
Citation: Dong ZZ, Gan YF, Zhang YN, Zhang Y, Li J, Zheng HH. The
clinical features of posterior scleritis with serous retinal detachment: a
retrospective clinical analysis. Int J Ophthalmol 2019;12(7):1151-1157
DOI:10.18240/ijo.2019.07.16
·Clinical Research·
The clinical features of posterior scleritis with serous retinal detachment: a retrospective clinical analysis
Zhi-Zhang Dong1, Yi-Feng Gan1, Yi-Nan Zhang1, Yu Zhang1, Juan Li2, Hai-Hua Zheng1
1Department of Ophthalmology, the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou 325027, Zhejiang Province, China
2Department of Ophthalmology, Shaanxi Ophthalmic Medical Center, Xi’an No.4 Hospital, Guangren Hospital Affiliated to School of Medicine of Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China
Co-first authors: Zhi-Zhang Dong and Yi-Feng Gan
Correspondence to: Hai-Hua Zheng. The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, 109W Xueyuan Road, Wenzhou 325027, Zhejiang Province, China. eyezhh@126.com
Received:
Abstract
AIM: To summarize the clinical features, systemic associations, risk factors and choroidal thickness (CT) changing in posterior scleritis (PS) with serous retinal detachment.
METHODS: This retrospective study included 23 patients diagnosed PS with retinal detachment from August 2012 to July 2017. All patients’ medical history and clinical features were recorded. The examinations included best corrected visual acuity (BCVA), intraocular pressure (IOP), fundus examination, and routine eye examinations. Posterior coats thickness (PCT) was determined by B-scan ultrasound, the CT was measured by enhanced depth imaging spectral-domain optical coherence tomography (EDI-OCT) and clinical data were compiled and analyzed.
RESULTS: After application of extensive exclusion criteria,
23 patients with PS remained (13 females, 10 males). The average age at
presentation was 29.5±9.24 years old. Ocular pain and blurred vision were the
two most common complained symptoms by patients. Anterior scleritis occurred in
12 patients, which was confirmed by ultrasound biomicroscopy (UBM) examination.
Despite all patients displaying serous retinal detachment in their macula, no
fluorescein leakage was observed in the macular area. Optic disc swelling was
documented in 10 of the 23 eyes. From B-scan ultrasound examination, the PCT
increased with fluid in Tenon’s capsule demonstrated as a typical T-sign. The average
PCT was 2.51±
CONCLUSION: PS with serous retinal detachment presented a variety of symptoms, such as pain, visual loss, and physical indicators. Typical T-sign detected by B-scan ultrasound is a useful confirmatory sign for PS diagnosis. Pathological increases in CT might be a potential predictive factor for inflammation.
KEYWORDS: choroidal thickness; scleritis; serous retinal detachment; clinical features; posterior scleritis
DOI:10.18240/ijo.2019.07.16
Citation: Dong ZZ, Gan YF, Zhang YN, Zhang Y, Li J, Zheng HH. The clinical features of posterior scleritis with serous retinal detachment: a retrospective clinical analysis. Int J Ophthalmol 2019;12(7):1151-1157
INTRODUCTION
Posterior scleritis (PS) is an uncommon and under-diagnosed condition caused by inflammation of the sclera (i.e. the fibrous outer layer of the eye)[1]. Due to its low incidence and variable clinical presentation, the mechanism underlying PS remains unclear and the majority of the research to date has focused instead on characterizing its clinical features, and optimizing diagnosis, treatment, and patient outcome[2, 3, 4]. Based on the anatomical location, PS presents with a range of clinical features, especially in fundus change, and is often misdiagnosed as intraocular inflammation[5], ocular tumors[6], or orbital inflammation[7]. Such misdiagnoses[8] increase the likelihood of incurring irreversible visual damage including eventual vision loss. Given the high rates of retinal detachment in PS[4], this manifestation can be easily misdiagnosed central serous chorioretinopathy (CSC), which is characterized by retinal detachment involving macula or not. There is currently a paucity of research dedicated to detailing the differences between these two diseases. These situation motivated our interest in the clinical features and risk factors involved in PS specifically with retinal detachment.
