or 24h. Compare with
Boxb group, the mRNA levels of LOX-1 (P<0.05; Figure
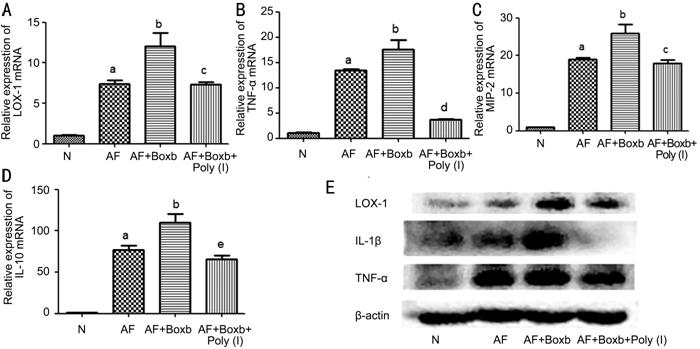
Figure 5 Effects of LOX-1 on HMGB1 proinflammatory effects in
macrophages In Boxb pretreated group, the mRNA
levels of LOX-1 (A), TNF-α (B), MIP-2 (C), IL-10 (D) were elevated, in Poly (I)
pretreated group, the mRNA levels of LOX-1 (A), TNF-α (B), MIP-2 (C), IL-10 (D)
were declined. In Boxb pretreated group, the protein levels of LOX-1, IL-1β,
TNF-α (E) were elevated, in Poly (I) pretreated group, the protein levels of
LOX-1, IL-1β, TNF-α (E) were declined. aP<0.001 vs
N group; bP<0.05 vs A. fumigatus group; cP<0.05;
dP<0.001; cP<0.01 vs Boxb+
A. fumigatus group.
As one of the danger associated molecular patterns, HMGB1 is an important
proinflammatory factor in innate immunity. HMGB1 is recognized by TLR2, 4, and
9 and functions as a proinflammatory factor[1,10]. HMGB1 is secreted by neutrophils, macrophages,
natural killer cells and dendritic cells[11].
HMGB1 is a target to elicit inflammatory effects in ocular surface inflammation[12]. In a mouse model of Pseudomonas aeruginosa
keratitis, blocking HMGB1 reduced clinical scores and a decreased of
inflammatory cytokine expression[13-15].
Liu et al[1] indicated that in A.
fumigatus keratitis, the pretreatment of corneas with Boxb leaded to severe
clinical manifestations and upregulation of inflammatory cytokine expression
after 1d of stimulation. Our data show that in A. fumigatus infected
groups, the clinical scores were higher after pretreated with Boxb, which are
consistent with previous data, explaining that additional HMGB1 plays a
proinflammatory magnification role in A. fumigates keratitis.
HMGB1 participates in the inflammatory response by selectively activating
multiple receptors on macrophages, neutrophils, eosinophils, fibroblasts, NK
cells, T cells and endothelial cells to produce inflammatory cytokines[16]. In chronic obstructive pulmonary disease (COPD)
immunity, HMGB1 siRNA reduced proinflammatory cytokine expression in A.
fumigatus-infected COPD alveolar macrophages compare with control alveolar
macrophages[5]. Neutrophils were also stimulated
by HMGB1 to produce more cytokines[17]. Previous
studies showed that in fungal keratitis, neutrophils, macrophages, and less T
cells are composed of the infiltrating cells[18].
To fullyconfirm the function of HMGB
Studies have shown that LOX-1 and Dectin-1 are important C-type lectin-like
receptors in A. fumigates keratitis. Both of these proteins play
proinflammatory roles in innate immunity[7-8,20]. To conform whether HMGB1 induced proinflammatory
cytokine production is related to LOX
In summary, our study demonstrates that HMGB1 promotes inflammation in
BALB/c mice corneas as well as in RAW264.7 macrophages and neutrophils. In
addition, inhibition of LOX-1 alleviates the proinflammatory effect of Boxb on
macrophages in BALB/c mice. Whether LOX-1 particcipate in HMGB1-induced
proinflammation effect directly or though HMGB1/TLR4 signal pathway, futher
studies will be expanded. These results indicate that HMGB1 exaerbate
inflammation mainly though neutrophils and macrophages and that LOX-1 functions
in HMGB1-mediated inflammation in A. fumigatus keratitis.
Fundations: Supported
by the National Natural Science Foundation of China (No.81470609; No.81500695;
No.81700800; No.81870632; No.81800800); Natural Science Foundation of Shandong
Province (No.ZR2017BH025; No.ZR2017MH008; No.ZR2013HQ007).
Conflicts of Interest: Jiang JQ, None; Li C, None; Cui CX,
None; Ma YN, None; Zhao GQ, None; Peng XD, None; Xu Q,
None; Wang Q, None; Zhu GQ, None; Li CY, None.
Citation: Jiang JQ, Li C, Cui CX, Ma YN,
Zhao GQ, Peng XD, Xu Q, Wang Q, Zhu GQ, Li CY. Inhibition of LOX-1 alleviates
the proinflammatory effects of high-mobility group box