
Citation: Reñones J,
Estévez B, González-Martín JM, Carreras H, Loro JF, Antón A. Effect of
femtosecond laser-assisted lens surgery on the optic nerve head and the macula.
Int J Ophthalmol 2019; 12(6):961-966
DOI:10.18240/ijo.2019.06.13
·Clinical
Research·
Effect of femtosecond laser-assisted lens surgery on the
optic nerve head and the macula
Josefina
Reñones de Abajo1,2, Beatriz Estévez Jorge1, Jesús María
González Martín3, Humberto Carreras Díaz1, Juan Francisco
Loro Ferrer2, Alfonso Antón López4,5,6
1Eurocanarias Oftalmológica, Las
Palmas de Gran Canaria 35004, Spain
2Universidad de Las Palmas de Gran
Canaria (ULPGC), Las Palmas de Gran Canaria 35001, Spain
3Department of Research, Hospital
Universitario de Gran Canaria Doctor Negrín, Las Palmas de Gran Canaria 35019,
Spain
4Universidad Internacional de
Cataluña (UIC), Barcelona 08195, Spain
5Institut Català de Retina (ICR),
Barcelona 08017, Spain
6Parc de salut Mar, Barcelona
08024, Spain
Correspondence to: Josefina
Reñones de Abajo. Department of Cataract and Refractive Surgery and Department
of Glaucoma, Clínica Eurocanarias Oftalmológica, León y Castillo 211, Las
Palmas de Gran Canaria 35004, Spain. re.josefina@gmail.com
Received:
Abstract
AIM: To
evaluate the effect of femtosecond laser-assisted lens surgery (FLALS; cataract
surgery or refractive lens exchange) on the structure of the optic nerve head
and the macula.
METHODS: This
prospective longitudinal study included healthy eyes undergoing FLALS. Eyes
with glaucoma or any other ocular disease that could alter optical coherence
tomography results were excluded. Retinal nerve fiber layer (RNFL), Bruch’s
membrane opening-minimum rim width (BMO-MRW) and macular thickness (MT) were
measured preoperatively, 1 and 6mo after surgery using spectral-domain optical
coherence tomography (SD-OCT). Changes between preoperative and postoperative
values were evaluated.
RESULTS: A total
of 87 eyes of 46 patients were included in this study. Preoperative RNFL,
BMO-MRW and MT in microns (µm) were 100.77±10.39, 330.31±49.99 and
276.30±33.39, respectively. Postoperative RNFL, BMO-MRW and MT were
104.74±11.55, 348.32±54.05 and 279.83±22.65 1mo after surgery and 102.93±11.17,
343.11±53.4 and 278.90±22.19 6mo after surgery, respectively; which equals an
increase of 3.93%, 5.45% and 1.27%, respectively, 1mo after surgery, and 2.14%,
3.87% and 0.94% 6mo after surgery. The differences between the preoperative and
the postoperative RNFL and BMO-MRW values were statistically significant (P<0.001).
Regarding MT values, there were not statistically significant differences (P=0.26).
CONCLUSION: Our
study suggests that FLALS does not have a negative impact on the structural
status of the optic nerve head in healthy eyes, assessed by SD-OCT. There is a
slight increase in the values of RNFL, BMO-MRW and MT 1mo and 6mo after
surgery.
KEYWORDS: femtosecond
laser-assisted cataract surgery; refractive lens exchange; optical coherence
tomography; optic nerve head; macula
DOI:10.18240/ijo.2019.06.13
Citation: Reñones J, Estévez B, González-Martín JM, Carreras H,
Loro JF, Antón A. Effect of femtosecond laser-assisted lens surgery on
the optic nerve head and the macula. Int J Ophthalmol
2019; 12(6):961-966
INTRODUCTION
Cataract surgery is the most commonly performed
surgical procedure in the world. Recently, femtosecond laser assisted cataract
surgery (FLACS) has gained popularity due to its advantages over conventional
phacoemulsification such as increased accuracy and reproducibility with better
refractive results, reduced endothelial cell loss, reduced effective
phacoemulsification time and reduced intraoperative complication rate[1-8]. Initially FLACS was only used in healthy
eyes, due to the lack of evidence regarding its effects on eyes with
pathologies. However, the benefits of FLACS in certain ocular conditions have
made this procedure expand its field of use. Nowadays FLACS is frequently the
chosen technique for eyes with low endothelial cell count, pseudoexfoliation,
narrow anterior chamber, dense cataracts, etc[1,5,9-10]. There is an increasing
number of publications stating that FLACS is useful in certain conditions
related to glaucoma, such as angle closure, nanophthalmos, Peters’ anomaly,
pseudoexfoliation or phacomorphic glaucoma[9,11-14], despite the fact that the
effect of FLACS on the optic nerve is unknown.
