
Citation: Li XT, Qin Y, Zhao JY, Zhang JS. Acute lens opacity
induced by different kinds of anesthetic drugs in mice. Int J
Ophthalmol 2019;12(6):904-908
DOI:10.18240/ijo.2019.06.04
·Basic Research·
Acute lens opacity induced by different kinds of
anesthetic drugs in mice
Xiao-Tong Li1,2, Yu
Qin1, Jiang-Yue Zhao1, Jin-Song Zhang1,2
1Department
of Ophthalmology, the Fourth Affiliated Hospital of China Medical University,
Eye Hospital of China Medical University, Key Lens Research Laboratory of
Liaoning Province, Shenyang 110005, Liaoning Province, China
2Aier Eye
Hospital, Shenyang 110000, Liaoning Province, China
Correspondence to: Jin-Song Zhang. Department of Ophthalmology, the Fourth Affiliated Hospital
of China Medical University, Eye Hospital of China Medical University, Key Lens
Research Laboratory of Liaoning Province, No.11 Xinhua Road, Heping District,
Shenyang 110005, Liaoning Province, China. cmu4h2_zjs@163.com
Received:
Abstract
AIM: To study whether specific
anesthetic drugs or tear layer evaporation was primarily responsible for the
acute cataract and what the change of lens structure is in anesthetized mice.
METHODS: Five groups were set up in the
experiment: Group A (topicamide and phenylephrine mixed eye drop+ chloral
hydrate), Group B (tropicamide and phenylephrine mixed eye drop+sevoflurane),
Group C (tropicamide and phenylephrine mixed eye drop), Group D (topicamide and
phenylephrine mixed eye drop+chloral hydrate, carbomer eye drop in the right
eyes), and Group E (tropicamide and phenylephrine mixed eye drop+sevoflurane,
carbomer eye drop in the right eyes). A simple classification system was used
to assess the severity of lens opacity. And a numerical value from 0 to 3 to
each grade was assigned for the cataract index calculation and data analysis.
The gross appearance and time course of development of lens opacity were
assessed. Hematoxylin and eosin staining was used to observe the lens structure
changes in the reversible cataract.
RESULTS: Tropicamide did not induce
lens opacification in mice. Lens opacity caused by inhaled sevoflurane was
similar to injected cholral hydrate. Both inhaled-anesthetic-induced lens
opacity and injected-anesthetic-induced lens opacity could be prevented by
carbomer eye drop. In the severe opacity lens, a wide range of lens fiber cell
structure had disordered. The fiber cells became uneven thickness.
CONCLUSION: The acute reversible lens
opacity can unilaterally develop or be induced by a local cause. The structure
of lens fiber cells changed in the lens opacity which may influence the
permanent connection of the lens fiber cells. This study was not only of
practical significance to help maintain lens transparency for eye research, but
also of the deeper consideration about the reversible lens opacification
phenomenon.
KEYWORDS: lens; opacity; anesthetic drugs;
tear film; mice
DOI:10.18240/ijo.2019.06.04
Citation: Li XT, Qin Y, Zhao JY, Zhang JS. Acute lens opacity induced
by different kinds of anesthetic drugs in mice. Int J Ophthalmol
2019;12(6):904-908
Outline
Mouse is the animal models commonly used in ophthalmology research[1-3], and general anesthesia is often
required in vivo experiment[4-5],
such as fund us examination or visual stimulate. Therefore, the feasibility of
iatrogenic lens opacity must be considered which may have a significant effect
on the result of measurement and even the experimental accuracy. It has been
reported that several drugs could induce the acute lens opacity in animal
models of ophthalmic research such as phenylephrine, sodium selenite,
naphthoquinone, xylazine and ketamine, etorphine, phenelzine and serotonin,
adrenaline, and chloral hydrate[6-9].
Early studies have shown that a range of exogenous factors can affect the
transparency of the lens, such as drugs, anesthetics, temperature, and so on[10-14]. Fluid homeostasis, especially
water cycle and ion exchange also have a critical effect on lens transparency[15-18]. As a result, it is still
challenging to clarify the exact causes of reversible lens opacification in the
anesthetized mice, the anesthetics or fluid homeostasis changes or both.
