
Citation: Zhang XT, Xu Z, Shi KP, Guo DL, Li H, Wang L, Zhu XB.
Elevated expression of TREK-TRAAK K2P channels in the retina of
adult rd1 mice. Int J Ophthalmol 2019;12(6):924-929
DOI:10.18240/ijo.2019.06.07
·Basic Research·
Elevated expression of TREK-TRAAK K2P channels
in the retina of adult rd1 mice
Xiao-Tong Zhang, Zhen Xu,
Kang-Pei Shi, Dian-Lei Guo, Han Li, Lei Wang, Xiao-Bo Zhu
State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun
Yat-sen University, Guangzhou, 510060, Guangdong Province, China
Correspondence to: Xiao-Bo Zhu. State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic
Center, Sun Yat-sen University, 54S Xianlie Road, Guangzhou 510060, Guangdong
Province, China. zhuxbo@mail.sysu.edu.cn
Received:
Abstract
AIM: To examine the expression of
Twik-related K+ channel 1 (TREK-1), Twik-related K+
channel 2 (TREK-2), and Twik-related arachidonic acid-stimulated K+
channel (TRAAK) in the retina of adult rd1 mice and to detect the protective
roles of TREK-TRAAK two-pore-domain K+ (K2P) channels
against retinal degeneration.
METHODS: Twenty-eight-day-old C57BL/6J
mice and 28-day-old rd1 mice were used in this study. Retinal protein, retinal
RNA, and embedded eyeballs were prepared from these two groups of mice.
Real-time quantitative polymerase chain reaction and Western blot analyses were
used to assess the gene transcription and protein levels, respectively. Retinal
structures were observed using hematoxylin and eosin (H&E) staining.
Immunohistochemistry was utilized to observe the retinal localization of
TREK-TRAAK channels. Current changes in retinal ganglion cells (RGCs) after
activation of TREK-TRAAK channels were examined using a patch-clamp technique.
RESULTS: Compared with C57BL/6J mice,
rd1 mice exhibited significantly higher retinal mRNA and protein expression
levels of TREK-1, TREK-2, and TRAAK channels. In both groups,
immunohistochemistry showed expression of TREK-TRAAK channels in retinal
layers. After addition of the TREK-TRAAK channel agonist arachidonic acid (AA),
whole-cell voltage step evoked currents were significantly higher in RGCs from
rd1 mice than in RGCs from control C57BL/6J mice, suggesting that TREK-TRAAK
channels were opened in RGCs from rd1 mice.
CONCLUSION: TREK-TRAAK K2P
channels’ expression is increased in adult rd1 mice. AA induced the opening of
TREK-TRAAK K2P channels in adult rd1 mice and may thus
counterbalance depolarization of RGCs and protect the retina from excitotoxicity.
TREK-TRAAK channels may play a protective role against retinal degeneration.
KEYWORDS: TREK-TRAAK
channels; arachidonic acid; retinal ganglion cells; retinal degeneration
DOI:10.18240/ijo.2019.06.07
Citation: Zhang XT, Xu Z, Shi KP, Guo DL, Li H, Wang L, Zhu XB.
Elevated expression of TREK-TRAAK K2P channels in the retina of
adult rd1 mice. Int J Ophthalmol 2019;12(6):924-929
Outline
Retinitis pigmentosa (RP) is an inherited retinal degenerative disease
involving the degeneration of rod photoreceptors followed by cone cells,
ultimately leading to blindness[1-2].
Several interventions can either delay photoreceptor degeneration or replace
lost photoreceptors[3-7].
However, the success of RP treatment largely relies on retinal ganglion cells
(RGCs), whose axons transmit visual information to the central nervous system[8-9]. Thus, it is essential to
understand the changes in these cells that accompany the degenerative loss of
photoreceptors.
Twik-related K+ channel 1 (TREK-1), Twik-related K+ channel
2 (TREK-2), and Twik-related arachidonic acid-stimulated K+ channel
(TRAAK) are two-pore-domain K+ (K2P) channels
that feature 4TMS/2P structures. TREK-TRAAK potassium channels can be strongly
activated by arachidonic acid (AA)[10]. In
addition to the neuronal functions of K2P channels[11], their roles in the rd1 mouse retina remain unknown.
A previous study reported that K2P channels are expressed in the
mouse retina[12]. However, the roles of these
channels in rd1 mice, which are characterized by rapid photoreceptor
degeneration, have not been clarified. The rd1 mouse is an RP model and
carries a loss-of-function mutation in the rod-specific Pde6β gene that leaves
a single layer of cone photoreceptors in the outer nuclear layer (ONL) by the
time the mouse reaches 4wk of age[13]. The rd1
model is widely used for studying retinal degeneration[13].
