
Citation: Zhang YH, Ren LM, Wang XY. Inhibitory effect of
Houttuynia cordata Thunb on LPS-induced retinal microglial activation. Int J
Ophthalmol 2019;12(7):1095-1100
DOI:10.18240/ijo.2019.07.07
·Basic Research·
Inhibitory effect of Houttuynia cordata Thunb on LPS-induced retinal microglial activation
Ying-Hui Zhang1, Le-Meng Ren2, Xiao-Yun Wang1
1The Second Hospital of Shandong University, Shandong University, Jinan 250033, Shandong Province, China
2The First Clinical Medical College, Lanzhou University, Lanzhou 730000, Gansu Province, China
Correspondence to: Xiao-Yun Wang. The Second Hospital of Shandong University, Shandong University, Jinan 250033, Shandong Province, China. wxiaoyunsd@163.com
Received:
Abstract
AIM: To identify the effect of Houttuynia cordata Thunb (HCT) on lipopolysaccharide (LPS)-induced microglial activation and investigate its possible molecular mechanisms.
METHODS: The primary retinal microglial cells were cultured from the retinas of newborn Sprague-Dawley rats and exposed to LPS, and/or HCT with different concentrations. The survival ability of retinal microglia cells was tested by standard MTT method. BrdU cell proliferation assay was used to evaluate the proliferation of retinal microglia. Inflammatory factors in the culture supernatants, including TNF-α, iNOS and IL-1β, were measured using ELISA. Microglia cells’ migration was determined with Transwell migration assay. The total p38-MAPK and phosphorylation of p38-MAPK (p-p38-MAPK) were detected with Western blot.
RESULTS: Primary retinal microglia in culture exposed to LPS to induce microglia activation. Pretreatment with HCT significantly inhibited the LPS-induced cell proliferation, but not the cell viability. LPS induced inflammatory reaction in microglia and cell migration. HCT significantly reduced LPS-stimulated release of pro-inflammatory factors and decreased the number of migrating cells substantially in a concentration-dependent manner. Moreover, the protein levels of p-p38 MAPK were identified as the up regulation and co-treatment with HCT obviously inhibited the upregulation of p-p38 MAPK, but had no effect on the levels of total p38-MAPK.
CONCLUSION: The data suggest that HCT inhibits LPS-induced retinal microglial activation via suppression of the p-p38-MAPK. HCT may be used for the treatment of ocular diseases characterized by over-activated microglia.
KEYWORDS: Houttuynia cordata Thunb; microglia activation; retina; inhibitory effect
DOI:10.18240/ijo.2019.07.07
Citation: Zhang YH, Ren LM, Wang XY. Inhibitory effect of Houttuynia cordata Thunb on LPS-induced retinal microglial activation. Int J Ophthalmol 2019;12(7):1095-1100
INTRODUCTION
Retinal microglia, resident immune cells of the retina, play a crucial role in the surveillance of their surrounding neural tissue and constantly monitor their environment in any exceptional conditions. When there is in the presence of foreign materials, cell debris or in the situation of inflammation, trauma, hypoxia, the resting retinal microglia can be rapidly activated. Moderate activation of microglia is beneficial for the maintainance of internal homeostasis in the retina. However, the over-activated microglia can secret numerous of inflammatory mediators that can further aggravate the retinal injury and even lead to the phagocytosis of the surrounding healthy retinal cells[1, 2]. Such deleterious action of the over-activated microglia has been observed in many ocular diseases, including glaucoma[3], retinal degenerative diseases[4, 5], diabetic retinopathy[6], etc. A great deal of research shows that microglia mediated neuroinflammation is an important contributor to the inflammatory injury and suppression of the over-activated microglia will exert neuroprotective effects. Therefore, a number of attempts have been undertaken so far to inhibit the excessive activation of microglia[7, 8, 9, 10].