In this study, we were to test whether specific anesthetic drugs or tear
layer evaporation was primarily responsible for the development of cataracts in
mice. More importantly, we showed what kind of changes took place in the lens
structure. This study was significant to help maintain lens transparency in the
ophthalmic research.
Ethical Approval All of the procedures involving
animals met the guidelines of the Association for Research in Vision and
Ophthalmology Statement for the Use of Animals in Vision and Ophthalmic
Research which were approved by the Animal Use Committee of the Institute of
Zoology, Chinese Academy of Science.
Animals Four-week-old male C57BL/6 mice were
obtained from Department of Laboratory Animal Science; China Medical University
and brought up in the specific pathogen- free circumstance which was maintained
at
Experimental Design Thirty-five mice were used in the
experiment (aged four weeks, weight 25±
Assessment of Lens Opacity The eyes were inspected by the slit
lamp at 0, 15, 30, 45, 60min after inhaled
Hematoxylin and Eosin Staining The eyeballs of mice in Group E were
got after cervical dislocation. They regulared in 4% paraformaldehyde at
Characteristics of Reversible Lens Opacity A rapid mydriasis was obtained by
insallation of tropicamide lasted for 6h in all mice. No opacity was noticed
after mydriasis in the five groups. After receiving the anesthetics, the lens
showed anterior subcapsular opacity from peripheral to central. Eventually, the
entire lens became opaque and showed a diffuse milk-white opacity (Figure 1).
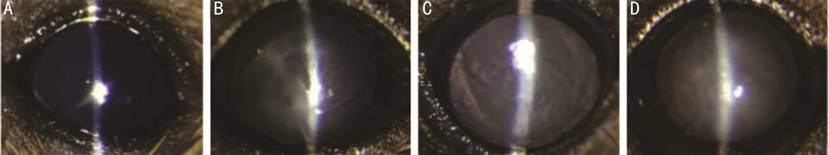
Figure 1 Four degrees opacification in lens A: No opacification: transparent lens
(numerical value=0); B: Mild opacification: opacification emerged in the
peripheral region (numerical value=1); C: Medium opacification: opacification
emerged in the medium region (numerical value=2); D: Severe opacification:
opacification emerged in the entire region (numerical value=3).
Development and Reversal of Opacification In Groups A and B lens opacification
was observed among most mice. No mice in Group C developed visible lens
opacity. In Group A, the lens opacity was noticed at 15min and progressed
rapidly, with 2/7 lens developed mild opacity, and 3/7 lens showed medium
opacity at 30min. All mice developed lens opacification at 60min. The entire
lens showed a milk-white suffused opacity. In Group B, the course of lens
opacity was the same as that in Group A. The 3/7 lens developed mild opacity, and
2/7 lens showed medium opacity at 30min. The 6/7 lens showed severe
opacification at 60min (Figure 2).
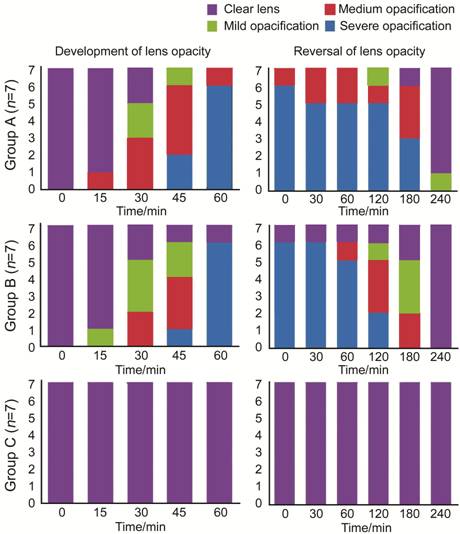
Figure 2 Time course of reversible lens opacity in the three groups (A-C)
are depicted by the column charts.