Femtosecond laser-assisted lens surgery (FLALS),
both in case of cataract surgery and in case of refractive lens exchange,
requires the application of a suction device to stabilize the laser head and
focus the laser beam accurately. As a result, there is an increase in
intraocular pressure (IOP), which poses potential risks, especially for
patients with glaucoma. Only a few studies have evaluated the changes in the
optic nerve head after a femtosecond laser procedure, as well as macular
changes, and most of them have been conducted with patients undergoing laser in
situ keratomileusis (LASIK)[15-16].
Therefore, there is a need for evidence stating whether FLALS causes changes in
the optic nerve head or in the macula.
With the introduction of optical coherence
tomography (OCT) both the optic nerve head and the macular structure have
become easily assessable by a direct and non-invasive method. The OCT device
acquires accurate measures of the retinal nerve fiber layer (RNFL), Bruch’s
membrane opening- minimum rim width (BMO-MRW) and macular thickness (MT), which
provide information about the structural status of the optic nerve head and the
macula[17-18].
The aim of this study was to evaluate the
structural changes in the optic nerve head and the macula after FLALS in
healthy eyes, assessed by OCT.
SUBJECTS AND METHODS
Ethical Approval The study
was performed in compliance with the tenets of the Declaration of Helsinki.
Written informed consent was obtained from all the subjects after receiving a
full explanation of the procedure.
Design and Study Group This
prospective longitudinal monocentric study included patients undergoing FLALS
from September 2016 to February
Inclusion-Exclusion Criteria Patients
undergoing FLALS between September 2016 and February 2017 were included.
Exclusion criteria were history of any ocular disease, particularly glaucoma,
ocular hypertension or any condition that could alter OCT results
(peripapillary atrophy, age related macular degeneration, difficulties in
fixation, etc.), as well as hyperopia superior to 3 diopters, myopia
superior to -3 diopters and astigmatism superior to 2 diopters.
Examinations
All patients underwent comprehensive slit lamp
examination before and 1d, 1wk, 1 and 6mo after surgery. Preoperative tests
included biometry (IOL Master® 700, Carl Zeiss Meditec, Jena, Germany),
Scheimpflug corneal topography (Pentacam Scheimpflug Image System, Oculus Inc.
Wetzlar, Germany) and Placido-based corneal topography (Allegro Topolyzer
Vario, WaveLight Technologie AG, Alcon Laboratories, Erlangen, Germany).
Corrected and uncorrected visual acuity and applanation IOP were recorded.
Prior to surgery Spectral-domain OCT (Spectralis-Glaucoma Module Premium
Edition, Heidelberg Engineering, Carlsbad, CA, USA) circle and radial scans
were acquired to provide RNFL and BMO-MRW measurements, respectively, as well
as horizontal scans to provide MT measurements. Circle and radial scans were
centered on the BMO and all scan types were aligned according to the
fovea-to-BMO-center (FoBMOC) axis using the automated anatomical positioning
system (APS) scan feature. The APS-based scans were repeated 1 and 6mo after
surgery using the automatic “follow-up” feature in order to provide RNFL,
BMO-MRW and MT measurements. Of all the measurements acquired, those used for
the analysis were average RNFL, average BMO-MRW and central retinal thickness
(CRT).
