
Citation: Lai J, Chen K, Shi HM, Zhuang L,
Zhou X, Xiao JJ, Li Y, Chen BB, Wang QP. B-scan ultrasound and cytology of the vitreous in primary
central nervous system lymphoma with vitreoretinal involvement. Int J
Ophthalmol 2019;12(6):1001-1007
DOI:10.18240/ijo.2019.06.20
·Clinical Research·
B-scan
ultrasound and cytology of the vitreous in primary central nervous system
lymphoma with vitreoretinal involvement
Jie Lai1, Kun Chen2, Hui-Min Shi3,
Lin Zhuang4, Xian Zhou1, Jian-Jiang Xiao3, Yi
Li3, Bo-Bin Chen4, Qing-Ping Wang 1
1Department
of Ophthalmology, Huashan Hospital, Fudan University, Shanghai 200040, China
2Department
of Clinical Laboratory, Huashan Hospital, Fudan University, Shanghai 200040,
China
3Department
of Ophthalmology, North Huashan Hospital, Fudan University, Shanghai 200040,
China
4Department
of Hematology, Huashan Hospital, Fudan University, Shanghai 200040, China
Co-first authors: Jie Lai and Kun Chen
Correspondence to: Qing-Ping Wang. Department of Ophthalmology,
Huashan Hospital, Fudan University, Shanghai 200040, China.
wangqingping71@163.com; Bo-Bin Chen. Department of Hematology, Huashan
Hospital, Fudan University, Shanghai 200040, China. bbchen@fudan.edu.cn
Received:
Abstract
AIM: To evaluate the diagnostic value of B-scan ultrasound and explore the
cytological characteristics of patients with vitreoretinal lymphoma (VRL) and
primary central nervous system lymphoma (PCNSL).
METHODS: The clinical data and pathologic specimens from patients
with VRL diagnosed at the North Huashan Hospital from 2016 to 2017 were
retrospectively reviewed. The patients were diagnosed by slit lamp
ophthalmoscopy, B-scan ultrasound, cytology of the vitreous, which was obtained
by vitrectomy, and cytokine measurements of interleukin (IL)-10 and IL-6.
RESULTS: Twenty-six eyes (19.4%) out of 134 eyes of 67
patients (47 men and 20 women) with PCNSL were diagnosed with VRL by B-scan
ultrasound, and 14 eyes (10.4%) were diagnosed by slit lamp ophthalmoscopy.
Twenty-four eyes (17.9%) of 17 patients were confirmed as having VRL with
cytology. No difference in the association between intracranial lesion location
and ocular involvement was found. VRL patients had higher levels of vitreous
IL-10 and IL-10/IL-6 when compared with macular hole cases, but the difference
was not statistically significant.
CONCLUSION: A total of 25.4% of the PCNSL patients had VRL,
B-scan ultrasound examination had characteristic features and is recommended
over slit lamp ophthalmoscopy for the screening diagnosis of PCNSL with
intraocular involvement. Moreover, the cytological and immunohistochemical
analyses performed after 25-gauge diagnostic vitrectomy were accurate
diagnostic techniques.
KEYWORDS: primary central nervous lymphoma;
intraocular lymphoma; B-scan ultrasound; vitrectomy; interleukin-10
DOI:10.18240/ijo.2019.06.20
Citation: Lai J, Chen K, Shi HM, Zhuang L,
Zhou X, Xiao JJ, Li Y, Chen BB, Wang QP. B-scan ultrasound and cytology of the vitreous in primary
central nervous system lymphoma with vitreoretinal involvement. Int J
Ophthalmol 2019;12(6):1001-1007
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is a rare
non-Hodgkin lymphoma that occurs in the brain, pia mater (dura mater), spinal
cord and eye and accounts for 2%-6% of the incidence of intracranial tumors[1-2]. Because of
the aggressiveness and high pathological and morphological heterogeneity of
this disease, PCNSL exhibits a poor prognosis and a strong possibility of
recrudescence[3]. Although PCNSL is categorized as a rare disease,
there has been a significant increase in the incidence of PCNSL in the past two
decades, and together with glioma, they have become the two most common primary
brain tumors[4].
