
·Letter to the Editor·
Increased
intracranial pressure and macular thickening: is there a link?
Hamid Sajjadi1,2, Hossein Poorsalman3,
Mohammad-Ali Abtahi2
1San Jose Eye
and Laser Medical Center, Cupertino 95104, California, USA
2Department of
Ophthalmology, Acacia Medical Center, Dubai 72298, UAE
3Department
of Ophthalmology, Iranian Red Crescent hospital, Dubai 02330, UAE
Correspondence
to: Hamid
Sajjadi. Acacia Medical Centre, Suite 111, Al Shafar Bldg. 7, Al Wasl Rd. P.O
Box: 72298, Dubai, UAE. hsajjadi@yahoo.com
Received:
DOI:10.18240/ijo.2019.06.29
Citation: Sajjadi
H, Poorsalman H, Abtahi MA. Increased intracranial pressure and macular
thickening: is there a link? Int J Ophthalmol 2019;12(6):1052-1055
Dear Editor,
I am Dr.
Hamid Sajjadi, director of Neuro-Ophthalmology at San Jose Eye and Laser
Medical Center in California USA and director of Department of Ophthalmology,
Acacia Medical Center, Dubai, UAE. I write to present three cases of macular
thickening (MT) and micro-papilledema associated with increased intracranial
pressure (IICP).
Since retina
is known as the anterior visible extension of the central nervous system, there
is growing evidence that several brain disorders affect this complex tissue. In
this regard, one of the best devices to detect early changes of central nervous
system disorders is retinal and optic nerve head (ONH) optical coherence
tomography (OCT)[1].
The hallmark
of IICP is the changes in the optic nerve that is called papilledema[2]. However in almost all these cases there were no overt
papilledema. Several authors have tried to not only grade the papilledema[3] but also to describe other associated funduscopic signs
of IICP[4-5] [e.g.
choroidal folds, macular star, macular hemorrhage and overt macular edema
(ME)]. Because of anatomic variations in ONH, papilledema grading may not always
have direct relation with the level of IICP[6].
Hence, changes of the optic nerve may be asymmetric[7]
and even subtle[8]. Recently, in a report we described
a large series of spinal tap (ST) proven IICP cases without overt papilledema
detected by ONH OCT[9].
There are
several causes associated with decreased vision in IICP[10]
, among which MT/ME can be addressed[5]. ME
related to IICP may also be either overt (44% in overt papilledema in a report)[6] or subtle[11].
Subclinical macular changes (only detected by macular OCT) were first described
as the cause of decreased vision in 7 out of 54 cases of papilledema in one
report[10].
We present 3
patients with decreased vision or other neurologic signs (headache, dizziness)
that were referred to us. First, we should mention that we followed principles
outlined in Declaration of Helsinki. Written informed consent was taken from
all subjects after explaining the study. The first 2 cases are young female
Caucasians and had a history of headache. The third one is older and African
male. All 3 patients were referred with loss of best corrected visual acuity
(BCVA) and 2 of them were obese [body mass index (BMI) more than 30]. Past
medical history was negative in regard to causes of MT/ME (diabetes,
hypertension, drugs, etc.). Macular and ONH OCT was performed, as a
result MT along with ONH nerve fiber layer thickening suspicious to IICP (as we
described in our previous article) was detected[9].
Case 2 had a highly asymmetric ONH and 1+ relative afferent pupillary defect
(RAPD) in right eye. Case 3 had a history of refractory ME labeled to be as a
result of cataract surgery in both eyes 20 months ago. He had no improvement of
vision despite months of topical dexamethasone and non-steroidal anti-inflammatory
drugs and scheduled for intravitreal Ozurdex. Since all 3 patients had normal
brain magnetic resonance imaging (MRI), we referred our patients to a
neurologist and he did ST in lateral decubitus position. As a result, all 3
patients showed IICP, were diagnosed to have idiopathic intracranial
hypertension (IIH) and were treated with oral acetazolamide (ACZ). After
follow-up period (more than 1y in 2 first cases and about 6mo in case 3) they
showed dramatic improvement in BCVA along with decrease in OCT thickness
profiles both in macula and ONH. Along with improvement in ONH appearance in
case 2 after only 3mo of treatment with ACZ, RAPD changed to negative. There
are reports of RAPD in IIH patients with unequal pressure on optic nerves from IICP.