Surgical Technique The
femtosecond laser platform used was LenSx (Alcon- LenSx Inc., Aliso Viejo, CA,
USA). Phacoemulsification was performed using Centurion® Vision
system (Alcon Laboratories Inc.). Corneal incisions were fixated at 45 and 135
degrees and capsulorhexis diameter was 5 millimetres. The nucleus fragmentation
pattern chosen can be seen in Figure 1. Postoperative treatment consisted of
topical application of antibiotic, steroids, nonsteroidal anti-inflammatory
drugs (NSAIDs) and artificial tears.
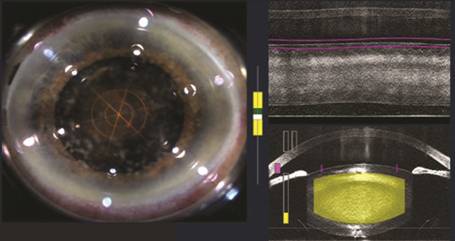
Figure 1 Femtosecond laser capsulorhexis and
phacofragmentation (circular and radial pattern).
Statistical Analysis Data were analysed
using R Core Team 2017 (R: A language and environment for statistical
computing, R Foundation for Statistical Computing, Vienna, Austria).
Shapiro-Wilk normality test was used to determine if the sample was normally
distributed. Mean and standard deviation (SD) of all parameters were
calculated. Linear regression analysis with its respective ANOVA test and post
hoc tests using the Bonferroni correction were applied. A P-value less
than 0.05 was considered statistically significant.
RESULTS
The study included 87 eyes of 46 patients, of which
50.5% were right eyes and 49.5% were left eyes. There were 30 women (65.2%) and
16 men (34.8%) with a mean age of 65.7±8.16y. Most of the patients had
cataracts (64.5%) and the rest of them aimed refractive lens exchange.
The mean preoperative values (±SD) of RNFL, BMO-MRW
and MT in microns (µm) were 100.77±10.39, 330.31±49.99 and 276.30±33.39,
respectively. There were no intraoperative or postoperative complications in
the study patients. In particular, clinically significant macular edema did not
appear in any of the patients in the postoperative period.
A slight increase in all parameters was observed
after surgery, which can be seen in Figure 2. This increase was greater at 1mo
than at 6mo post surgery. Postoperative RNFL, BMO-MRW and MT were 104.74±11.55,
348.32±54.05 and 279.83±22.65 1mo after surgery and 102.93±11.17, 343.11±53.4
and 278.90±22.19 6mo after surgery, respectively; the percentage difference is
shown in Table 1. The differences between the preoperative and the
postoperative RNFL and BMO-MRW values were statistically significant (P<0.001).
Regarding MT values, there were not statistically significant differences (P=0.26).
For those parameters that showed a P value smaller than 0.05 paired
comparisons with Bonferroni correction were performed; Table 2 showed the
groups with statistically significant differences and the confidence intervals
(CI).
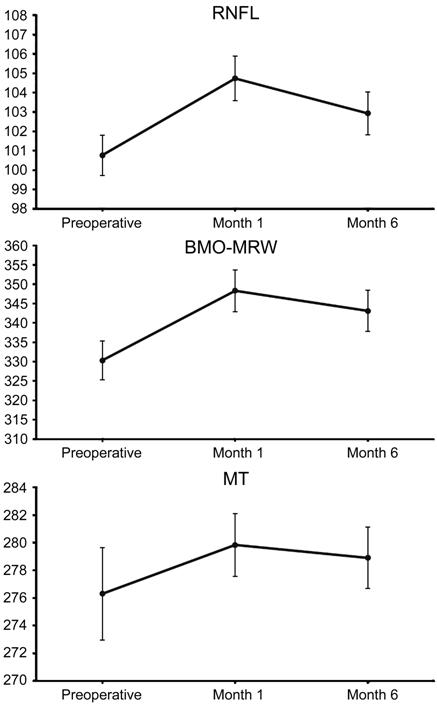
Figure 2 Changes in RNFL, BMO-MRW and MT 1mo and
6mo after surgery (mean±SD, µm).
