
Citation: Noh HJ, Kim ST. Combined treatment of phacoemulsification
and single-port limited pars plana vitrectomy in acute angle-closure glaucoma.
Int J Ophthalmol 2019;12(6):974-979
DOI:10.18240/ijo.2019.06.15
·Clinical
Research·
Combined treatment of phacoemulsification and single-port
limited pars plana vitrectomy in acute angle-closure glaucoma
Ha Jeong
Noh, Seong Taeck Kim
Department of Ophthalmology, Chosun University Hospital, Dong-gu, Gwang-ju
501-717, Republic of Korea
Correspondence to: Seong Taeck Kim, Department of Ophthalmology, Chosun University Hospital,
365 Pilmundaero, Dong-gu, Gwang-ju 501-717, Republic of Korea.
s20age@hanmail.net
Received:
Abstract
AIM: To evaluate the efficacy of
combined treatment of phacoemulsification (PE) and micro-incisional single-port
transconjunctival limited pars plana vitrectomy (PPV) in acute angle-closure
glaucoma (AACG).
METHODS: A retrospective study included
26 patients who underwent PE diagnosed with AACG. Among them, 16 patients (16
eyes) underwent PE alone, 10 patients (10 eyes) underwent combined limited
vitrectomy and PE. Then we compared intraocular pressure (IOP), anterior
chamber angle, anterior chamber depth, central corneal thickness and corneal
endothelial cell count before and after surgery, and effective PE time during
cataract surgery.
RESULTS: Effective PE time was shorter
in the combined surgery group than in the single surgery group (P=0.040).
There was no statistically significant difference in IOP and best-corrected
visual acuity between the two groups postoperatively. At 6mo postoperatively,
there was no difference in the anterior chamber angle, anterior chamber depth,
and central corneal thickness between two groups, but corneal endothelial cell
count was higher in the combined surgery group than in the single surgery group
(P=0.046). No complication such as vitreoretinal disease,
endophthalmitis, bullous keratopathy was noted.
CONCLUSION: Combined micro-incisional
single-port transconjunctival limited PPV and PE are more effective and safer
than PE alone because of less operation time and fewer complications for
management of AACG.
KEYWORDS: limited
vitrectomy; phacoemulsification; acute angle-closure glaucoma
DOI:10.18240/ijo.2019.06.15
Citation: Noh HJ, Kim ST. Combined treatment of phacoemulsification
and single-port limited pars plana vitrectomy in acute angle-closure glaucoma.
Int J Ophthalmol 2019;12(6):974-979
INTRODUCTION
Acute angle-closure glaucoma (AACG) is caused by the relative pupillary
block or a sudden closure of the anterior chamber angle, which result in a
severe intraocular pressure (IOP) rise. Laser iridotomy is a safe and effective
nonsurgical treatment to resolve the relative pupillary block and to widen the
anterior chamber angle[1]. However, laser
iridotomy is not always effective in controlling IOP, and in some cases it
often fails to control IOP[2]. One of the causes
of this result is that the lens pushes the peripheral iris forward, making the
anterior chamber shallower[3]. Old patients with
AACG are more likely to be associated with cataracts. In this case,
phacoemulsification (PE) may result in the increase of the anterior chamber
depth and the decrease of IOP. So, many studies have reported that cataract
surgery in patients with AACG can lower IOP[4].
However, cataract surgery in patients with AACG has difficulties due to
anatomical problems such as shallow anterior chamber, high IOP, small pupil,
corneal edema, and weak zonule[5]. High-vitreous
pressure in such eyes can result in capsulorhexis extension, iris prolapse,
zonular dialysis or posterior capsular rupture with subsequent vitreous loss
and possibly suprachoroidal haemorrhage[6].
Extreme caution is recommended when operating on eyes with shallow anterior
chamber. Therefore, cataract surgery in patients with AACG results in prolonged
PE ultrasound time and increased corneal endothelial damage[7-8].
If the vitreous pressure is lower before performing cataract surgery, the
cataract surgery can be performed much easier and safer. In 2001, Chang[9] performed vitreous aspiration to lower the vitreous
pressure using a 20-gauge vitrectomy cutter in PE for management of AACG.