Recently some studies have suggested that choroidal expansion might play an important role in PS[9, 10]. Due to advances in ophthalmic imaging technology, choroidal thickness (CT) can be noninvasively measured by enhanced depth imaging spectral-domain optical coherence tomography (EDI-OCT). While studies have implicated the importance of CT in PS, but none have focused on how CT is involved in PS with retinal detachment[10, 11]. Furthermore, CT involvement is increasingly recognized as a feature of PS. However, little is known about the association between CT and other ocular biometric parameters, such as visual acuity, axial length (AL) and posterior coats thickness (PCT), in PS, let alone in PS with retinal detachment. Therefore, this study aimed to fully characterize the clinical features, systemic associations, and risk factors in PS with retinal detachment and to investigate the clinic findings and association of CT in PS with serous retinal detachment by a retrospective clinical analysis.
SUBJECTS AND METHODS
Ethical Approval This study was approved by the Ethical Review Committee of the Second Affiliated Hospital of Wenzhou Medical University, Wenzhou, China. All procedures adhered to the tenets of the Declaration of Helsinki for research involving human subjects. Written informed consent was obtained from all participants enrolled in this study. All potential study participants were from Chinese Han population and patients of the aforementioned clinic.
Subjects and Enrollment Criteria A retrospective review of patient medical records from the ophthalmological clinic at the Second Affiliated Hospital of Wenzhou Medical University between August 2012 and July 2017 was performed to identify patients with diagnosis of PS. PS was diagnosed by presence of: 1) acute or sub-acute symptom onset; 2) eye pain with or without decreased visual acuity, accompanied by various fundus changes (e.g. optic disc edema, retinal phlebectasia, exudative retinal detachment); 3) posterior sclerochoroidal wall thickening and fluid in the Tenon’s capsular with low echo and fascial sac edema in the eye coats, defined as typical T-sign[3, 4, 12], revealed by B-scan ultrasound examination.
Patients with any of the following conditions were excluded: incomplete clinical information, infection with acute orbital cellulitis, tuberculosis, or syphilis; ocular trauma or tumor; the orbital inflammatory pseudotumor; Graves’ ophthalmopathy; macular retinal detachment with vascular leakage in fundus fluorescein angiography (FFA); rhegmatogenous retinal detachment; any obvious cataract leading to an intumescent lens; a history of intraocular surgery; clinically relevant opacities of the optic media; and low-quality images due to unstable fixation or a severe cataract.
Of the 69 records initially identified, 16 were excluded because of insufficient clinical detail to ensure a correct diagnosis or lack of satisfactory ultrasonography data. Thirty PS patients were not included because no sub retinal fluid was detected in our research. Finally 23 patients were included in this study. All enrolled patients were diagnosed with PS presenting serous retinal detachment over the macula.
Study Measurements Demographic data of age, sex, and blood pressure were collected. Detailed clinical information regarding original diagnosis, disease onset, clinical presentation, ultrasound data, and systemic associations was recorded. All patients were regularly tested with regular blood test, C reactive protein (CRP), erythrocyte sedimentation rate (ERS), P- and C-anti-neutrophil cytoplasmic antibodies (ANCA), antinuclear antibody, PANA, antistreptolysin O antigen test, dsDNA antibody, SS-A and SS-B antibody, complement series, serum amyloid A (SAA), rheumatoid factor, immunoglobulin G and M, and TORCH test (for Toxoplasma gondii, Rubella virus, Cytomegalovirus, Herpessimplex virus). All subjects underwent a thorough ophthalmic evaluation, including the best corrected visual acuity (BCVA), slit-lamp biomicroscopy, intraocular pressure (IOP) measurement (applanation tonometry), fundus examination, ultrasonographic biomicroscopy (UBM), ultrasound B-mode scanning (B-scan, Bio-vision Echosens, France), FFA, refractive error examination, and axial length (AL) measured with partial optical coherence interferometry (IOLMaster, Carl Zeiss Meditec, German). All examinations were performed upon diagnosis by experienced ophthalmologists who were blinded to the patients diagnosis.
EDI-OCT
Examination As previously described[13], CT was determined using the Spectralis device
(Heidelberg Engineering, Heidelberg, Germany), by using the device’s automatic
averaging (about 100 real-time frames were averaged) and eye-tracking features.
