
Citation: Tang WY, Zhang T, Shu QM, Jiang CH, Chang Q, Zhuang H, Xu
GZ. Focal choroidal excavation complicated with choroidal
neovascularization in young and middle aged patients. Int J Ophthalmol
2019;12(6):980-984
DOI:10.18240/ijo.2019.06.16
·Clinical Research·
Focal
choroidal excavation complicated with choroidal neovascularization in young and
middle aged patients
Wen-Yi Tang1,2, Ting Zhang1,2,
Qin-Meng Shu1,2, Chun-Hui Jiang1, Qing Chang1,
Hong Zhuang1,2, Ge-Zhi Xu1, 2
1Department
of Ophthalmology, Eye and ENT Hospital of Fudan University, Shanghai 200031,
China
2Key
Laboratory of Visual Impairment, Restoration of Shanghai and Key Laboratory of
Myopia of State Health Ministry, Fudan University, Shanghai 200031, China
Co-first
authors: Wen-Yi Tang
and Ting Zhang
Correspondence
to: Hong
Zhuang. Department of Ophthalmology, Eye and ENT Hospital of Fudan University,
83 Fenyang Road, Shanghai 200031, China. zhuang_hong1008@126.com
Received:
Abstract
AIM: To investigate the clinical and optical coherence tomography (OCT)
features of focal choroidal excavation (FCE) complicated with choroidal
neovascularization (CNV) in young and middle aged patients.
METHODS: We performed a retrospective review of 26 patients
with FCE accompanied by CNV. All patients underwent a complete ophthalmic
examination. We analyzed the clinical characteristics of patients, focusing on
the spectral-domain OCT features. All patients received intravitreal injection
of anti-vascular endothelial growth factor (anti-VEGF) agents. And we assessed
the changes of central retinal thickness and best-corrected visual acuity
(BCVA) after anti-VEGF therapy.
RESULTS: The mean age of 26 patients was 35.5±7.3y (range,
21-48y). Of the 26 FCE lesions, 11 were located subfoveal, 6 were parafoveal,
and 9 were extrafoveal. The mean FCE depth was 129.8±50.3 μm, and the mean width was
901.3±306.0 μm. The
FCE depth was correlated positively with the width, but not correlated with age
or refractive error. CNV was located within the excavation (19 eyes) or
adjacent to the excavation (7 eyes). After anti-VEGF therapy, the central
retinal thickness was significantly reduced and the BCVA was significantly
improved. In the absorption process of subretinal fluid, we found that the
fluid in the excavations needed to be absorbed at the last. A small amount of
residual fluid could still be seen in a few deep excavations even after a
long-term follow-up.
CONCLUSION: FCE may be an important reason to cause CNV.
Especially in young patients with idiopathic CNV, we should pay attention to
the use of OCT to check the presence of FCE. Anti-VEGF therapy is generally
effective for CNV associated with FCE.
KEYWORDS: focal choroidal excavation;
choroidal neovascularization; optical coherence tomography
DOI:10.18240/ijo.2019.06.16
Citation: Tang WY, Zhang T, Shu QM, Jiang CH, Chang Q, Zhuang H, Xu
GZ. Focal choroidal excavation complicated with choroidal
neovascularization in young and middle aged patients. Int J Ophthalmol
2019;12(6):980-984
INTRODUCTION
Focal
choroidal excavation (FCE) is an idiopathic clinical entity, which can be
clearly displayed by spectral-domain optical coherence tomography (OCT). In
2006, Jampol et al[1] firstly reported a
case with unusual choroidal excavation at the macula using time-domain OCT. But
due to the limitation of imaging resolution and scanning depth, it’s difficult
to make precise analysis of this clinical entity using time-domain OCT. Until 2011,
Margolis et al[2] analyzed a series of 12
patients using spectral-domain OCT and firstly proposed the term “focal
choroidal excavation”. They defined FCE as an area of macular choroidal
excavation without evidence of posterior staphyloma or scleral ectasia. In
2014, our group reported a series of Chinese patients with FCE[3]. These reports suggested FCE may be a congenital
abnormality, and mainly diagnosed in young and middle-aged patients[2-4]. Most FCE lesions were found in
normal eyes, but a few cases were diagnosed with concurrent central serous
chorioretinopathy or choroidal neovascularization (CNV)[5-8].