Intraocular involvement occurs in approximately 15%-25%
of PCNSL cases[5]. The most common phenotype of
intraocular involvement is vitreoretinal lymphoma (VRL). In the United States,
approximately 380 new VRL cases are reported each year[6].
VRL patients usually have blurred vision that affects their quality of life. In
the PCNSL Guidelines for Baseline Evaluation for Clinical Trials published by
the International PCNSL Collaborative Group (IPCG), slit lamp examination and
indirect ophthalmoscopy are suggested for evaluation in clinical practice,
whereas invasive examinations, including vitreous biopsy, subretinal
fine-needle aspiration biopsy (FNAB) and/or central serous biopsy, are
suggested as diagnostic techniques[7-8].
MYD88 gene analysis is a helpful ancillary tool for diagnosing VRL as well[9]. If involvement of the optic nerve is suspected, then
optic nerve biopsy should be conducted according to the patient’s clinical
condition[10].
Although the slit lamp exam is still advised for
diagnostic examination by the IPCG Guidelines, the experience and technical
skill of ophthalmologists differ; thus, the results lack objectivity and
reproducibility. Moreover, the slit lamp examination is not a good choice for
follow-up because it is not quantitative. B-scan ultrasound is a useful
adjunctive diagnostic technique for the detection and differential diagnosis of
degeneration in the vitreous[11]. B-scan
ultrasound can reveal retinal detachment and additional mass lesions. Recently,
B-scan ultrasound has become a routine examination for PCNSL cases to diagnose
intraocular involvement and rule out other conditions, such as uveal melanoma,
metastatic carcinoma and choroidal hemangioma[12].
Biopsy remains one of the most important diagnostic methods. Specimens are
obtained by fine needle vitreous aspiration or pars plana vitrectomy (PPV), and
25-gauge PPV is the primary choice. However, the role of B-scan ultrasound in
the diagnosis of PCNSL has yet to be undetermined.
The aim of our study was to determine the value of B-scan
ultrasound compared with slit lamp ophthalmoscopy for the screening diagnosis
of VRL and to describe the biological characteristics in PCNSL with
vitreoretinal involvement.
SUBJECTS AND METHODS
Ethical Approval This study
received approval from the Ethics Committee of the Institutional Review Board
of Huashan Hospital, Fudan University and was performed in compliance with the
tenets of the Declaration of Helsinki. All the patients voluntarily
participated in this study and provided informed consent.
Subjects In this study, 67 patients who
pathologically confirmed PCNSL at the Huashan Hospital from April 2016 to
January 2017 were recruited. The patients’ demographic and clinical data were
collected, including age, gender, date of cerebral diagnosis and ophthalmic
diagnosis, eye (left/right), visual acuity, intraocular pressure, clinical
symptoms and duration of ocular symptoms.
B-scan Ultrasound Examination All patients received eye examinations, including slit
lamp (MediWorks, S350), indirect
ophthalmoscopy examinations, B-scan ultrasound examinations (SOUER, SW-2100,
B-10MHz), visual field tests, optical coherence tomography (OCT) and
funduscopy. All the examinations were performed by doctors who were blinded to
the patients’ diagnoses. When yellow deposits in the retina were observed by
slit lamp, clusters of moderately condensed punctate echoes or eccentric masses
were observed on ultrasound, it indicated suspected PCNSL with intraocular
involvement.
Diagnostic Vitrectomy and Pathology A 25-gauge
diagnostic vitrectomy was performed in patients with suspected intraocular
involvement. Within one hour after the vitrectomy, the vitreous cells were sent
to the cytology laboratory for further examination.
Wright’s staining and immunohistochemical staining were
performed on 1 mL of undiluted vitreous humor or 5 mL of diluted sediment of vitreous
humor (when 1 mL of vitreous humor could not be pipetted). Primary antibodies,
including those for CD3, CD20, PAX-5, BCL-2 and BCL-6 (Shanghai Sangon Biotech,
Shanghai, China), secondary antibodies and chromogen (DAKO, Denmark) were
obtained. The EnVision two-step method and Diaminobenzidine (DAB) color
development were adopted. The appearance of brown particles in the cell
membrane or cytoblast was considered to indicate a positive result. Due to the
limited cell count in the vitreous humor, a semi-quantitative method was
applied to analyze the results. If the number of tumor cells was greater than
10% of the total cell count, the PCNSL patient was considered to have
intraocular involvement.