First of all, in order to estimate optical magnification and achieve accurate
comparisons across individuals, keratometry readings and the refraction data
were collected and entered into the Spectralis software program. For the aim of
ensuring high-quality images, the quality of choroidal imaging was judged
according tothe signal-to-noise ratio, and only images ratios ≥20 dB were
collected and used for further analysis. The resulting scans were visualized
and measured by the measuring software, the standard Spectralis OCT (v
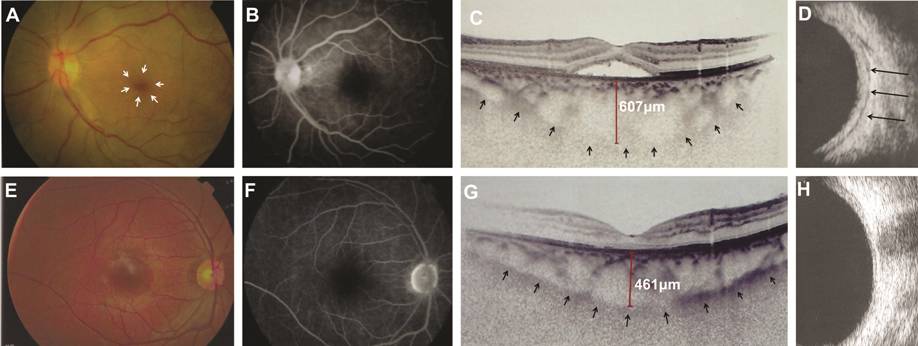
Figure 1 Clinical findings of posterior
scleritis in case
Statistical Analysis Data were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Levene’s test was used to confirm normally distribution. A paired t-test was used to determine changes in macular CT. Pearson’s correlation analysis was performed to evaluate the relationships between the changes in CT, BCVA, AL, and PCT. P<0.05 was considered statistically significant.
RESULTS
Demographic Characteristics During the five-year period from August 2012 to July 2017, nearly 300 000 patients were referred to the Ophthalmology Department of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University. Of these, 69 patients (0.023%) had PS and 33.33% of the patients with PS also presented with retinal detachment; therefore, a total of 23 patients (23 eyes) included in the study population (Table 1).
Table 1 Demographic characteristics of the 23 patients
No. |
Sex |
Age, y |
Ocular pain |
Visual acuitya |
Anterior scleritis |
AC cell or flare |
Retinal phlebectasia |
Annular retinal detachment |
Optic nerve swelling |
Systemic factors |
1 |
M |
23 |
Yes |
1 |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
2 |
M |
23 |
Yes |
0.1 |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
3 |
F |
41 |
Yes |
0.5 |
Yes |
No |
Yes |
Yes |
Yes |
ANA+ |
4 |
F |
24 |
Yes |
0.2 |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
5 |
F |
19 |
Yes |
0.7 |
No |
Yes |
Yes |
Yes |
Yes |
No |
6 |
M |
24 |
Yes |
0.4 |
Yes |
Yes |
Yes |
Yes |
No |
No |
7 |
F |
36 |
Yes |
0.7 |
No |
No |
Yes |
Yes |
No |
No |
8 |
F |
57 |
Yes |
0 |
No |
No |
No |
Yes |
No |
No |
9 |
F |
28 |
Yes |
0.5 |
No |
No |
Yes |
Yes |
Yes |
No |
10 |
F |
26 |
Yes |
1.1 |
No |
No |
No |
Yes |
No |
No |
11 |
M |
27 |
Yes |
0 |
Yes |
Yes |
Yes |
Yes |
No |
No |
12 |
M |
25 |
Yes |
0.5 |
No |
No |
No |
Yes |
No |
No |
13 |
M |
21 |
Yes |
1.2 |
No |
No |
No |
Yes |
Yes |
Rhinallergosis |
14 |
F |
26 |
Yes |
0.2 |
Yes |
No |
No |
Yes |
No |
No |
15 |
M |
37 |
No |
1.7 |
Yes |
No |
Yes |
Yes |
No |
No |
16 |
F |
19 |
No |
1 |
No |
No |
Yes |
Yes |
No |
Hypertension |
17 |
F |
38 |
Yes |
0.4 |
No |
No |
Yes |
Yes |
No |
No |
18 |
F |
46 |
No |
2 |
No |
No |
No |
Yes |
No |
SS |
19 |
M |
23 |
Yes |
0.1 |
No |
No |
No |
Yes |
No |
No |
20 |
F |
22 |
Yes |
0.2 |
Yes |
No |
No |
Yes |
No |
No |
21 |
F |
22 |
Yes |
1.3 |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
22 |
M |
35 |
No |
0 |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
23 |
M |
36 |
No |
0.1 |
Yes |
No |
Yes |
Yes |
Yes |
No |
n (%) |
18 (78.26) |
20 (86.96) |
12 (52.17) |
8 (34.78) |
15 (65.22) |
23 (100) |
10 (43.48) |
4 (17.39) |
||
AC: Anterior chamber; ANA: Antinuclear antibody; SS: Sjögren syndrome. alogMAR best corrected visual acuity.