There were
only a few literature reports about FCE complicated with CNV. Lee et al[9] studied 16 patients who had FCE complicated with CNV.
This case series had a wide range of age distribution (28 to 86y). And Xu et
al[10] reported a series of 12 patients. The
same limitation of these two studies was the small number of included patients.
Then Kuroda et al[11] analyzed the
characteristics of FCE in elderly patients (aged over 55y) with neovascular
age-related macular degeneration (AMD). However, it’s still unclear about the
detailed characteristics of FCE complicated with CNV in younger patients.
Therefore,
our study aims to investigate the clinical and OCT features of FCE complicated
with CNV in young and middle aged patients, and observe the efficacy of
anti-vascular endothelial growth factor (anti-VEGF) therapy.
SUBJECTS AND METHODS
Ethical
Approval We performed a retrospective review
of the patients with FCE accompanied by CNV, who visited Eye and ENT Hospital
of Fudan University from January 2015 to December 2016. The research followed
the tenets of the Declaration of Helsinki and was approved by the Ethics
Committee of Eye and ENT Hospital of Fudan University. All patients underwent a
complete ophthalmic examination, including slitlamp biomicroscopy, indirect
ophthalmoscopy and measurements of best-corrected visual acuity (BCVA),
refractive error and intraocular pressure. Ancillary tests included fundus
photography, spectral-domain OCT and fundus fluorescein angiography (FFA).
FCE was
defined as a macular lesion of choroidal excavation detected on spectral-domain
OCT without evidence of posterior staphyloma or scleral ectasia. The diagnosis of
CNV was based on the fundus manifestation, OCT and angiography findings. The
age range of included patients was from 20 to 50y. Exclusion criteria included
choroidal inflammation, AMD, and any history of ocular trauma or intraocular
surgery.
All OCT images
were obtained by a spectral-domain OCT instrument (Spectralis; Heidelberg
Engineering, Heidelberg, Germany). During the follow-up period, the eyes were
scanned by the eye-tracking-based follow-up function in Spectralis OCT. The
depth and width of each FCE was measured with a built-in caliper tool. The
distance between the center of FCE and the fovea was measured. According to the
distance measurement, the location of FCE was classified into subfoveal
(<200 μm), parafoveal (200-500 μm) and extrafoveal (>500 μm). The
positional relationship between CNV and FCE was evaluated. The central retinal
thickness was also manually measured with a built-in caliper tool. The central
retinal thickness at the fovea was measured from the vitreoretinal interface to
the inner border of retinal pigment epithelium (RPE).
All patients
received intravitreal injection of anti-VEGF agents (ranibizumab 0.5 mg).
Anti-VEGF treatment was performed on an as-needed basis, after either an
initial 2 or 3 consecutive injections or a single injection. Intravitreal
anti-VEGF injection was repeated, if an increase of central retinal thickness
was detected on follow-up OCT.
Statistical
Analysis The spherical equivalent of
refractive error was analyzed. Myopia was classified according to degree of
myopic diopter (D) as follows: low myopia (-0.5 to -3.0 D), moderate myopia
(-3.0 to -6.0 D), high myopia (greater or equal to -6.0 D). The measured
decimal visual acuity was converted to the logarithm of the minimum angle of
resolution (logMAR) for statistical analysis. The statistical analysis was
performed with Stata 11.0 statistical software (Stata Corporation, College
Station, TX, USA). The correlation between two parameters was analyzed by
Spearman correlation coefficient. The changes of central retinal thickness and
BCVA after anti-VEGF therapy were analyzed by Wilcoxon matched-pairs
signed-rank test. A two-tailed P value of <0.05 was considered
statistically significant.
RESULTS
A total of
26 patients (26 eyes) with FCE accompanied by CNV were included in this
retrospective study. All patients were Chinese, including 14 female patients
and 12 male patients. The mean age was 35.5±7.3y (range, 21-48y). These 26 eyes
included 5 emmetropic eyes, and 21 myopic eyes (mean -3.35±1.57 D; range, -1.0
to -6.5 D). Of the 21 myopic eyes, 7 were mildly myopic, 12 were moderately
myopic, and only 2 were highly myopic.