The concentrations of IL-10 and IL
Statistical Analysis
We conducted a descriptive
statistical analysis to determine the demographic, tumor, and treatment
characteristics, including a 2-tailed t-test, analysis of variance, a
Chi-squared test and Fisher’s exact test. The data were analyzed using SPSS
Statistics version 23.0. The null hypothesis was rejected if the P-value
was less than 0.05. Continuous data are presented as the median (range) or
number (%) as applicable.
RESULTS
Clinical Features Among the 67
PCNSL patients, 47 were male patients, and 20 were female patients. The median
age was 55y (range 20-76y). Only a few PCNSL patients complained of specific
ocular symptoms, and their courses of disease were varied. The most common
ocular symptom was blurred vision, with 14 of the 67 patients (20.90% of the
total number of patients) reporting this symptom. The median duration of the
disease was 11mo. As for other ocular symptoms, 5 patients (7.47%) reported
fundic hemorrhage or exudate, 4 (5.97%) had conjunctival congestion, and 2
(2.99%) had limitations in eye movement. In most cases, the vitreous body and
retina were involved in the PCNSL. Only nebulous and flaky turbidity of the
vitreous body was observed, whereas no abnormalities except for yellow
subretinal lesions were detected on OCT scanning (Figure 1).

Figure 1 Photos of PCNSL with intraocular involvement A: Clusters
of moderately or highly condensed punctate echoes were observed in the B-scan
ultrasound examination of the eyes; B: Yellow deposits in the retina were
observed; C: Normal central foveal thickness was detected through OCT scanning;
D: Suspected eccentric masses were observed on ultrasound but not on slit lamp
examination.
Two patients had anterior segment involvement in the
PCNSL and actively sought medical advice at the Ophthalmology Department.
Examinations indicated that the intraocular pressure was >
Table 1 Clinical characteristics of PCNSL patients
|
Parameters |
Total (n=67) |
Vitreoretinal involved (n=17) |
Non-ocular involved (n=50) |
|
Gender (M/F), n |
47/20 |
9/8 |
38/12 |
|
Age (y), median (range) |
55 (20-76) |
58 (45-71) |
55 (20-76) |
|
Ocular symptoms, n (%) |
|
||
|
Blurred vision |
14 (20.9) |
10 (58.8) |
4 (8.0) |
|
Conjunctival congestion |
4 (5.97) |
4 (23.5) |
0 |
|
Fundic hemorrhage or exudate |
5 (7.46) |
5 (29.4) |
0 |
|
Limitations of eye movement |
2 (2.99) |
2 (11.8) |
0 |
Even the PCNSL patients who received systemic
chemotherapy plus monocular chemotherapy were likely to exhibit invasion of the
other eye during the course of treatment. One patient was diagnosed with VRL
during received systemic chemotherapy. Two patients were diagnosed with VRL
during follow-up period when successfully relieved the clinical intracranial
symptoms. One patient presented with involvement of the left eye during the
first visit to the Ophthalmology Department. However, after four months of
local chemotherapy of the left eye, the right eye was also found to be
involved.
Diagnosis Rate of B-scan Ultrasound Compared with Slit
Lamp Ophthalmoscopy Vitreous flocculence and nebulous
turbidity were observed in 12 cases (15 eyes) with slit lamp indirect
ophthalmoscopy in patients with dilated pupils. Yellow fundic lesions were also
detected, indicating suspected intraocular infiltration of PCNSL, and 14 eyes
were diagnosed with intraocular infiltration. Among these 14 eyes, 13 were
diagnosed with VRL by cytology. The diagnosis rate of slit lamp ophthalmoscopy
was 10.4%, which was lower than the cytological diagnosis rate (17.9%).