Clinical Characteristics The main characteristics of the study participants are presented in Table 1. This study included 13 female and 10 male patients from 19 to 57 years old (mean age 29.5±9.24y), 78.26% of which were between 21 and 40 years old. Unilateral involvement was present in all of the patients. Ocular pain (78.26%) and vision loss (86.96%) were the two most commonly reported symptoms. Interestingly, there were 2 patients (8.96%) without symptoms identified just through routine physical examination. Around 26.09% patients were diagnosed during their first visit, while the remaining 73.91% patients were initially diagnosed with and treated for other retinal diseases (e.g. CSC with 7 cases, neuroretinitis with 10 cases). Sixteen patients (69.5%) visited the clinic within one month of symptom onset. At the time of presentation, four patients (17%) were documented as having systemic associations including ANA+, hypertension, allergic rhinitis, and Sjögren syndrome. Eighteen patients (78.26%) had a single episode of PS and five patients had multiple episodes of PS, less than 4 episodes .
Visual
Acuity The BCVA (logMAR) was determined for
all patients. As described in Table 2, three (13.04%) patients had a BCVA of 0
logMAR at presentation, 11 (47.83%) patients were below 0.5 logMAR, 3 (13.04%)
patients between 0.5-1 logMAR, and 6 (26.09%) patients over 1 logMAR. No
patients were found to be amaurosis or light perception using this test. The
mean of the logMAR visual acuity was 0.61±0.54 logMAR. In their other eyes, 20
(86.96%) patients had a BCVA of 0 logMAR and 3 (13.04%) patients were between
0.08-0.2 logMAR (average 0.017±0.045). As expected, the BCVA in PS-affected
eyes was worse than in the unaffected eyes. The BCVA decrease in PS was not
related to the unaffected eyes according to Pearson’s correlation coefficient (r=0.105; P=0.635).
Table 2 The basic clinical features of posterior scleritis in 23 cases
Clinical features |
Eyes (%) |
Age |
|
≤20y |
2 (8.70) |
21-40y |
18 (78.26) |
>40y |
3 (13.04) |
Gender |
|
Male |
10 (43.48) |
Female |
13 (56.52) |
Complaint pain |
18 (78.26) |
Complaint vision loss |
20 (86.96) |
Diagnosis rate |
6 (26.09) |
Onset time (1mo) |
16 (69.57) |
Visual acuity (logMAR) |
|
0 |
3 (13.04) |
>0-0.5 |
11 (47.83) |
>0.5-1 |
3 (13.04) |
>1 |
6 (26.09) |
Total |
23 (100) |
Anterior
Segment PS occurred in 12 (52.17%) patients
with associated anterior scleritis, which can cause symptoms of conjunctival
congestion and tenderness in the superficial sclera (Figure
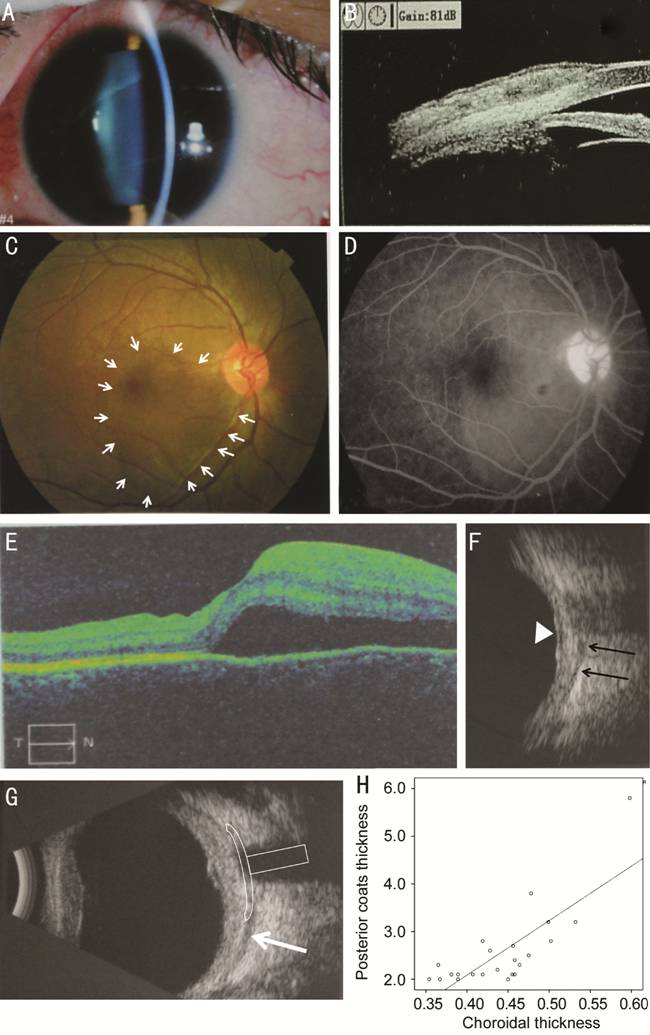
Figure 2 Clinical findings of posterior