On fundus
photos, we could see the submacular hemorrhage and exudates caused by CNV
(Figure 1). FFA could show hyperfluorescent CNV and blocked fluorescence
corresponding to submacular hemorrhage (Figure 1). But the FCE lesion could not
be identified on fundus photos or FFA. In our series, all eyes had a single FCE
lesion detected by spectral-domain OCT. Of the 26 FCE lesions, 11 were located
subfoveally, 6 were parafoveal, and 9 were extrafoveal. The mean FCE depth was
129.8±50.3 μm (range, 60-247 μm), and the mean width was 901.3±306.0 μm (range,
437-1372 μm). The FCE depth was correlated positively with the width (r=0.60,
P<0.01). The FCE depth was not correlated with age (P=0.45) or
refractive error (P=0.38). CNV was located mainly within the excavation
(19 eyes) or adjacent to the excavation (7 eyes; Figure 1).
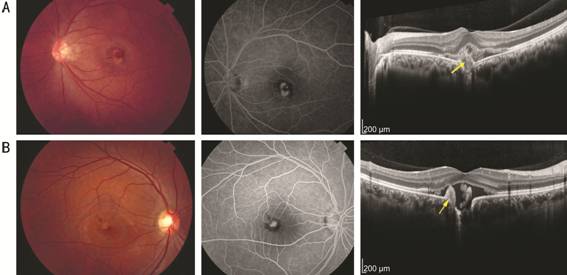
Figure 1
Fundus photos, FFA and OCT images of two patients at the time of
presentation The fundus photos of these two
patients both show submacular hemorrhage and exudates caused by CNV. FFA images
of these two patients show hyperfluorescent CNV and blocked fluorescence
corresponding to submacular hemorrhage. The OCT images reveal the positional
relationship between CNV and FCE.
A: The CNV lesion is located mainly within the excavation; B: The CNV
lesion is adjacent to the excavation. The hyperreflective CNV lesions on the
OCT images are indicated by arrows.
All patients
received intravitreal injection of anti-VEGF agents. Twenty-six patients
respectively received 1 injection (4 patients), 2 injections (9 patients), 3
injections (9 patients), and 4 injections (4 patients). The mean follow-up
duration was 14.0mo (range, 5-22mo). All of patients responded well to
intravitreal anti-VEGF therapy, the CNV lesions regressed and the macular edema
resolved. After the patients received anti-VEGF therapy, the central retinal
thickness was significantly reduced from baseline (304.0±93.4 μm) to last visit
(180.8±33.7 μm; P<0.001). The mean BCVA at baseline (0.43±0.24
logMAR) was significantly improved to 0.21±0.20 logMAR at the last visit (P<0.001).
In the absorption process of subretinal fluid, we found that the fluid in the
excavations needed to be absorbed at the last (the representative case was
shown in Figure 2). A small amount of residual fluid could still be seen in a
few deep excavations (4 patients) even after a long-term follow-up (the representative
case was shown in Figure 3).
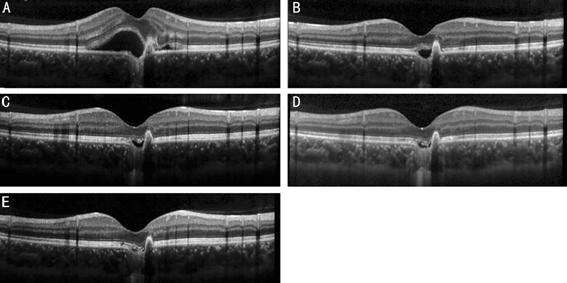
Figure 2 OCT
scans from a 28-year-old male patient showing the gradual absorption of
subretinal fluid after 3 anti-VEGF injections A: At the initial visit, the OCT
image revealed a CNV lesion adjacent to the excavation, with obvious subretinal
fluid; B: One month after the 1st intravitreal injection, the
subretinal fluid was reduced. But the choroidal excavation was full of fluid.
C: One month after the 2nd intravitreal injection; D: One month
after the 3rd intravitreal injection, the residual fluid in the
excavation was still seen; E: Three months after the 3rd
intravitreal injection, the fluid in the excavation was completely absorbed at
last.