Table 1 Percentage difference between preoperative
and postoperative values of RNFL, BMO-MRW and MT %
|
Percentage difference |
RNFL |
BMO-MRW |
MT |
|
1mo postop.-preop. |
3.93 |
5.45 |
1.27 |
|
6mo postop.-preop. |
2.14 |
3.87 |
0.94 |
Table 2 Results of paired comparisons with
Bonferroni correction for the groups
|
Groups |
Difference
(µm) |
SE |
95%CI |
P |
|
RNFL |
|
|
|
|
|
1mo postop.-preop. |
3.97 |
0.29 |
3.29 to 4.64 |
<0.001 |
|
6mo postop.-preop. |
2.16 |
0.29 |
1.48 to 2.84 |
<0.001 |
|
6mo postop.-1mo postop. |
-1.80 |
0.29 |
-2.48 to -1.13 |
<0.001 |
|
BMO-MRW |
|
|
|
|
|
1mo postop.-preop. |
18.01 |
0.92 |
15.85 to 20.17 |
<0.001 |
|
6mo postop.-preop. |
12.80 |
0.92 |
10.64 to 14.96 |
<0.001 |
|
6mo postop.-1mo postop. |
-5.21 |
0.92 |
-7.37 to -3.05 |
<0.001 |
DISCUSSION
The main purpose of our study was to evaluate the
effect of FLALS on the optic nerve head in healthy eyes, and secondarily to
assess its effect on the macula. It has been proved that FLALS induces neither
more macular thickening nor higher rates of cystoid macular edema than
conventional cataract surgery[19-22].
Nevertheless, there is a lack of scientific evidence regarding the effect of
FLALS on the optic nerve head. Our results show a small but statistically
significant increase in RNFL and BMO-MRW 1mo after surgery with a tendency to
return to baseline values after 6mo. A slight increase in MT was also observed
one month after surgery, with the same tendency towards reduction after 6mo.
This increase in MT is similar to that published in previous studies[19-25]. The fact that RNFL and BMO-MRW
values were not reduced after surgery suggests that FLALS does not have a
negative impact on the structural status of the optic nerve head. Zhang et
al[15] evaluated the RNFL before and after a
different femtosecond laser-assisted procedure, femto LASIK, which causes
greater IOP elevation, and they found no significant changes after surgery
(mean RNFL 106.34±10.45 preoperatively, 106.01±10.35 after 1mo, P>0.05).
This is especially relevant for patients with glaucoma, in whom femtosecond
laser-assisted procedures have been contraindicated so far.
There is a concern about whether the increase in
IOP caused by the suction ring during the femtosecond laser procedure could be
long and/or intense enough to damage the optic nerve head. Several authors have
studied the changes in IOP induced by the different femtosecond platforms,
demonstrating that those with flat and curved interfaces (LenSx and Victus) cause
greater IOP increases than those with liquid interfaces (Ziemer LDV Z8 and
Catalys)[26-29]. The estimated
IOP increase for Victus, Ziemer LDV Z8 and Catalys is 42, 30 and <
The effect of FLALS on the measurements of RNFL,
BMO-MRW and MT is the combination of the effect of 1) the femtosecond laser
procedure itself, which causes the IOP increase previously discussed; 2) the effect
of the phacoemulsification procedure, which also causes an intraoperative IOP
increase; 3) the intraocular lens (IOL) implantation. Interestingly, the IOP
levels reached during phacoemulsification have not been studied by many
authors. A prospective randomized study of 80 eyes by Vasavada et al[35] demonstrated that the maximum IOP during
phacoemulsification was 69±3.0 and 85±
This study is subject to certain limitations, such
as the relatively small sample size and the fact that there was no control
group. Furthermore, given the fact that the thickening observed in the three
OCT parameters decreased over time, with a long-term surveillance the results
shall show if the values return to baseline eventually or if the slight
increase remains stable after six months, suggesting that there are new
baseline values after FLALS.
In conclusion, this study showed that FLALS does
not seem to cause any deterioration in the structural status of the optic nerve
head in healthy eyes. Since the postoperative values of RNFL, BMO-MRW and MT
are slightly superior to the preoperative values, new baseline measurements
should be acquired after FLALS in order to continue the follow-up in an
accurate manner. Further studies are necessary to assess if there are any long
term implications and/or different results in ocular hypertension and glaucoma
patients.