We sought to examine the role of TREK-TRAAK K2P channels in rd1
mice, with a particular focus on RGCs after photoreceptor degeneration. In this
study, real-time quantitative polymerase chain reaction (RT-qPCR), Western
blot, hematoxylin and eosin (H&E) staining, immunohistochemistry and
patch-clamp recording were used to analyze TREK-TRAAK channels in the rd1 mouse
retina. Our aim was to reveal correlations between changes in TREK-TRAAK
potassium channels expression and retinal degeneration.
Ethical Approval Animal experiments were performed in
accordance with the ARVO Statement for the Use of Animals in Ophthalmic and
Vision Research and were approved by the Animal Ethical Committee of Zhongshan
Ophthalmic Center. For this study, rd1 (C3H/HeJ) mice were obtained from
Nanjing University, and C57BL/6J mice were provided by Zhongshan Ophthalmic
Center.
Animal Use and Welfare At postnatal day 28 (P28), rd1 and
C57BL/6J mice were sacrificed to detect TREK-TRAAK expression in the retina.
C57BL/6J mice of the same age were used as the controls.
RT-qPCR C57BL/6J mice and rd1 mice were
euthanized at P28, and the eyes were enucleated. Total RNA was extracted from
the retina with TRIzol (Takara, Japan) and converted into cDNA using PrimeScript
RT Master Mix (Takara). The primer sequences were as follows: TREK-1 forward,
Western Blotting Retina were removed from rd1 and
C57BL mice at P28. Protein was extracted with RIPA lysis buffer containing
protease inhibitors. Protein samples were separated by SDS-PAGE and transferred
to PVDF membranes. The membranes were blocked with 5% skim milk at
Hematoxylin and Eosin Staining H&E staining was performed as
described previously[3]. Eyeballs were removed
from normal C57BL/6J mice and rd1 mice at 28d and processed to create paraffin
sections. The sections were then stained with H&E using standard methods.
After H&E staining, the slides were dehydrated.
Immunohistochemistry Eyes were fixed in 4% paraformaldehyde,
embedded in paraffin wax, and deparaffinized according to standard procedures.
The sections were incubated with 0.3% H2O2 at room
temperature for 1h and blocked with bovine serum albumin for 30min. They were
then incubated with primary antibody at
Patch-clamp Recordings The retina were carefully dissected
from the pigment epithelium in artificial cerebrospinal fluid (ACSF).
Whole-cell currents in response to voltage step stimuli were recorded in
voltage-clamp mode with real-time P/N leak subtraction. To activate the
TREK-TRAAK channels, 10 µmol/L AA was added to the perfusing ACSF and directly
applied to the retina. The pipette solution contained 120 mmol/L potassium
gluconate (with 120 mmol/L potassium chloride used instead to measure
spontaneous synaptic current), 5 mmol/L NaCl, 10 mmol/L KCl, 1 mmol/L MgCl2,
1 mmol/L EGTA, 10 mmol/L HEPES, 2 mmol/L ATP, and 0.5 mmol/L GTP and was
adjusted to pH 7.2 with 1 mol/L KOH.
The currents evoked by voltage
steps from -80 mV to +60 mV were measured under control and AA-treated
conditions. For each cell, the current under the AA condition was normalized to
the corresponding current under the control condition.
Statistical Analysis The data are presented as the
means±SEM with n≥3 and were analyzed by GraphPad Prism (version 7.0, USA).
Between-group differences were compared using Student’s t-test.
Expression of TREK-TRAAK in the Retina of P
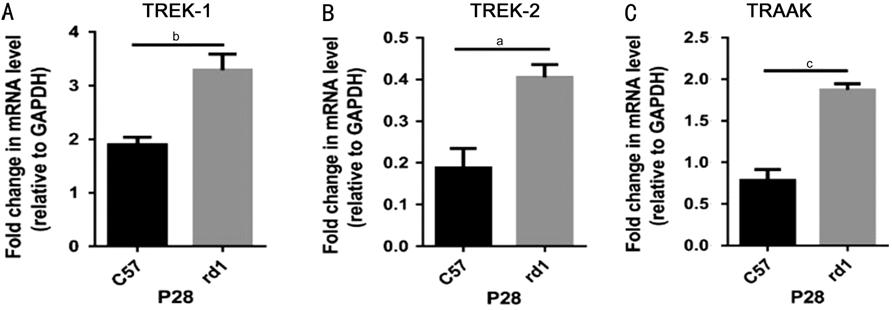
Figure 1 Relative mRNA expression
of TREK-TRAAK in the retinae of P
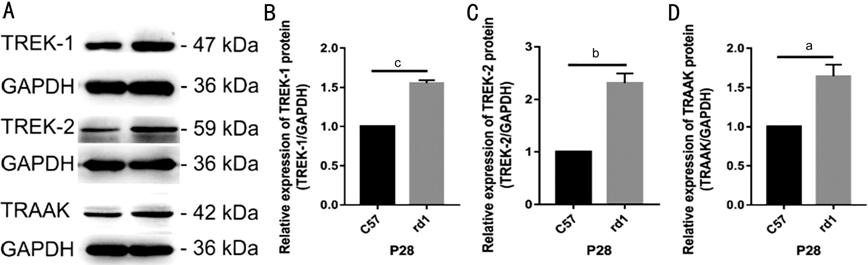
Figure 2 Western blot quantification of TREK-TRAAK protein expression in
the retinae of C57BL/6J (C57) and rd1 mice at P28 aP<0.05, bP<0.01,
cP<0.001.