After the mice recovering from the anesthesia (Figure 2), the lens
opacification started to be reversed. Both in Groups A and B, the opacity
needed more time to reverse than develop. The lens opacification had not
changed in 60min. In Group A, one lens completely reversed at 120min and 6/7
lens was transparent at 240min. In Group B, all lens completely reversed at
240min. The lens opacification had no apparent difference between Groups A and
B (Figure 2). Both injected or inhaled anesthetic drugs could cause the acute
reversible cataract.
Both in Groups D and E (Figures 3-5), the left eyes of mice natural which
were exposured all developed lens opacification while in the right eyes used
carbomer eye drop, no lens developed opacification. It demonstrated that tear
layer evaporation might be the main cause of the reversible lens opacification
in anesthetized mice.
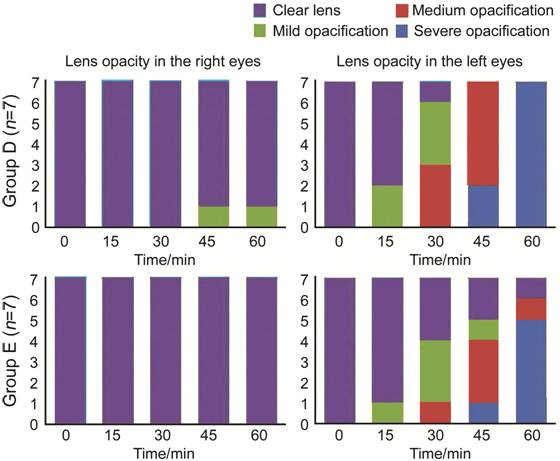
Figure 3 Time
course of reversible lens opacity in Groups D and E Carbomer eye drop coverage (right eyes);
natural exposure (left eyes).

Figure 4 An example in Group E Carbomer eye drop coverage (right
eyes); natural exposure (left eyes). No opacification and opacification were in
the same one.
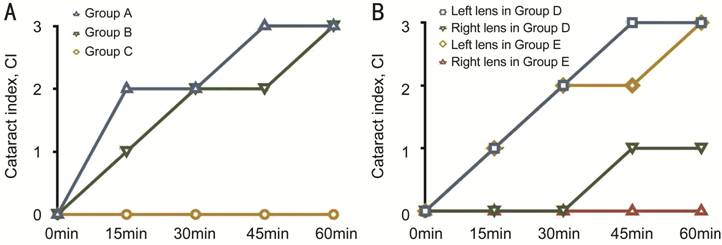
Figure 5 Time course of the reversible lens opacity in the different
groups.
Structure Changes of the Reversible Lens Opacity In Figure 6, a wide range of lens
fiber cells distributed disorderedly in the left lens of Group E. The fiber
cells became uneven thickness.
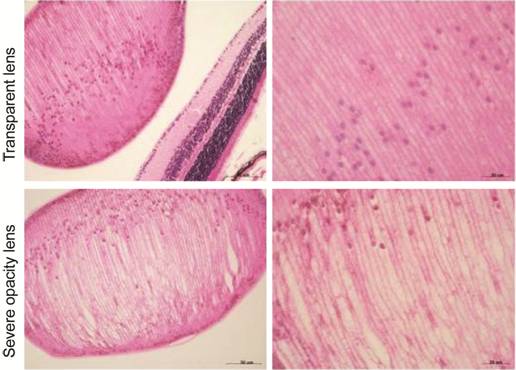
Figure 6 The lens structure in Group E
The right
lens were transparent, and the left were severe opacity lens.
Topical phenylephrine induced lens opacity was reported[9].
Hubert et al[22] used 3000 mice to dilated
in the research, and 19% of the mice had cataract. However, this might be known
as naturally lens opacification, and did not seem to be related to the drug. In
this study, tropicamide did not induce cataract in a mouse. It seems safe to
use tropicamide in the ophthalmic research. Inhaled sevoflurane and injected
chloral hydrate could affect the transparency of lens in most cases. The lens
showed anterior subcapsular opacity from peripheral to central and progressed
rapidly until the entire lens became opaque. The lens opacification started to
develop about 15min after anesthesia. After the mouse recovered from the
anesthesia, the cataracts gradually recovered. It has been reported that
several drugs could induce the acute lens opacity in animal models of
ophthalmic research including phenylephrine, sodium selenite, naphtoquinone,
xylazine and ketamine, etorphine, phenelzine and serotonin, and adrenaline[6,7,10-11,14]. However the symptom caused by different types of
drugs was similar[6-11,16].