However, vitreous aspiration could be a good technique in vitrectomized eye,
but could fail to aspirate the vitreous in non-vitrectomized eye. If vitreous
was aspirated strongly, the risk of complications such as retinal detachment
and vitreous hemorrhage were increased. In addition, vitreous aspiration was
required a large sized needle and conjunctival peritomy was necessary to suture
the sclerotomy, which may affect the poor outcome of glaucoma surgery in the
future[10]. Currently, micro-incisional
transconjunctival sutureless pars plana vitrectomy (PPV) is widely used in
vitreoretinal surgery due to the development of the instrument. The performance
of the PPV with a higher cutting rate and the smaller diameter of vitrectomy
cutter has been improved so that the vitreous traction can be effectively reduced
and the possibility of making on iatrogenic retinal tear can be reduced. Dada et
al[11] in 2007 described a
single-port-limited PPV without infusion cannula using a 23-gauge vitrectomy probe
inserted through a sclerotomy incision
SUBJECTS AND METHODS
Ethical Approval The study protocol was approved by
the institutional review board (IRB) of the Chosun University Hospital and
followed the Declaration of Helsinki. All participants signed an informed
consent. And all participants didn’t receive a stipend.
Study Design We retrospectively reviewed the
medical records of 26 patients who underwent PE to manage AACG from March 2011
to October 2016. Of these, 16 patients (16 eyes) underwent PE alone, and 10 patients (10
eyes) underwent combined pars plana vitrectomy and phacoemulsification
(PPV+PE). Secondary glaucoma, such as neovascular glaucoma and uveitic
glaucoma, was excluded from the study. Patients with corneal disease and
vitreoretinal disease that may affect visual acuity were also excluded.
Surgical Technique In patients with AACG, to lower IOP,
we performed laser iridotomy. We performed cataract surgery emergently in AACG
patients who have difficulty performing laser iridotomy due to severe corneal
edema, and can’t lower IOP after laser iridotomy and medication. Surgery was
performed by one surgeon (Kim ST) who had been adopting the use of this
technique for several years. Retrobulbar local anaesthetic mixture of lidocaine
1% and bupivacaine 0.5% was injected. In combined surgery group, limited PPV
was performed prior to the PE to lower posterior vitreous pressure. Patient
selection for PE alone or combined surgery is conducted randomly selection
without specific criteria. A 25-gauge trocar was inserted at a distance of
Retrospective Chart Review Preoperative and demographic data
were retrieved. Preoperative and postoperative best-corrected visual acuity,
IOP, and complications were reviewed. Recorded preoperative and postoperative
Snellen best-corrected visual acuity was converted to logarithmic minimal angle
of resolution (logMAR) units for statistical analysis. Preoperative axial
length had been measured by IOL Master® (Carl Zeiss Meditec, Dublin,
CA, USA). Preoperative and postoperative anterior chamber depth, and central
corneal thickness had been measured by Visante OCT® (Carl Zeiss
Meditec Inc., Dublin, CA, USA). Anterior chamber angle, anterior chamber depth,
central corneal thickness and corneal endothelial cell count were measured at
6mo postoperatively. Postoperative corneal endothelial cell counts were
measured by non-contact specular microscope (SP-2000P®, Topcon,
Tokyo, Japan). It was difficult to measure the preoperative corneal endothelial
cell counts due to corneal edema. Intraoperative data such as EPT and
intraoperative complications, if any, were collected. Postoperative
complications such as corneal edema, hyphema, and posterior capsular rupture
were also reviewed.
Statistical Analysis Statistical analysis was performed
using SPSS 21.0 for windows (version 17.0 SPSS Inc., Chicago, IL, USA).
Means±standard deviations (SD) were used for descriptive data. Statistical
evaluation was based on an independent samples t-test, Mann-Whitney U-test
and Pearson’s Chi-square test. P<0.05 was considered significant.
RESULTS
The mean age of the patients was 68.69±7.46y in the PE single surgery
group, and 67.70±9.42y in the PPV+PE combined surgery group (P=0.769).
The mean preoperative IOP was 52.56±
Table 1 Preoperative clinical characteristics
mean±SD
|
Parameters |
PE (n=16) |
PPV+PE (n=10) |
P |
|
Age (y) |
68.69±7.46 |
67.70±9.42 |
0.769 |
|
Sex (M/F) |
3/13 |
1/9 |
0.566 |
|
IOP (mm Hg) |
52.56±7.14 |
51.40±9.56 |
0.726 |
|
BCVA (logMAR) |
1.78±0.44 |
1.67±0.41 |
0.604 |
|
Axial length (mm) |
22.16±0.70 |
22.26±0.73 |
0.725 |
|
ACD (mm) |
2.17±0.24 |
2.07±0.30 |
0.339 |
|
CCT (μm) |
611.50±61.71 |
628.70±31.76 |
0.425 |
IOP: Intraocular pressure; BCVA: Best corrected visual acuity; ACD:
Anterior chamber depth; CCT: Central corneal thickness; PE:
Phacoemulsification; PPV: Pars plana vitrectomy.