ACKNOWLEDGEMENTS
The abstract of this study was presented in the 13th
Congress of the European Glaucoma Society (EGS).
Conflicts of Interest: Reñones
J, None; Estévez B, None; González-Martín JM, None; Carreras
H is consultant for Alcon; Loro JF, None; Antón A is
consultant for Santen, Thea, Aerie, Alcon and Bausch+Lomb.
REFERENCES
|
1 Al-Mohtaseb Z, He X, Yesilirmak N, Waren D,
Donaldson KE. Comparison of corneal endothelial cell loss between two
femtosecond laser platforms and standard phacoemulsification. J Refract Surg
2017;33(10):708-712. |
|
|
|
|
|
2 Chen XY, Chen KL, He JL, Yao K. Comparing the
curative effects between femtosecond laser-assisted cataract surgery and
conventional phacoemulsification surgery: a meta-analysis. PLoS One
2016;11(3): e0152088. |
|
|
|
|
|
3 Popovic M, Campos-Möller X, Schlenker MB, Ahmed
II. Efficacy and safety of femtosecond laser-assisted cataract surgery
compared with manual cataract surgery: a meta-analysis of 14 567 eyes.
Ophthalmology 2016;123(10):2113-2126. |
|
|
|
|
|
4 Kanellopoulos AJ, Asimellis G. Standard manual
capsulorhexis/Ultrasound phacoemulsification compared to femtosecond
laser-assisted capsulorhexis and lens fragmentation in clear cornea small
incision cataract surgery. Eye Vis (Lond) 2016;3:20. |
|
|
|
|
|
5 Mayer WJ, Klaproth OK, Hengerer FH, Kohnen T. Impact
of crystalline lens opacification on effective phacoemulsification time in
femtosecond laser-assisted cataract surgery. Am J Ophthalmol
2014;157(2):426-432.e1. |
|
|
|
|
|
6 Abell RG, Kerr NM, Howie AR, Mustaffa Kamal MA,
Allen PL, Vote BJ. Effect of femtosecond laser-assisted cataract surgery on
the corneal endothelium. J Cataract Refract Surg 2014;40(11):1777-1783. |
|
|
|
|
|
7 Rivera RP, Hoopes PC Jr, Linn SH, Hoopes PC.
Comparative analysis of the performance of two different platforms for
femtosecond laser-assisted cataract surgery. Clin Ophthalmol
2016;10:2069-2078. |
|
|
|
|
|
8 Chen M, Swinney C, Chen M. Comparing the intraoperative
complication rate of femtosecond laser-assisted cataract surgery to
traditional phacoemulsification. Int J Ophthalmol 2015;8(1):201-203. |
|
|
|
|
|
9 Roberts TV, Lawless M, Sutton G, Hodge C. Update
and clinical utility of the LenSx femtosecond laser in cataract surgery. Clin
Ophthalmol 2016;10:2021-2029. |
|
|
|
|
|
10 Taravella MJ, Meghpara B, Frank G, Gensheimer W,
Davidson R. Femtosecond laser-assisted cataract surgery in complex cases. J
Cataract Refract Surg 2016;42(6):813-816. |
|
|
|
|
|
11 Martin AI, Hughes P, Hodge C. First report of
femtosecond laser cataract surgery in a nanophthalmic eye. Clin Exp
Ophthalmol 2014;42(5):501-502. |
|
|
|
|
|
12 Hou JH, Crispim J, Cortina MS, Cruz Jde L.
Image-guided femtosecond laser-assisted cataract surgery in Peters anomaly
type 2. J Cataract Refract Surg 2015;41(11):2353-2357. |
|
|
|
|
|
13 Grewal DS, Basti S. Intraoperative reverse
pupillary block during femtosecond laser-assisted cataract surgery in a
patient with phacomorphic angle closure. J Cataract Refract Surg
2014;40(11):1909-1912. |
|
|
|
|
|
14 Kránitz K, Takács ÁI, Gyenes A, Filkorn T,
Gergely R, Kovács I, Nagy ZZ. Femtosecond laser-assisted cataract surgery in management