However, 26 eyes were observed to have clusters of condensed punctate echoes
(vitreous hemorrhage was not observed) on B-scan ultrasound, indicating
suspicion for vitreoretinal infiltration of PCNSL. The diagnosis rate of B-scan
ultrasound was 19.4% and was similar to the cytological diagnosis rate.
Relevance of PCNSL Ocular Involvement with Location of
Intracranial Lesion When comparing VRL with cases without
ocular involvement, we found no difference in the location of the intracranial
lesions (Table 2). Regardless of whether or not there was ocular involvement,
almost one-half of the patients presented with a single intracranial lesion,
and the other half presented with multiple lesions. The highest proportion of
patients presented with hemispheric lesions when we analyzed the different
intracranial distributions. However, we found no difference between the two
groups (P>0.5). Similarly, the different locations of the hemispheric
lesions were not different between the two groups.
Table 2 Characteristics of PCNSL patients and
intracranial lesion locations
n (%)
|
Location |
Total (n=67) |
Vitreoretinal involvement (n=17) |
No ocular involvement (n=50) |
P |
|
Single lesion |
34 (50.7) |
10 (58.8) |
24 (48.0) |
|
|
Multiple lesions |
33 (49.3) |
7 (41.2) |
26 (52.0) |
0.58 |
|
Intracranial distribution |
|
0.65 |
||
|
Hemisphere |
48 (71.6) |
13 (76.5) |
35 (70.0) |
|
|
Ventricle |
14 (20.9) |
2 (11.8) |
12 (24.0) |
|
|
Basal ganglia |
12 (17.9) |
3 (17.6) |
9 (18.0) |
|
|
Corpus callosum |
12 (17.9) |
2 (11.8) |
10 (20.0) |
|
|
Brainstem |
12 (17.9) |
5 (29.4) |
7 (14.0) |
|
|
Thalamus |
10 (14.9) |
2 (11.8) |
8 (16.0) |
|
|
Right or left hemisphere distribution |
0.41 |
|||
|
Right hemisphere |
16 (23.9) |
3 (17.6) |
13 (26.0) |
|
|
Left hemisphere |
22 (32.8) |
8 (47.1) |
14 (28.0) |
|
|
Both hemispheres |
10 (14.9) |
2 (11.8) |
8 (16.0) |
|
|
Hemisphere distribution |
|
0.99 |
||
|
Frontal lobe |
31 (46.3) |
8 (47.1) |
23 (46.0) |
|
|
Temporal lobe |
16 (23.9) |
4 (23.5) |
12 (24.0) |
|
|
Parietal lobe |
18 (26.9) |
4 (23.5) |
14 (28.0) |
|
|
Occipital lobe |
8 (11.9) |
2 (11.8) |
6 (12.0) |
|
|
Insular lobe |
1 (1.5) |
0 |
1 (2.0) |
|
Cytology A 25-gauge diagnostic vitrectomy was performed
on 17 PCNSL patients (26 eyes) based on the diagnosis made with B-scan
ultrasound, including 3 patients who reported postoperative complications
during the early stages of sutureless vitrectomy. After the operation, the
intraocular pressure was tested. Patients with lower intraocular pressure were
required to have sclerotic sutures placed, and no postoperative complications
were reported.
Among the 17 patients with vitreoretinal involvement, 24
eyes contained atypical lymphomas in the vitreous humor based on Wright’s
staining. Additionally, a significant multiplication of mononuclear
macrophages, primitive lymph cells or abnormally shaped lymphoblasts were
observed, and the proportions of these cells were 51%, 30% and 19%,
respectively. A typical lymphoid cell had large, irregular, nuclear, loosely
arranged chromatin, prominent nucleoli and scant basophilic cytoplasm (Figure
2). In terms of immunohistochemical staining, the results for the specific
lymph cells were as follows: CD20 was positive, PAX-5 was positive, CD3 was
negative, and Bcl-2 was partially positive. All 67 cases were diffuse large B
cell-lymphomas, and this finding was consistent with the pathological results
of the intracranial lesions.