scleritis with serous retinal detachment A: Anterior segment examination showed conjunctival congestion and
anterior scleritis with tenderness in the upper part of the sclera; B: UBM
examination detected swelling and hyperemia in the superficial fasciitis and
sclera at the 12 o’clock; C: Posterior segment examination revealed retinal
detachment at the macula (white arrow); D: FFA illuminated the serous retinal
detachment at the macula but no fluorescein leakage was detected; E: The OCT
scanning confirmed serous retinal detachment at the macula; F: B-scan
ultrasonography confirmed scleral thickening (black arrows indicate the
posterior boundary of the sclera and the retinal detachment is indicated by the
white triangle); G: B-scan ultrasonography showing diffuse thickening of the
posterior coats of the eye (
Posterior
Segment A number of abnormalities were
observed in PS-affected eyes. All of the patients displayed dome-shaped macular
retinal detachment (Figures
Ultrasonography
Examination B-scan ultrasonography is the most
useful confirmatory analysis for PS diagnosis[12],
so all eyes had been examined with B-scan ultrasound. The longitudinal B-mode
ultrasound scan showed diffuse PCT with fluid both in the Tenon’s capsule and
around the nerve sheath (Figures 1D,
Table 3 Clinical measurements in posterior scleritis and fellow eyes n=23, mean±SD
Measurements |
Posterior scleritis |
Fellow eyes |
Difference, mean (95%CI) |
t |
aP |
Visual acuity, logMAR |
0.61±0.54 |
0.017±0.045 |
0.593 (0.36 to 0.83) |
5.03 |
0.000 |
IOP, mm Hg |
14.21±6.61 |
15.1±2.36 |
-0.89 (-2.5 to -0.69) |
-1.19 |
0.248 |
AL, mm |
22.94±1.24 |
24.4±0.58 |
-1.46 (-1.98 to -0.92) |
-5.64 |
0.000 |
Choroid thickness, μm |
442.61±55.61 |
246±42.31 |
196.61 (183.78 to 209.43) |
31.8 |
0.000 |
Posterior coats thickness, mm |
2.51±0.81 |
1.09±0.29 |
1.48 (1.26 to 1.93) |
9.8 |
0.000 |
AL: Axial length; IOP: Intraocular pressure; CI: Confidence interval. aP<0.05 was considered statistically significant.
Choroidal
Thickness Changes in the Macula The CT of the subfoveal area was
examined by EDI-OCT (Figure
DISCUSSION
This study analyzed the clinical features of PS with serous retinal detachment in 23 Han Chinese patients. Demographically, participants in our study were much younger at disease onset (29.5y, 78% below 40 years old) compared to a previous study performed in England (49.3y)[12] but similar to a report from Yang et al[2] (29.2y) which also based on Chinese patients. This finding suggests that young adults might have a higher risk of developing PS in the Chinese population.