Figure 1 Effective phacoemulsification (PE) time between PE and combined
pars plana vitrectomy and phacoemulsification (PPV+PE) Effective PE time was shorter in the
PPV+PE than in the PE (P=0.040).
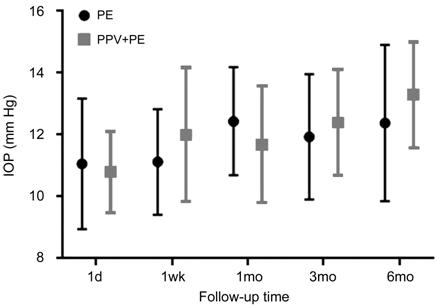
Figure 2 Postoperative intraocular pressure between phacoemulsification
(PE) and combined pars plana vitrectomy and phacoemulsification (PPV+PE) There was no significant difference
between two groups (by Mann-Whitney U-test).
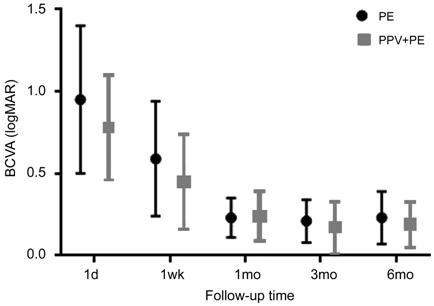
Figure 3 Postoperative best-corrected visual acuity between phacoemulsification
(PE) and combined pars plana vitrectomy and phacoemulsification (PPV+PE) There was no significant difference
between two groups (by Mann-Whitney U-test).
Table 2 Postoperative clinical characteristics
mean±SD
|
Parameters |
PE (n=16) |
PPV+PE (n=10) |
P |
|
ACA (degree) |
18.50±4.41 |
17.80±4.05 |
0.689 |
|
ACD (mm) |
3.08±0.36 |
3.27±0.41 |
0.143 |
|
CCT (μm) |
516.56±16.90 |
513.60±21.20 |
0.697 |
|
ECC |
1912.75±457.72 |
2317.70±510.29 |
0.046 |
ACA: Anterior chamber angle; ACD: Anterior chamber depth; CCT: Corneal
central thickness; ECC: Corneal endothelial cell count; PE:
Phacoemulsification; PPV: Pars plana vitrectomy.
Table 3 Complication
n (%)
|
Complication |
PE (n=16) |
PPV+PE (n=10) |
|
Intraoperative |
|
|
|
Iris prolapse |
4 (25) |
1 (10) |
|
Capsulorhexis extension |
2 (12.5) |
1 (10) |
|
PC rupture |
2 (12.5) |
0 |
|
Zonulolysis |
1 (6.25) |
0 |
|
Dropped nuclear fragment |
0 |
0 |
|
Postoperative |
|
|
|
Corneal edema |
12 (75) |
5 (50) |
|
Cyclitic membrane |
6 (37.5) |
3 (30) |
|
Hyphema |
2 (12.5) |
1 (10) |
|
Retinal detachment |
0 |
0 |
|
Endophthalmitis |
0 |
0 |
|
Bullous keratopathy |
0 |
0 |
PC: Posterior capsule; PE: Phacoemulsification; PPV: Pars plana vitrectomy.
DISCUSSION
In the treatment of AACG, it is important to restore angle to prevent the
peripheral anterior synechia[13]. Laser iridotomy
is a treatment of choice for AACG. However, Aung et al[14] reported that long-term normal IOP range was
maintained only in 41.8% of Asian eyes when laser iridotomy was performed for AACG.
The reason why IOP may not well controlled after laser iridotomy is that the
iris is thick and dark brown color in Asian. Therefore, when laser iridotomy is
performed, there is a high possibility of closing the trabecular meshwork
because of inflammatory response and dispersion of iris pigment. The second
reason is that the lens becomes thicker with aging, which is likely to cause an
angle-closure anatomically[15]. For this reason,
there has been a report that it is possible to lower IOP by cataract surgery
that can remove the factor of phacomorphic characteristic[16].