Recently, many chinese medicinal herbs have been found to have anti-inflammatory effect and can inhibit microglia activation, like Curcumin[11], Gastrodin[10], hispidulin[12] , and so on. Houttuynia cordata Thunb (HCT), present in a Chinese medicinal plant, is widely distributed throughout China and well known for its medicinal properties. HCT has been shown to suppress the inflammatory response and oxidative stress through inhibiting the inflammatory pathways in various diseases in recent times[13, 14]. Retinal microglia are the main immune cells in the retina, however, there has been no report of HCT on microglia activation.
In the current study, primary retinal microglia was cultured and used for studying microglial activity. Lipopolysaccharide (LPS) is used as a tool to study the process of microglia activation. We firstly examined whether HCT could prevent LPS-induced microglia activation and investigated the possible mechanisms.
MATERIALS AND METHODS
Ethical Approval All the research procedures were approved by the Ethical Review Committee of the Second Hospital of Shandong University and strictly conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Preparation
of Houttuynia Cordata Thunb Ethanol Extract HCT was purchased from Tong Ren Tang
Group Co., Ltd. (Jinan, Shandong Province, China). The procedures for HCT
ethanol extract were in accordance with the previously published articles from
methods[15]. After extraction, the powder from
the extract was dissolved in DMSO and stored at
Primary Retinal Microglia Culture Newborn Sprague-Dawley rats (1 to 3 days old) were used
for the cell culture. A total of 60 rats were used in our study. The retinal
microglial cells were cultured according to the extensively used technique,
with minor modifications[16]. In brief, the
newborn Sprague-Dawley rats were sacrificed and then the eyes were enucleated.
And, the retinas were carefully dissected under a microscope. Six retinas were
polled together and they were incubated in 2% papain in Hanks balanced salt
solutions (HBSS) at
LPS-induced Microglial Activation and Treatment with
Houttuynia Cordata Thunb Harvested microglia were seeded in
To study a dose-response curve for different concentrations of HCT on LPS stimulation, the microglia were exposed to 10, 50, 100 μmol/L HCT for 1h before administering LPS.
Cell
Viability Assay The standard MTT [3-(4,5-
dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Roche, Mannheim,
Germany] method was used to evaluate the survival ability of retinal microglial
cells. Briefly, the cells were treated with HCT at serially diluted concentrations
10, 50, 100 μmol/L, LPS (Sigma, Deisenhofen, Germany; 1 μg/mL) for 24h. Then
the cells were washed twice with phosphate buffered solution (PBS) and
incubated with 5 mg/mL MTT solution at
BrdU Cell Proliferation Assay The cells were pretreated with HCT (10, 50, and 100 μmol/L) for 1h and then incubated with or without LPS (1 μg/mL) for another 48h. One commercial cell proliferation assay kit (Cell Signaling) was performed to measure the incorporation of BrdU during DNA synthesis, following the manufacturer’s instructions. Absorbance was immediately assessed at 450 nm via a microplate reader (Tecan, Reading, UK) and XFluor software (Version V 4.51, Tecan).
Enzyme-linked Immunosorbent Assay Retinal microglia were cultured in 96-well plates at a density of 2×105 cells/mL for 24h and then incubated with various concentrations of HCT (10, 50, and 100 μmol/L) for 4h; LPS (1 μg/mL) was added and cultured for another 48h. The culture media were harvested at the end of the culture period. Inflammatory factors in the culture supernatants, including TNF-α, iNOS and IL-1β, were determined by enzyme linked immunosorbent assay (ELISA) kits (Beyotime Biotechnology, China) based on the product description. The color development was measured at 450 nm using a multi-well plate reader (Thermo Fisher Scientific GmbH, Schwerte, Germany). All measurement was performed thrice, and the mean values of the determinations were used for further statistical analysis.