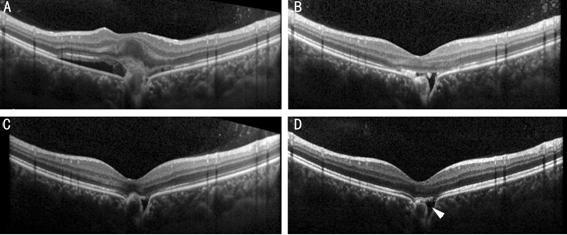
Figure 3 OCT
scans from a 29-year-old female patient who received 2 intravitreal injections
of anti-VEGF agents A: At the initial visit, the OCT
image revealed a CNV lesion in a deep choroidal excavation, with obvious
subretinal fluid; B: One month after the 1st intravitreal injection,
the CNV atrophied and the subretinal fluid was reduced; C: Three months after
the 2nd intravitreal injection, the subretinal fluid continued to be
absorbed, but the residual fluid in the excavation was still seen; D: Twelve
months after the 2nd intravitreal injection, a small amount of
residual fluid (arrowhead) could still be seen in the deep excavation.
DISCUSSION
In this
study, we reported the clinical characteristics of FCE complicated with CNV in
young and middle aged patients, focusing on the spectral-domain OCT features.
The spectral-domain OCT provides high-resolution image of the retina and
choroid[12-13], therefore it’s
able to clearly display the FCE lesion. FCE is considered to be a congenital
abnormality, arising from the focal defect of chorioretinal differentiation[3,8]. But sometimes FCE could be
acquired from choroidal inflammation or infection[14-16], so we have excluded these secondary factors in our
study.
Kuroda et
al[11] previously reported the mean depth of
FCE in elderly patients was 53.3 μm (range, 22-106 μm). But our study found
that the depth of FCE was 129.8 μm (range, 60-247 μm), which was greater than
the data reported by Kuroda et al[11]. The
inconsistence of FCE depth could be explained by age difference between the two
studies. The RPE atrophy and choroidal thinning occurred in elderly patients[17-19], which may influence the
morphology of the FCE and shallow the depth of the FCE. Therefore, our study of
younger patients can avoid the aging influence on the characteristics of
FCE.
In our
study, we analyzed the proportion of different degrees of myopia in the
patients who had both FCE and CNV. We found most eyes were emmetropic or mildly
to moderately myopic, and only a few were highly myopic. The results indicated
that the formation of FCE was different from posterior scleral staphyloma in
high myopia.
All of CNV
lesions grew within the excavation or near the margin of excavation. This close
positional relationship suggested that the structure of FCE play an important
role in the development of CNV. Choroidal excavation can result in focal
choroidal thinning, and then may induce ischemic changes and development of CNV[6]. Another explanation is that the Bruch membrane may be
impaired in the area of choroidal excavation[9].
The degeneration and break of the Bruch membrane can lead to the CNV formation[20-21]. Thus, the young patients
previously diagnosed as idiopathic CNV may be reconsidered, and OCT can be used
to check whether FCE exists.
Due to the
limitation of this retrospective study, the anti-VEGF treatment did not follow
a consistent standard protocol. Even though, we could see that all of patients
responded well to intravitreal anti-VEGF therapy and had good prognosis. And we
found the absorption process of subretinal fluid followed certain rules.
Because of the special structure of choroidal excavation, the fluid in the
excavations needed to be absorbed at the last. Although the CNV lesion
atrophied after treatment, a small amount of residual fluid still existed in a
few deep excavations after a long-term follow-up.
In
conclusion, FCE may be an important reason to cause CNV. Especially in young
patients with idiopathic CNV, we should pay attention to the use of OCT to
check the presence of FCE. Anti-VEGF therapy is generally effective for CNV
associated with FCE.
ACKNOWLEDGEMENTS
Foundations:
Supported by
the National Natural Science Foundation for Young Scholar of China
(No.81600739); the Shanghai Hospital Development Center (No.SHDC12016116); the
Science and Technology Commission of Shanghai Municipality (No.16411953700).
Conflicts of
Interest: Tang WY, None; Zhang T, None; Shu QM, None; Jiang CH, None; Chang
Q, None; Zhuang H, None; Xu GZ, None.
REFERENCES