This may be related to the size of optic nerve canal or anatomy of optic nerve
at lamina cribrosa[12]. Findings and treatment results are
summarized in Table 1 and Figures 1-3.
Table 1
Summary of examinations and follow-up of 3 cases
|
Parameters |
Case 1 |
Case 2 |
Case 3 |
|
Demographics |
18-year-old Caucasian female |
24-year-old Caucasian female |
57-year-old African male |
|
Presentation |
Blurry vision and dizziness since 1 year ago |
Decrease vision in right eye and severe headache |
Blurry vision since 2 years ago; no DM, no HTN, no headaches |
|
Past history |
Severe headaches diagnosed as Migraine, since 4 years ago |
Severe headache since 5 years ago |
Uncomplicated cataract surgery 20mo before: no vision improvement |
|
Ocular exam (presentation) |
BCVA: 20/25 (she was insisting it was not as clear as it used to be);
normal anterior segment; PCIOP: |
BCVA 20/200 OD and 20/25 OS; normal anterior segment; PCIOP: |
BCVA: 20/200 OU; 2+ PCO OD & 1+ OS; PCIOP: |
|
Other examinations |
ONH OCT: Pattern 1: that raised suspicion to IICP; macular OCT: central
foveal sparing MT with normal structure; BMI:37.5; normal MRI; ST: |
ONH OCT: overt papilledema OD, pattern 2 OS; macular OCT: MT in right eye
more than in left eye with normal structure and central foveal sparing;
BMI:24; normal brain MRI; ST: |
ONH OCT: overt IICP pattern OD and Pattern 1 specific to IICP OS; macular
OCT: MT OU, the fluid pattern was pointing toward optic nerve with more
prominence in temporal macula; BMI:31; normal brain MRI; ST: |
|
Treatment |
Oral ACZ (1 gram daily) |
Oral ACZ (1 gram daily) |
At presentation: Cosopt b. i. d., Alphagan-P 0.1% b. i. d. and
Xalatan in OU; Then ACZ was started ( |
|
Follow-up, final exam |
15mo F/U: BCVA improved :20/20 (patient stating that her previous sharp
vision had returned); ONH and macular OCT showed thickness improvement;
headaches totally improved; loss of |
1.5y F/U: BCVA improved :20/25 OU; resolution of micro-papilledema and MT |
6mo F/U: CMT improved dramatically, from 324 and 278 μm to 278 and 212 μm
respectively |
ONH: Optic
nerve head; OCT: Optical coherence tomography; IICP: Increased intracranial
pressure; ST: Spinal tap; MT: Macular thickening; ME: Macular edema; BCVA: Best
corrected visual acuity; IOP: Intraocular pressure; BMI: Body mass index; MRI:
Magnetic resonance imaging; ACZ: Acetazolamide; IIH: Idiopathic intracranial
hypertension; RAPD: Relative afferent pupillary defect; CME: Cystoid macular
edema; PCIOP: Pachymetry corrected intraocular pressure; CMT: Central macular
thickness; PCO: Posterior capsule opacification; IV: Intravitreal; OD: Right
eye; OS: Left eye; OU: Both eyes; NSAID: non-steroidal anti-inflammatory drugs;
F/U: Follow-up; DM: Diabetes mellitus; HTN: Hypertension.