Transwell Migration Assay The effect of HCT on the migration ability of microglia
was evaluated using the Costar Transwell System (pore size 8 μm; Costar, Cambridge, MA, USA). Of 0.2 mL cell suspension
in serum-free medium were plated in the upper well (4×105 cells per
well) and 0.6 mL DMEM with 1 μg/mL LPS, 1 μg/mL LPS+HCT (10, 50 or 100 μmol/L) were added to the lower chamber. After incubation at
Western Blot Analysis Retinal microglia cells were seeded in 6-well plates at a
density of 5×106 cells/well and pretreated with HCT (10, 50, and 100 μmol/L) for 4h before being exposed to LPS (1 μg/mL) for another 48h. After treatment, the total cells
were collected and immediately homogenized in RIPA lysis buffer (Beyotime
Biotechnology, China). After centrifugation at 12
Statistical Analysis SPSS (version 22.0; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data were expressed as the mean±SD from three independent experiments. The differences between different groups were calculated by One-way analysis of variance, followed by Tukey’s multiple comparison test. A P value of <0.05 was indicated as statistically significant difference.
RESULTS
Effect of Houttuynia Cordata Thunb on the Viability of
Retinal Microglia Retinal microglia were collected from the primary mixed
glial cells by shaking the flask. Over 98% of the collected cells showed
positive immunofluorescence staining of IBA1 (Figure 1). The standard MTT assay
was performed to assess the cytotoxicity of HCT. As the Figure

Figure 1 Primary retinal microglial cells were identified with immunofluorescence staining of IBA-1.

Figure 2 Effect of HCT on the viability of retinal
microglia A: Cell viability was measured using the
standard MTT assay; B: The effect of HCT on proliferation of retinal microglial
cells was evaluated using the BrdU assay. aP<0.05, bP<
Effect of Houttuynia Cordata Thunb on the Cell Proliferation Effect of HCT on the proliferation of retinal microglia was tested using the BrdU assay. Treatment with LPS (1 μg/mL) significantly increased the cell proliferation and pretreatment with HCT (10, 50, 100 μmol/L) significantly inhibited this increased proliferation (Figure 2B).
Effect of Houttuynia Cordata Thunb on the Production of Inflammatory Factors LPS stimulation could induce inflammatory reaction in microglia, resulting in the release of inflammatory factors. In order to investigate the influence of HCT on the production of LPS-induced inflammatory factors, we used the ELISA kits to measured the expression levels of TNF-α, iNOS and IL-1β released into the culture medium. As shown in Figure 3, incubation with LPS (1 μg/mL) obviously increased the secretion of TNF-α, iNOS, and IL-1β in comparison with the control group. Preliminary treatment of HCT significantly decreased the levels of inflammatory factors in a dose-dependent manner as compared to LPS group, suggesting the anti-inflammatory effect of HCT.

Figure 3 Effect of HCT on the inflammatory factors in the
supernatant measured by ELISA assays aP<0.05, bP<
Effect of Houttuynia Cordata Thunb on the Migration of Retinal Microglia To evaluate the influence of HCT on the migration of retinal microglia, we performed Transwell migration assay. The results indicated that LPS induced the migratory ability of the microglia in comparison with the control group and cotreatment with HCT significantly reduced the number of migrating cells substantially in a density-dependent pattern (Figures 4, 5).

Figure 4 Transwell migration assay was performed to evaluate the effect of HCT on the motility of retinal microglia cells induced by LPS A: Control; B: LPS; C: LPS+10 μmol/L HCT; D: LPS+50 μmol/L HCT; E: LPS+100 μmol/L HCT.
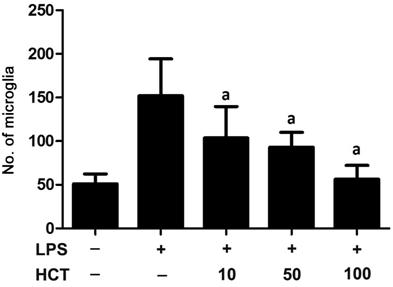
Figure 5 HCT remarkably inhibited the migration of
LPS-induced microglial cells in a concentration-dependent manner aP<
Effect of HCT on the LPS-stimulated Phosphorylation of p38-MAPK in Retinal Microglia As well known, p38-MAPK pathway is important for the migration of microglia[17, 18]. Therefore, we further detected the levels of total p38-MAPK and phosphorylated-p38 MAPK (p-p38-MAPK) in the microglial cells using Western blot. The result showed that the levels of p-p38-MAPK were significantly enhanced in LPS-stimulated microglia. As expected, HCT treatment decreased the level of p-p38-MAPK in a concentration-dependent pattern but not the total p38-MAPK (Figure 6).