It had been reported that systemic or local diseases may contribute to the development of PS[2]. The present study found that 17% of patients with PS had systemic or local diseases, which was lower than described in studies conducted in England[12] and Singapore (37%)[4] but, again, consistent with the study in China (13%)[2]. These results suggest that the association between PS and autoimmune diseases may be weaker in China than in England and Singapore.
In the
present study, we found that while blurred vision was one of the most common
symptoms of PS, but the BCVA (logMAR) remained below
Many studies have indicated that PS is highly associated with anterior scleritis at rates that range from 20% to 80% overall[3, 4, 12]; specifically, 19% in Singapore[4], 59% in England[12], 24% in China[2] and 52% in this current study. The incidence of comorbidity between PS and anterior scleritis seems to be higher in this report than previously documented. However, Yang et al[2] and Wieringa et al[14] used the term “panscleritis” to describe the scleritis that involves both the anterior and posterior segments. This difference in the definition of PS might contribute to the inconsistencies observed between these studies. Taken together, these results beg the question of whether the inflammation of the anterior scleritisis secondary to the posterior segments and which risk factors are involved.
As expected, physical manifestations of PS varied in this study and included swollen discs, retinal neuroepithelial folds, macular edema, serous retinal detachment, and more. Importantly, we found 74% patients were initially misdiagnosed. This observation hints at the importance of differential diagnosis for PS with retinal detachment. In this and previous studies[12, 15], B-scan documented a range of ocular abnormalities in patients with PS corresponding to the fundus examination, thereby, the typical T-sign and PCT detected by ultrasonography were confirming signs, potentially necessary for the accurate diagnosis of PS. However, posterior coats thickening can occur under other conditions (e.g. presence of a tumor[16] or orbital inflammatory disease, but the typical symptoms and signs could help us make the right differential diagnosis), so we suggest that PS should be diagnosed using well-established symptoms and B-scan results.
Recently, an abnormal increase in CT has been hypothesized to contribute to CSC[17], primary open angle glaucoma[18], and primary angle closure glaucoma[13]. Given its anatomical location, scleral inflammation can spread to the choroid, so CT was supposed to be affected due to inflammation in PS. In a previous study, Uchihori et al[11] reported a PS patient that the subfoveal CT had grown to 418 μm in PS-affected eye by the initial examination, which is in line with our work (average of 442.61 μm). These observations further support that the increased CT might result from inflammation in PS but future studies that focus on the role of the immune system are needed to draw causal connections. Research on other diseases has revealed associations between pathological CT increasesand clinical factors, such as AL, choroidal inflammation, and IOP[19]. Based on data from this study, CT correlated with AL and PCT but not with IOP and BCVA. Given that the CT measurement included portions of the eyeball, the correlation between CT and PCT is intuitive. Since PCT was expected to positive correlate with inflammation in PS[12], CT likely represents an additional and promising predictive factor. Some researchers have reported CT increases during the active inflammation period that decreased with anti-inflammation treatment over time[10, 11]. Despite the promising nature of the CT results for monitoring inflammation in PS, it does not seem to be related to BCVA. However, due to the limitations imposed by this study’s sample size, further efforts are needed to elucidate the role of CT in PS pathology.
In addition to small study size, this study has some limitations. The retrospective nature of this study greatly limits the significance. And we rule out the infectious factor which was supposed to be associated with PS[20]. Furthermore, most patients were referred by other hospitals and our study population may, therefore, represent the most serious cases.
In conclusion, our study characterized the clinical features of PS with retinal detachment, which frequently manifested as ocular pain and blurred vision in symptom, while serous retinal detachment and optic nerve swelling in sign. Typical T-sign detected by B-scan ultrasound provided a critical indicator for diagnosis. Pathological increases in CT and PCT might be a potential predictive factor for inflammation.
ACKNOWLEDGEMENTS
Foundation: Supported by the Fund of Natural Science Foundation of Zhejiang Province (No.LY18H120009).
Conflicts of Interest: Dong ZZ, None; Gan YF, None; Zhang YN, None; Zhang Y, None; Li J, None; Zheng HH, None.
REFERENCES