Retinal Layers of C57BL/6J and rd1 Mice at P28 Observed by H&E
Staining H&E staining showed the retinal
structures of rd1 and C57BL mice (Figure 3). In C57BL mice, at P28, the
observed retinal layers were the ganglion cell layer (GCL), inner plexiform
layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), ONL, photoreceptor
cell layer (PCL), and retinal pigment epithelium (RPE). In rd1 mice, at P28,
the photoreceptors had completely degenerated, and the observed retinal layers
were the GCL, IPL, INL, ONL and RPE. Compared to that in the C57BL/6J mice, the
total retinal thickness was lower in rd1 mice (216.7±3.50 μm vs 103.8±4.95
μm, P<0.0001).
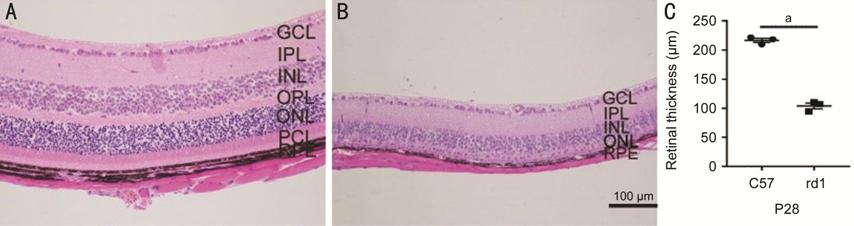
Figure 3 H&E-stained retinal layers of C57BL/6J (C57) and rd1 mice at P
Expression of TREK-TRAAK in the Retina of P
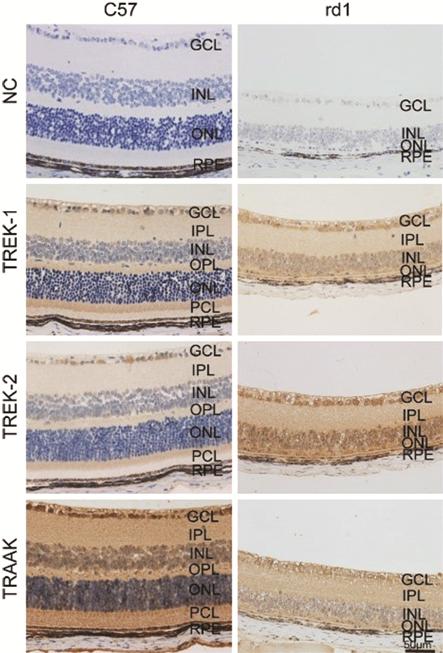
Figure 4 Expression of TREK-TRAAK channels in the retina of C57BL/6J (C57)
and rd1 mice NC: Negative control.
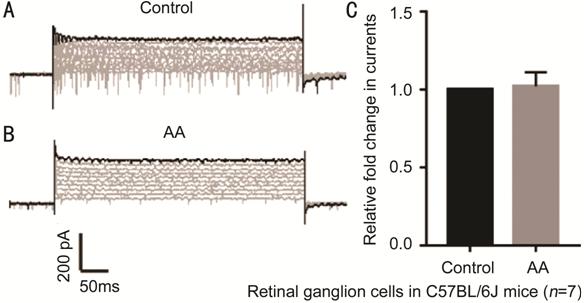
Figure 5 Current response of C57BL/6J (C57) mouse RGCs to voltage step
stimuli A: Whole-cell currents under the
control condition; B: Whole-cell currents under the AA-treated condition; C:
Difference in current between cells under the control and AA-treated
conditions. AA: Arachidonic acid. P=0.813.