Figure 6 Effect of HCT on the levels of total p38-MAPK and phosphorylated-p38-MAPK (p-p38-MAPK) in the retinal microglia cells was tested using Western blot (A) Statistical analysis showed that HCT treatment significantly decreased the level of p-p38-MAPK in a concentration-dependent manner but not to total p38-MAPK (B, C). aP<0.001 compared with the LPS-treated group.
DISCUSSION
HCT (Family: Saururaceae), a herbaceous perennial plant, has been long used in Asian countries, especially in China, for the treatment of inflammation diseases. HCT has a wide range of biological activities and has shown to have strong anti-inflammatory and anti-oxidative properties. More importantly, HCT did not have any toxicity in vivo and in vitro models[13]. HCT has been reported to attenuate LPS-stimulated synthesis of iNOS and TNF-α protein in the mouse peritoneal macrophages[19]. In the study, we constructed the model of microglia activation induced by LPS and observed that HCT could inhibit the LPS-induced cell proliferation, but did not affect cells’ viability. Furthermore, HCT elicited a dose-dependent inhibition of the production of inflammatory factors. HCT was also found to decrease the number of migrating cells and suppress the LPS-stimulated phosphorylation of p38-MAPK. These results suggested that the anti-inflammatory effect of HCT against LPS-induced inflammatory response in primary retinal microglia cells involving the p38-MAPK pathway.
Microglia are the intrinsic immune cells in the retina tissue. In general, the retinal microglia are quiescent and play a very important role in keeping the homeostasis of the retina. However, in the situation of pathological conditions, the quiescent microglia can be rapidly activated and release an amount of neurotoxic factors, like TNF-α and iNOS, which magnify and perpetuate the local inflammatory response to induce the death of retinal ganglion cells (RGCs). Recent experimental evidence demonstrates that anti-neuroinflammation mediated by microglia have neuroprotective effects on RGCs in various eye disease models[10, 20, 21]. Recently, many herbs and their active components have showed as inhibitors of microglia activation[9]. In our study, we found that HCT suppressed the LPS-induced production of inflammatory factors such as TNF-α, iNOS and IL-1β in microglia. Many studies have suggested that these pro-inflammatory cytokines were recognized to neurodegeneration and overproduction of the inflammatory mediators in the retina can result in tissue damage and RGCs death. Reduction of the inflammatory mediators might present a promising therapy for ocular diseases concerning the death of RGCs. Therefore, we speculate that HCT has neuroprotective effect on RGCs through inhibiting microglia mediated neuroinflammation.
The migration of microglia cells is a hallmark in response to inflammation. Prevention of the migration is another important strategy to minimize the damage of RGCs during inflammation[19]. Due to the anti-inflammatory effect of HCT, we further clarified the effect of HCT pretreatment on the migration of retinal microglia. Our data suggested that HCT treatment obviously inhibited the migration of retinal microglia. Plenty of evidence indicates that the p38-MAPK signaling pathway is vital for cell migration, which can be activated by stresses and proinflammatory factors. The phosphorylation of p38-MAPK eventually results in cell migration. To further interpret the possible mechanism of HCT on the migration of retinal microglial cells, we examined the levels of p-p38-MAPK in the retinal microglia using Western blot. Results showed that the expression levels of p-p38-MAPK in microglia treated with HCT were significantly decreased in a concentration-dependent manner.
In conclusion, our results showed that HCT not only suppressed the production of inflammatory factors, but also inhibited the migration of retinal microglial cells, which are involved in the inhibition of p38-MAPK phosphorylation. Therefore, our data indicate that HCT has anti-neuroinflammatory properties and could be used as a therapeutic agent applicable to microglia-mediated neuroinflammation.
ACKNOWLEDGEMENTS
Conflicts of Interest: Zhang YH, None; Ren LM, None; Wang XY, None.
REFERENCES