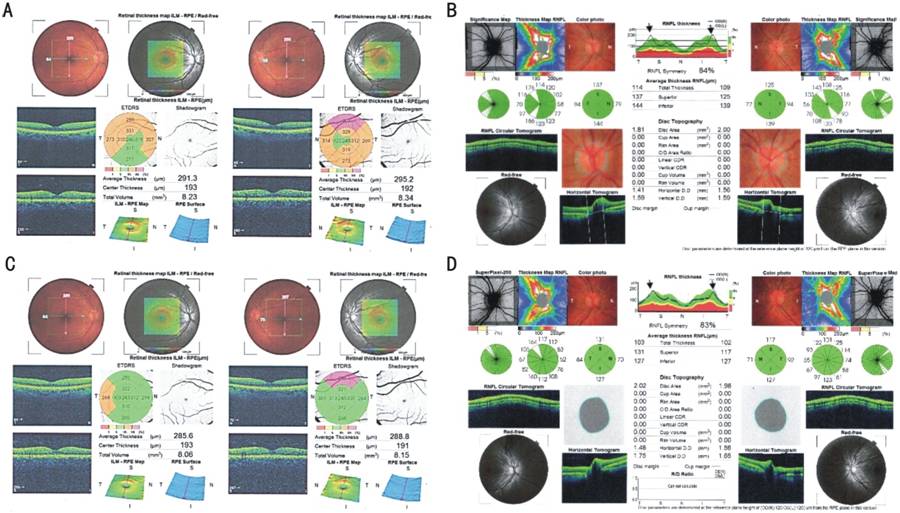
Figure 1
Case
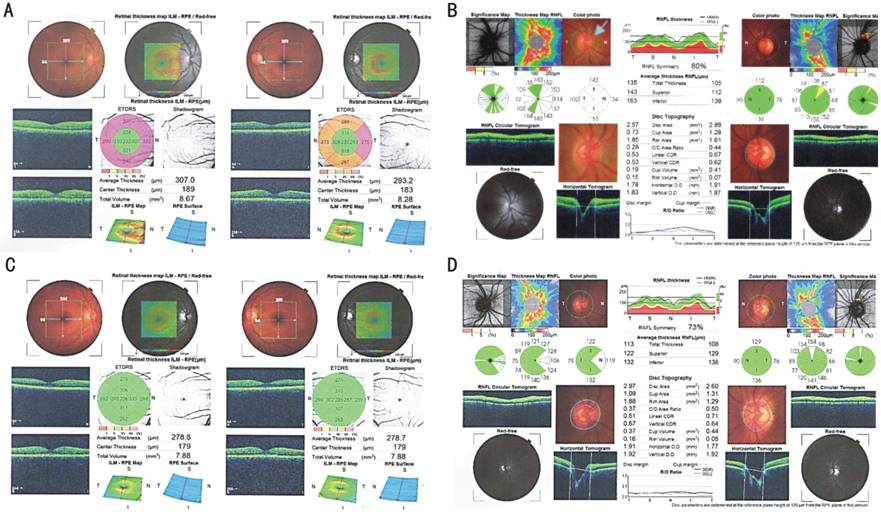
Figure 2
Case
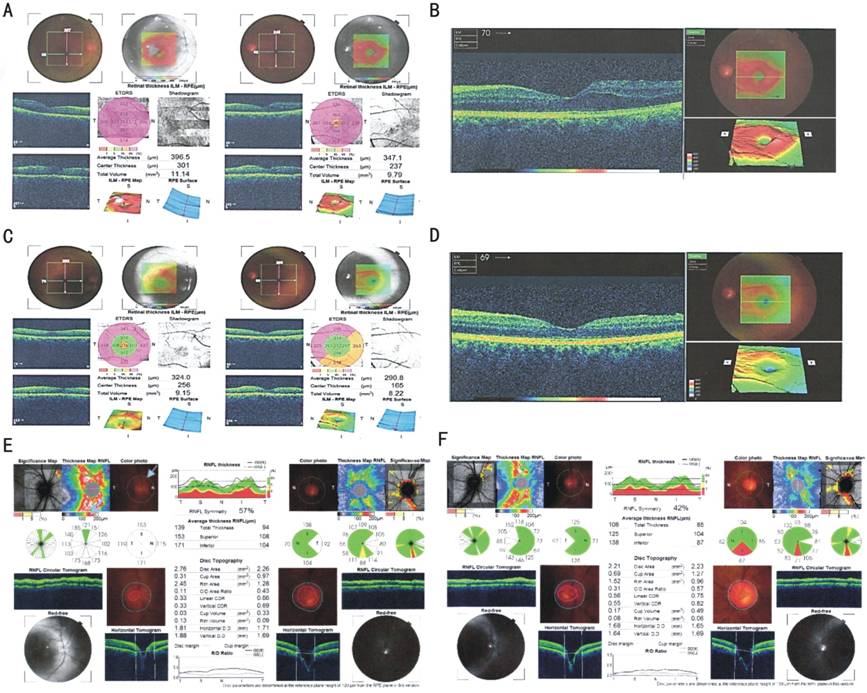
Figure 3
Case
Although an
overt papilledema may indicate IICP, the severity of papilledema may not have a
direct relationship with the level of ICP[6]. This
notion relies on of variations in width of CSF space surrounding the optic
nerve as a rout of communication between the optic canal and the intracranial
space[11]. There are several reports regarding
IICP without papilledema in adults[8] and children[2]. We have recently reported 171 cases of IICP-suspects
diagnosed by OCT before imaging and or ST. Study consisted of 3 tumors and 148
IIH cases diagnosed by OCT; 144 without overt papilledema[9].
We defined 4 patterns of micro-papilledema, among which the most specific type
was pattern 1 (raised temporal and depressed nasal wings of the ONH). We
suggested that IICP could be detected in the absence of overt papilledema with
the help of OCT device.
Macular
changes have been reported to be associated with elevated intracranial pressure
and papilledema[4-5]. To our
knowledge, the first paper that evaluated MT/ME in association with papilledema
with the aid of first generation OCT[10],
mentioned that the cases did not show overt ME but decreased vision. Therefore,
they performed OCT for evaluation of macula. The authors believed that
decreased vision that may be present in some patients with papilledema and IICP
can be attributed to subclinical macular changes. Another common point between
our and this case report was rapid resolution of MT/ME in response to oral ACZ[10]. This is completely in agreement with a recent cohort
in which measured average total peri-papillary retinal thickness, ONH volume
and the ganglion cell plus inner plexiform layer thickness (macula) by OCT in