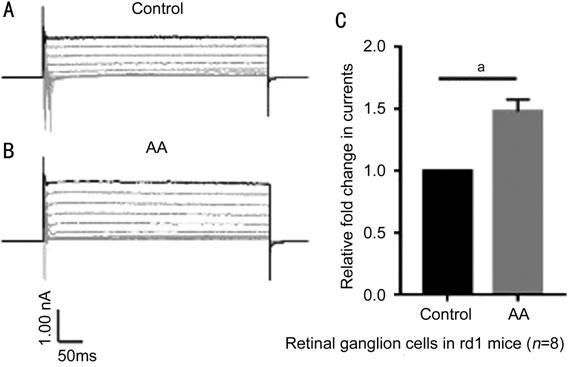
Figure 6 Current response of rd1 mouse RGCs to voltage step stimuli A: Whole-cell currents under the
control condition; B: Whole-cell currents under the AA-treated condition; C:
Difference in current between cells under the control and AA-treated
conditions. AA: Arachidonic acid. aP<0.001.
Given the variety of biological effects of K2P potassium
channels, great interest has developed in identifying the protective roles of
these channels against diseases[15-17].
Previous research studied the localization of K2P channels in
the adult C57BL/6J mouse retina[12], while this
research assessed TREK-TRAAK ion channels in the retina of rd1 mice. Both the
mRNA and protein expression levels of TREK-TRAAK were higher in rd1 mice than
in C57BL/6J mice. TREK-TRAAK channels are associated with resting potential and
cellular excitability[18]. In retinal
degeneration, TREK-TRAAK K2P channels may be meaningful targets for
suppressing pathological hyperactivity in RGCs[9,12,19]. Upregulation of TREK-TRAAK
channels may produce K+ currents and hyperpolarize the resting
membrane potential, leading to decreased cellular excitability in rd1 mice.
Thus, in such mice, upregulation of TREK-TRAAK channels in RGCs might suppress
the excitability of these cells and play a protective role. In a previous
study, increased expression of TREK-1 was found to be a protective feedback mechanism
under pathological conditions. TREK-1 has also previously been observed to be
upregulated in the dorsal root ganglion (DRG) of rats with detrusor
overactivity; this upregulation might suppress the excitability of DRG neurons
and protect the bladder from overactivity[20]. In
the current study, we found that TREK-TRAAK levels were upregulated in retinal
tissue from rd1 mice compared to that from control mice. In RP, a family of
blinding diseases that result in photoreceptor degeneration, approximately 20%
of RGCs were reduced in rd mutant mice[21]. In
vision, RGCs ultimately project light information to retinorecipient areas of
the brain. Given the critical role of RGCs in the visual pathway, it is
necessary to delay the functional decay of RGCs, a process that can be studied
in rd1 mice[8].
A previous study has reported the effects of TREK-TRAAK channels in human
RPE cells under oxidative stress[22], whereas our
research concentrated on RGCs in retinal degeneration. The previous study did
not show patch-clamp recording-based evidence of protective effects. In
patch-clamp experiments, the TREK-TRAAK agonist AA was chosen to explore the
functional expression of TREK-TRAAK channels in RGCs in retinal degeneration.
In RGCs from rd1 mice, addition of the TREK-TRAAK channel agonist AA
significantly increased whole-cell voltage step evoked currents, suggesting
that TREK-TRAAK channels were opened. Thus, AA induced the opening of K2P
channels in adult rd1 mice and may therefore limit RGC depolarization and
protect the retina from excitotoxicity. Our results show that TREK-TRAAK
channels may protect the retina from degeneration.
Our study had certain limitations.
The ways in which overexpression of TREK-TRAAK channels regulates the
excitability of RGCs remain unknown and warrant further investigation.
Moreover, we did not detect whether upregulation of potassium ion channels
increased the action potential threshold of RGCs. To better elucidate the
function of TREK-TRAAK channels in retinal degeneration, future studies should
include the use of specific inhibitors of these channels.
In summary, this study showed marked upregulation of TREK-TRAAK K+
channels in the retina of rd1 mice after photoreceptor degeneration; this
upregulation might suppress the excitability of RGCs and play a protective
role against RP. Upregulation of TREK-TRAAK potassium channels in the retina
may be a form of protective feedback in response to retinal degeneration in rd1
mice. Therefore, it is likely that increased expression of TREK-TRAAK K+ channels
plays a protective role against retinal degeneration in rd1 mice. Whether
TREK-TRAAK channels can be a potential interventional target in the treatment
of RP needs additional investigation.
Foundation: Supported
by National Natural Science Foundation of China (No.81271012).
Conflicts of Interest: Zhang XT, None; Xu Z, None; Shi KP, None; Guo DL, None;
Li H, None; Wang L, None; Zhu XB, None.
|
|