of phacomorphic glaucoma. J Refract Surg 2013;29(9):645-648. |
|
|
|
|
|
15 Zhang J, Zhou YH, Zheng Y, Liu Q, Zhai CB, Wang Y.
Effect of suction on macular and retinal nerve fiber layer thickness during
femtosecond lenticule extraction and femtosecond laser-assisted laser in situ
keratomileusis. J Cataract Refract Surg 2014;40(12):1994-2001. |
|
|
|
|
|
16 Hosny M, Zaki RM, Ahmed RA, Khalil N, Mostafa
HM. Changes in retinal nerve fiber layer thickness following mechanical
microkeratome-assisted versus femtosecond laser-assisted LASIK. Clin
Ophthalmol 2013;7:1919-1922. |
|
|
|
|
|
17 Sharma P, Sample PA, Zangwill LM, Schuman JS. Diagnostic
tools for glaucoma detection and management. Surv Ophthalmol
2008;53(Suppl1):S17-S32. |
|
|
|
|
|
18 Rebolleda G, Casado A, Oblanca N, Muñoz-Negrete
FJ. The new Bruch's membrane opening-minimum rim width classification
improves optical coherence tomography specificity in tilted discs. Clin
Ophthalmol 2016;10:2417-2425. |
|
|
|
|
|
19 Ecsedy M, Miháltz K, Kovács I, Takács A, Filkorn
T, Nagy ZZ. Effect of femtosecond laser cataract surgery on the macula. J
Refract Surg 2011;27(10):717-722. |
|
|
|
|
|
20 Nagy ZZ, Ecsedy M, Kovács I, Takács Á, Tátrai E,
Somfai GM, Cabrera DeBuc D. Macular morphology assessed by optical coherence
tomography image segmentation after femtosecond laser-assisted and standard
cataract surgery. J Cataract Refract Surg 2012;38(6):941-946. |
|
|
|
|
|
21 Levitz L, Reich J, Roberts TV, Lawless M.
Incidence of cystoid macular edema: femtosecond laser-assisted cataract
surgery versus manual cataract surgery. J Cataract Refract Surg
2015;41(3):683-686. |
|
|
|
|
|
22 Conrad-Hengerer I, Hengerer FH, Al Juburi M,
Schultz T, Dick HB. Femtosecond laser-induced macular changes and anterior
segment inflammation in cataract surgery. J Refract Surg 2014;30(4):222-226. |
|
|
|
|
|
23 Asena BS, Karahan E, Kaskaloglu M. Retinal and choroidal
thickness after femtosecond laser-assisted and standard phacoemulsification.
Clin Ophthalmol 2017;11:1541-1547. |
|
|
|
|
|
24 Mursch-Edlmayr AS, Bolz M, Luft N, Ring M,
Kreutzer T, Ortner C, Rohleder M, Priglinger SG. Intraindividual comparison
between femtosecond laser-assisted and conventional cataract surgery. J
Cataract Refract Surg 2017;43(2):215-222. |
|
|
|
|
|
25 Yu YH, Chen XY, Hua HX, Wu MH, Lai KR, Yao K.
Comparative outcomes of femtosecond laser-assisted cataract surgery and manual
phacoemusification: a six-month follow-up. Clin Exp Ophthalmol
2016;44(6):472-480. |
|
|
|
|
|
26 Schultz T, Conrad-Hengerer I, Hengerer FH, Dick HB.
Intraocular pressure variation during femtosecond laser-assisted cataract
surgery using a fluid-filled interface. J Cataract Refract Surg
2013;39(1):22-27. |
|
|
|
|
|
27 Talamo JH, Gooding P, Angeley D, Culbertson WW,
Schuele G, Andersen D, Marcellino G, Essock-Burns E, Batlle J, Feliz R,
Friedman NJ, Palanker D. Optical patient interface in femtosecond
laser-assisted cataract surgery: contact corneal applanation versus liquid
immersion. J Cataract Refract Surg 2013;39(4):501-510. |
|
|
|
|
|
28 Wu BM, Williams GP, Tan A, Mehta JS. A
comparison of different operating systems for femtosecond lasers in cataract
surgery. J Ophthalmol 2015;2015:616478. |
|
|
|
|
|
29 Williams GP, Ang HP, George BL, Liu YC, Peh G,
Izquierdo L, Tan DT, Mehta JS. Comparison of intra-ocular pressure changes
with liquid or flat applanation interfaces in a femtosecond laser platform.