Jacobi et al[17] reported that high IOP
was effectively controlled in 75% of patients with AACG by cataract surgery
alone. It reported that 1.40±
Corneal endothelial cell loss is a main concern due to the closer distance
between PE tip and corneal endothelium. In addition, during performing cataract
surgery in AACG, the ultrasound PE time may be long, and the corneal
endothelial cell damage increases[19-21].
Severe corneal endothelial damage can result in corneal edema, which can lead
to permanent visual loss[22]. The normal corneal
endothelial cell density is 2500 cells/mm2, and corneal edema and
corneal decompensation can occur when the corneal endothelial cell density is
reduced under 500 cells/mm2[23]. It is known that
corneal endothelial cell density decreases from 0.89% to 1% per year even
naturally, and the rate of decrease is about 2% per year after cataract surgery[24]. Factors affecting corneal endothelial cell damage in
cataract surgery include patient-related factors such as age and severity of cataract,
and operative factors such as surgeon factor, viscoelastic materials,
ultrasound emulsification time, and ultrasound emulsification energy[25]. The lower the anterior chamber depth is, the higher
damage of the corneal endothelial cell becomes. Igarashi et al[26] reported corneal endothelial damage by the surgical
instrument, which is related to anterior chamber depth. Lee et al[27] reported the corneal endothelial cell density would
be less than 1900 cells/mm
Therefore, performing limited PPV to remove small volume of vitreous is
considered the only possible way to successfully deepen the anterior chamber. This
technique makes it easier to perform cataract surgery and reduce the incidence
of complications. In addition, the risk of corneal endothelial cell damage can
be reduced. In comparison with that, in our study, corneal endothelial cell
density in the single surgery group was 1912 cells/mm2 at
postoperative 6mo, corneal endothelial cell density of the patients in the
combined surgery group are more than in single surgery group. Although it may
be more reasonable to compare corneal endothelial cell density before and after
surgery, but it is difficult to measure preoperative corneal endothelial cell
density because of corneal edema in glaucoma attack. Another factor affecting
corneal endothelial cell damage during cataract surgery is the total amount of
PE ultrasound used. If the effective PE time is shortened during cataract
surgery, the amount of ultrasound energy can be reduced to minimize
complications such as corneal edema and corneal endothelial cell loss[28]. Baradarb-Rafii et al[29]
reported that total ultrasound use during cataract surgery was significantly
associated with loss of corneal endothelial cell. In our study, effective
ultrasound time was shorter in the combined surgery group than in the single
surgery group, which means that the combined limited PPV is effective for
corneal endothelial cell protection. Thus, limited PPV is an effective method
to prevent problems occurring in PE alone for AACG. In addition, recent
micro-incisional limited PPV may be performed without a conjunctival peritomy
and suture. Therefore, even if glaucoma surgery is necessary in the future,
preservation of the conjunctiva does not interfere with the success rate of the
glaucoma surgery. The disadvantage of this combined surgery is the possibility
of retinal tear or retinal detachment due to traction from PPV. However,
micro-incisional PPV, which has been widely used recently, has been decreasing
the risk because of the increased cutting rate of vitreous cutter. In case of
having taken combined, it is recommended to perform fundus examination until
the first month after surgery to avoid retinal tears or retinal detachment.
There are some reports on partial PPV combined with cataract surgery without
any serious complication for malignant glaucoma[30].
The limitations of this study are the follows. First, the number of
patients is low. Second, this study is a retrospective study. Third, there can
be a possibility of the selection bias. Fourth, preoperative corneal
endothelial cell density could not be measured due to corneal edema. There have
been many reports on the effects of laser iridotomy and PE in AACG. However,
there has been no analysis on the effect of limited PPV combined with PE.
In this study, we compared the single PE, and the combined limited PPV and
PE. As a result, there was no significant difference in visual acuity, IOP,
anterior chamber depth, and central corneal thickness before and after surgery,
but the difference of corneal endothelial cell density measured at 6mo
postoperatively was statistically significant. In conclusion, we confirmed that
in patients with AACG, performing the micro-incisional single-port sutureless
limited PPV followed by PE is more effective than PE alone because of less
surgical complications and less corneal endothelial damage.
ACKNOWLEDGEMENTS
Authors’ contributions: All the authors contributed to the conception or design of the work, the
acquisition, analysis and interpretation of data, drafting the work, revising
it critically for important intellectual content and gave final approval of the
version to be published.
Foundation: Supported by
Research Fund from Chosun University, 2018.
Conflicts of Interest: Noh HJ, None; Kim ST, None.
REFERENCES