patients with overt papilledema and IIH. Results were dramatically lower in ACZ
therapy group compared to placebo in 6mo follow-up[6].
There is another common interesting point that all 3 cases share a common
pattern of thickening, which is more prominent in peripapillary region and
spares the central foveal region. Despite this doughnut shaped pattern that
spares central fovea, all three patients had decreased BCVA. In this regard we
think that thickening of retinal layers mainly sparing central fovea in
association of decreased vision or headache may be considered one of the
harbingers of IICP. There is a theory about origin of this MT/ME. In one
report, authors suggested that the origin of this fluid may originate from
cerebrospinal fluid (CSF)[10]. We are proposing
that because of the high ICP flow of the CSF is slowed down and therefore
axonal flow in the optic nerve neurons are slowed and thus cause a thickening
of the peri-macular retina where it is the richest in ganglion cell layers. In
this regard, we could name this type of MT/ME as CSFME.
This
highlights the need for further studies to evaluate subtle changes associated
with IICP. The OCT of macula alone can be a sign of increased ICP in early
cases and it will show thinning in
advanced cases or after treatment. This is a substantial new finding that may
indicate the future of early neurological diagnosis may be in the macular OCT
of the eyes.
ACKNOWLEDGEMENTS
We should
thank Dr. Habib Dezhagagah by his contribution in adding some new concepts to
the manuscript.
Authors’ contributions: Sajjadi H and Poorsalman H visited the patients and did the follow-up of
the patients. Abtahi MA and Sajjadi H had a major contribution in writing the
manuscript. All authors read and approved the final manuscript.
Conflicts of
Interest: Sajjadi
H, None; Poorsalman H, None; Abtahi MA, None.
REFERENCES