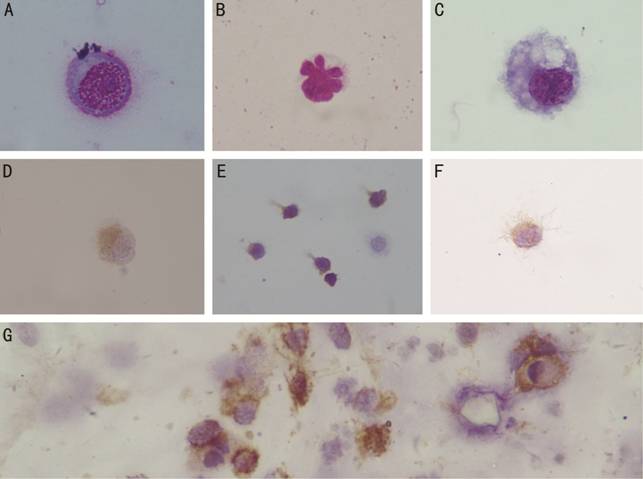
Figure 2 Wright’s staining (100×) and immunohistochemical
staining (40×) of vitreous cells A: Abnormally large B-cells with clear
nucleoli and loosely arranged chromatin; B: T-cell; C: Macrophage; D-G: Immunohistochemical
staining showing PAX-5+, CD3+, Bcl-2+ and CD20+ expression.
IL-10 and IL-10/IL-6 Levels in the Vitreous The level of IL
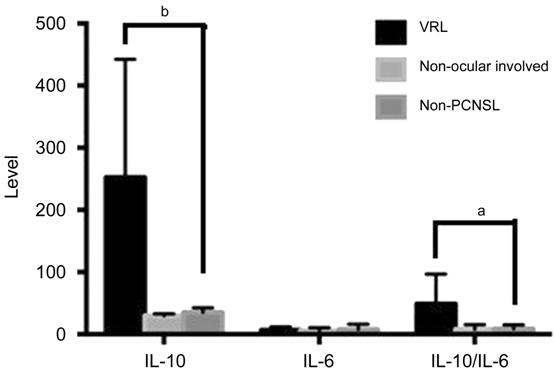
Figure 3 Levels of IL-10 and IL-10/IL
Table 3 IL-10 and IL-10/IL-6 levels
|
Cytokine |
PCNSL patients |
Non-PCNSL patients (n=14) |
|
|
VRL (n=13) |
Non ocular involvement (n=2) |
||
|
IL-10 (pg/mL) |
252.40 |
29.81 |
35.29 |
|
IL-6 (pg/mL) |
6.81 |
5.81 |
7.96 |
|
IL-10/IL-6 |
48.85 |
8.16 |
8.85 |
DISCUSSION
Our study indicated that PCNSL with intraocular involvement
had a slow onset and resembled non-infectious or infectious uveitis, white dot
syndrome or other metastatic carcinomas, and these patients experienced
progressively blurred vision. However, the visual acuity of these patients
generally exceeded expectations. The typical symptoms, including vitreous
opacity and yellow subretinal lesions, could have led to retinal detachment and
affected the visual perception of these patients. For PCNSL patients, if only
the posterior segment of the eye is involved, then they are unlikely to
complain of ocular discomfort. Similarly, this study also found that
intraocular involvement in PCNSL patients could occur at any time during the
development of the disease, including during treatment with systemic chemotherapy,
during the follow-up process after clinical relief of central nervous system
signs or even during systemic chemotherapy plus monocular chemotherapy. At
present, the pathogenesis of PCNSL remains unclear. The eyes are likely to
become sites for the “storage” of lymphomas, as observed in human
immunodeficiency virus (HIV)[13]. In general, due
to the development of intraocular disease, both patients and clinicians should
attach greater importance to follow-up examinations of the eyes[13-14].
Presently, according to the guidelines introduced in the
United States, a slit lamp examination and indirect ophthalmoscopy are
recommended methods for screening for PCNSL with intraocular involvement.
However, in practice, many other routine ocular examinations can facilitate the
diagnosis. Because slit lamp and indirect ophthalmoscope examinations largely
depend on the experience and technical skill of the ophthalmologist, and the
results cannot be recorded for filing, these techniques fail to support the
follow-up clinical diagnosis of intraocular involvement in PCNSL patients,
which adversely affects the diagnosis and treatment of these patients.