Therefore, this phenomenon was more likely to be caused by the common side
effects of the anesthetic drugs.
Early studies had shown that various factors affect the transparency of the
lens, such as oxygen, pH, calcium, dehydration, and temperature[10-14]. Further studies were conducted
on generalization and mainly focused on narcotic effects, corneal dehydrationan
and temperature. Koehn et al[23] proposed
that anesthesia can affect intraocular pressure and develop corneal lesions. In
addition, fluid homeostasis[24], especially water
cycle and ion flow had a critical effect on lens transparency[15,25-26]. Thus, we
applied carbomer eye drop to guard against tear evaporation or corneal
dehydration. Finally, it could vaildly prevent opacification. This study
demonstrated that this opacification was more likely induced by local reasons
than systemic factors. The anesthetic drugs could retract the eyelids, restrain
the blink reflex and injure the tear film. Therefore, it is more likely that
the side effects of anesthetic affect the microcirculation of the eyes, thus
affecting the lens, rather than the direct effect of anesthetic on the lens. In
this study, the strategy which could prevent the evaporation of tear and the
dehydration of cornea could prevent opacification formation. Thus, tear film
stability might be more appropriate to explain this phenomenon.
As we know, lens proteins represent 30%-35% of the total mass; the
remaining 65%-70% is water compared to 95% water found in non-lenticular cells.
The main constituents of the lens proteins are water soluble structural
proteins, referred to as crystallins. The transparency and high refractive
index of mammalian lenses are due to very high concentrations of crystallins in
the lens fiber cells. However, crystallins are stabilized under appropriate
conditions of osmotic pressure and PH[27]. The
early study had shown that the corneal exposure could induce sodium
concentration in aqueous and lens changes[28].
Variation of aqueous or osmolarity in lens influenced lens opacity[29]. These studies proved regular circulation, stable
water and ion exchange in lens were significant to safeguard transparency.
Besides, we observed the lens fiber cells distributed disorderedly in the
cloudy lens. Also the fiber cells became uneven thickness. So the rapid
reversible lens opacity can be restored in four hours. So we speculated that
the changes in liquid environment interrupt the stability of lens, and then a
reversible change occurred in lens fiber cells. As far as we know, this is the
only research to report the lens structure changes in the reversible cataract.
In conclusion, both the inhaled anesthetics and the injected anesthetics
could induce the lens opacification. However, carbomer eye drop could prevent
the acute lens opacity. These supported that this opacification could
independently developed by a local reason. Furthermore, we found the structural
of lens fiber cells changed in the lens opacity. The structure changes of lens
fiber cells might influence the permanent connection of the lens fiber cells.
It was worthy considering whether these changes could affect the forward
transparency of the lens. More importantly, this kind of lens opacity might be
not really reversible. The research was not only of the practical significance
to keep lens transparency, but also of the more in-depth consideration of the
reversible lens opacification.
The study was performed in the Lens Research Laboratory of Liaoning
Province, China. Xiao-Tong Li conceived and carried out experiments,
interpreted data and wrote manuscript. Yu Qin and Jiang-Yue Zhao carried out
the animal raising and obtained research funding. Jin-Song Zhang conceived
experiments, obtained research funding and modified the manuscript.
Foundations: Supported by National Natural Science Foundation of China (No.81470617;
No.81270988; No.81371003); Youth Project of National Natural Science Foundation
of China (No.81600717); Natural Science Foundation of Liaoning Province
(No.201602851).
Conflicts of Interest: Li XT, None; Qin Y, None; Zhao JY, None; Zhang
JS, None.
Citation: Li XT, Qin Y, Zhao JY, Zhang JS. Acute lens opacity
induced by different kinds of anesthetic drugs in mice. Int J
Ophthalmol 2019;12(6):904-908
DOI:10.18240/ijo.2019.06.04