Sci Rep 2015;5:14742. |
|
|
|
|
|
30 Baig NB, Cheng GP, Lam JK, Jhanji V, Chong KK,
Woo VC, Tham CC. Intraocular pressure profiles during femtosecond
laser-assisted cataract surgery. J Cataract Refract Surg 2014;40(11):
1784-1789. |
|
|
|
|
|
31 Kerr NM, Abell RG, Vote BJ, Toh T. Intraocular
pressure during femtosecond laser pretreatment of cataract. J Cataract
Refract Surg 2013;39(3):339-342. |
|
|
|
|
|
32 Ibarz M, Hernández-Verdejo JL, Bolívar G, Tañá
P, Rodríguez-Prats JL, Teus MA. Porcine model to evaluate real-time
intraocular pressure during femtosecond laser cataract surgery. Curr Eye Res
2016;41(4):507-512. |
|
|
|
|
|
33 Sperl P, Strohmaier C, Kraker H, Motloch K,
Lenzhofer M, Moussa S, Reitsamer HA. Intraocular pressure course during the
femtosecond laser-assisted cataract surgery in porcine cadaver eyes. Invest
Ophthalmol Vis Sci 2017;58(14):6457-6461. |
|
|
|
|
|
34 Darian-Smith E, Howie AR, Abell RG, Kerr N,
Allen PL, Vote BJ, Toh T. Intraocular pressure during femtosecond laser
pretreatment: comparison of glaucomatous eyes and nonglaucomatous eyes. J
Cataract Refract Surg 2015;41(2):272-277. |
|
|
|
|
|
35 Vasavada V, Raj SM, Praveen MR, Vasavada AR,
Henderson BA, Asnani PK. Real-time dynamic intraocular pressure fluctuations
during microcoaxial phacoemulsification using different aspiration flow rates
and their impact on early postoperative outcomes: a randomized clinical
trial. J Refract Surg 2014;30(8):534-540. |
|
|
|
|
|
36 Khng C, Packer M, Fine IH, Hoffman RS, Moreira
FB. Intraocular pressure during phacoemulsification. J Cataract Refract Surg
2006;32(2): 301-308. |
|
|
|
|
|
37 Mauschitz MM, Roth F, Holz FG, Breteler MMB,
Finger RP. The impact of lens opacity on SD-OCT retinal nerve fiber layer and
bruch's membrane opening measurements using the anatomical positioning system
(APS). Invest Ophthalmol Vis Sci 2017;58(5):2804-2809. |
|
|
|
|
|
38 Celik E, Cakır B, Turkoglu EB, Doğan E, Alagoz
G. Effect of cataract surgery on subfoveal choroidal and ganglion cell
complex thicknesses measured by enhanced depth imaging optical coherence
tomography. Clin Ophthalmol 2016;10:2171-2177. |
|
|
|
|
|
39 Barrancos C, Rebolleda G, Oblanca N, Cabarga C,
Muñoz-Negrete FJ. Changes in lamina cribrosa and prelaminar tissue after deep
sclerectomy. Eye (Lond) 2014;28(1):58-65. |
|
|
|
|
|
40 Yang HS, Lee J, Choi S. Ocular biometric
parameters associated with intraocular pressure reduction after cataract
surgery in normal eyes. Am J Ophthalmol 2013;156(1):89-94.e1. |
|
|
|
|
|
41 Poley BJ, Lindstrom RL, Samuelson TW, Schulze R
Jr. Intraocular pressure reduction after phacoemulsification with intraocular
lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a
causal relationship between the natural lens and open-angle glaucoma. J
Cataract Refract Surg 2009;35(11):1946-1955. |
|
|
|
|