Therefore, a larger study must be conducted to determine a screening method
with an expanded range of application for PCNSL with intraocular involvement.
In this study, B-scan ultrasound examination demonstrated a higher diagnosis
rate than slit lamp ophthalmoscopy, but the diagnosis rate was not much
different than that achieved by cytology. In addition, B-scan ultrasound allows
objective measurement with good repeatability, descriptiveness and ease of
follow-up. Therefore, the B-scan ultrasound examination of the eyes should
provide a basis for the clinical diagnosis and follow-up of PCNSL with
intraocular involvement as a cost-effective method with high accuracy and
efficiency for diseases that have a high level of malignancy and low incidence.
Various diagnostic methods are available for PCNSL with intraocular
involvement, including cytological examination of the vitreous humor,
immunohistochemical detection, FNAB, flow cytometry analysis, PCR-based
monitoring of immunoglobulin gene rearrangement and cytokine detection.
However, MRI or CT scans were less useful for the diagnosis of ocular lymphoma.
Cytological examination is considered the gold standard, and the other methods
are considered auxiliary methods to improve the diagnosis rate[12]. Sufficient specimens for the cytological diagnosis
of VRL can obtained through vitrectomy, and 25-gauge diagnostic vitrectomy
tends to outperform 20-gauge diagnostic vitrectomy in terms of diagnosis rate;
furthermore, genetic mutations can be assessed in vitrectomy samples as a
valuable tool to improve the diagnostic yield of vitreous aspirates[14-15]. Diffuse B-cell lymphomas are
the main pathological type; however, the limited cell counts in the vitreous
humor and the effects of chemotherapy and vitrectomy on the tumor cells have
become major obstacles to diagnosis. Jiang et al’s[16]
study showed that corticosteroids could decrease the viability of lymphomas and
destroy the cellular structure, and the speed of vitrectomy could also affect
the viability of the cells. Specifically, the viability began to fall as the
speed reached 600 cpm and remained at its lowest level at 2500 cpm. Thus,
during diagnostic vitrectomy, the speed and pressure should remain low, and the
specimens should be collected quickly and immediately sent for preparation for
smears and staining[12,17].
Malikova et al[1] studied
in the characteristics of cranial MRI in 54 PCNSL patients. They showed that
PCNSL presented either as multiple lesions that enhanced homogenously or as
diffuse infiltrative brain involvement, often with involvement of the basal
ganglia and optic pathways. However, there are no reports about whether the
location of the intracranial lesions is a risk factor for intraocular
involvement. Our studies suggested no correlation between the distribution of
intracranial lesions and ocular involvement.
At present, vitreous cytokine examinations are known to
effectively facilitate the diagnosis of primary intraocular lymphoma (PIOL).
The high expression of IL
In conclusion, the analysis of the clinical data,
screening and pathological features of PCNSL with intraocular involvement
showed that PCNSL with intraocular involvement accounted for 25.37% of the
PCNSL cases, with VRL being the most common cause of PCNSL with intraocular
involvement. Additionally, this study showed that B-scan ultrasound examination
increased the potential for diagnosis of intraocular lymphoma in PCNSL cases.
The use of 25-gauge diagnostic vitrectomy combined with a cytological
examination and immunohistochemical staining represents a safe and effective
method for the diagnosis of intraocular involvement in PCNSL patients.
ACKNOWLEDGEMENTS
We would like to express my warmest gratitude to all my
partners, who planned the protocols, examined the vitreous cytology, performed
the examinations and surgeries on the patients, and analyzed the data. We are
also greatly indebted to all my teachers who helped in performing the protocols
and reviewed the manuscript with regard to hematological and ophthalmic
diseases. Last but not least, we thank the staff of the laboratory at Huashan
Hospital for their help.
Foundations: Supported by the National
Natural Science Foundation of China (No.81700123); the Shanghai Hospital
Development Centre (No.16CR2043B).
Conflicts of Interest: Lai J, None; Chen K, None; Shi HM, None; Zhuang
L, None; Zhou X, None; Xiao JJ, None; Li Y, None; Chen
BB, None; Wang QP, None.
REFERENCES