
Citation: Zhang P, Tang LJ, Gao HH, Zhang WX, Lin JX, Yang HS.
Immunohistochemical features of carcinoma ex pleomorphic adenoma and
pleomorphic adenoma in the lacrimal gland. Int J Ophthalmol
2019;12(8):1238-1242. DOI:10.18240/ijo.2019.08.02
·Basic Research·
Immunohistochemical features of
carcinoma ex pleomorphic adenoma and pleomorphic adenoma in the lacrimal gland
Ping Zhang1, Li-Juan Tang1,
Huan-Huan Gao1, Wen-Xin Zhang1, Jian-Xian Lin1,
Hua-Sheng Yang2
1Department of Ocular Pathology,
State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen
University, Guangzhou 510060, Guangdong Province, China
2Department of Orbital Disease and
Oncology, State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center,
Sun Yat-sen University, Guangzhou 510060, Guangdong Province, China
Correspondence to: Ping Zhang. Department of Ocular
Pathology, State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center,
Sun Yat-sen University, Guangzhou 510060, Guangdong Province, China.
zhangping@gzzoc.com
Received:
Abstract
AIM: To investigate C-myc, Ki-67, pan-cytokeratin, and vimentin
immunohistochemical features of carcinoma ex pleomorphic adenoma (Ca-ex-PA) and
pleomorphic adenoma (PA) in the lacrimal gland in order to find some clues in
the differential diagnosis between them.
METHODS: We reviewed microscopic slides and clinical records
of 64 cases of PA and 15 cases of Ca-ex-PA in the lacrimal gland. Immunohistochemical
antibodies for C-myc, Ki-67, pan-cytokeratin, and vimentin were employed.
RESULTS: Median age of PA was 43.2y (from 21 to 75). The 35
patients (54.7%) were male and 29 patients (45.3%) were female. For the PAs,
the average positivity of C-myc was 4.6%; the average proliferation index of
Ki-67 was 3.2%; pan-cytokeratin was positive in ductal cells, and vimentin was
positive in myoepithelial cells. Median age of Ca-ex-PA was 54.3y (from 26 to
76). There were 7 male patients (46.7%) and 8 female patients (53.3%). Among 15
Ca-ex-PAs, there were 6 myoepithelial carcinomas, 4 adenocarcinomas, 3
epithelial-myoepithelial carcinomas, and 2 squamous cell carcinomas. For the
Ca-ex-PAs, the average positivity of C-myc was 36.4%; the average proliferation
index of Ki-67 was 29.2%; pan-cytokeratin was positive in all cases, and
vimentin was positive in myoepithelial carcinomas.
CONCLUSION: PA has a lower positivity of C-myc and Ki-67, while
Ca-ex-PA had a higher positivity of these two biomarkers. These four biomarkers
as a set could provide valuable clues in the differential diagnosis between
Ca-ex-PA and PA. Our results indicate that the activation of C-myc could play
an important role in the pathogenesis of Ca-ex-PA and PA.
Keywords: carcinoma ex pleomorphic adenoma;
pleomorphic adenoma; C-myc; immunohistochemistry
DOI:10.18240/ijo.2019.08.02
Citation: Zhang
P, Tang LJ, Gao HH, Zhang WX, Lin JX, Yang HS. Immunohistochemical features of
carcinoma ex pleomorphic adenoma and pleomorphic adenoma in the lacrimal gland.
Int J Ophthalmol 2019;12(8):1238-1242
INTRODUCTION
Pleomorphic adenoma (PA, also called
mixed tumor) is the most common tumor in the lacrimal gland, consisting 50% of
epithelial lacrimal gland tumors[1-2].
Although lacrimal PA is benign, it is inclined to recur after incomplete
surgical resection, and has the possibility to transform into carcinoma ex
pleomorphic adenoma (Ca-ex-PA) with a poor prognosis[3].
Ca-ex-PA is a kind of infiltrative carcinoma arising in a PA.
Ki-67 is a marker for showing cells
DNA synthesis before mitosis. Numerous studies have demonstrated that
malignancies usually have high ki-67 expression related to high cellular
proliferation. C-myc is a key protein in cell cycle regulation. A nuclear
phosphoprotein encoded by MYC gene works as a kind of DNA-binding factor which
activate or repress the transcription of a great quantity of genes. The
aberrations of MYC result in its constitutive activation in many tumors[4].
In order to identify diagnostic
factors for Ca-ex-PA and PA, we evaluated the expression of intermediate
filaments vimentin, pan-cytokeratin, C-myc protein, as well as a proliferation
marker Ki67 antigen in Ca-ex-PA and PA. The study is intended to find some
immunohistochemical biomarkers that could provide assistance in the
differential diagnosis between Ca-ex-PA and PA.
Although many markers have been
researched for their expressions in salivary gland tumors, only several
literatures were found about C-myc expression in Ca-ex-PA and PA[5-7]. We chose pan-cytokeratin,
vimentin, Ki-67 and C-myc to test their capability to describe useful general
diagnostic differences between PA and Ca-ex-PA in the lacrimal gland. This
paper has a purpose to set up a baseline of some immunohistochemical markers
which could provide aid in the diagnosis of controversial or difficult cases in
some circumstances.
SUBJECTS AND METHODS
Ethical Approval This was a retrospective,
noninterventional study, which was performed on the basis of the principles of
the Declaration of Helsinki. Informed consent was waived due to the
retrospective nature of the study.
Tissues PA tissues were collected from the
archives of Zhongshan ophthalmic center in the period 2015-2018. Ca-ex-PA
tissues were collected in the period 2012-2018. We obtained the clinical
information from the medical records. Sections were cut from the
formalin-fixed, paraffin-embedded specimens and were stained with hematoxylin
and eosin.
Immunohistochemistry Formalin-fixed paraffin-embedded
specimens were cut at a thickness of 4 μm and mounted on coated slides for
immunohistochemical staining. The following antibodies were utilized: C-myc
(clone Y69; rabbit monoclonal; Abcam, prediluted), Ki-67 (clone 7B11; mouse
monoclonal; Abcam, prediluted), pan-cytokeratin (clone AE1/AE3; mouse
monoclonal; Abcam, prediluted), vimentin (clone V9; mouse monoclonal; Abcam,
prediluted). The sections were processed using Leica Bond Max autostainer at
our Department of Pathology. Positive controls and negative controls were
carried out respectively. The negative controls were omitted the primary
antibodies. The tissues were stained with chromogen diaminobenzidine and were
counterstained with hematoxylin. The positive cells with brown nucleuses of
Ki-67 and C-myc were counted in three representative high-power fields. Then
the results were averaged.
Statistical Analysis The independent-samples t
tests were conducted for analyzing data. SPSS software version 22 was used for
the analyses. The statistical tests were two-sided. And a P value of
0.05 or less was considered statistically significant.
Results
There were 64 cases of PA and 15
cases of Ca-ex-PA in the lacrimal gland in all. The mean age of patients with
PA was 43.2y (range from 21 to 75). Among them 35 patients (54.7%) were male
and 29 patients (45.3%) were female. And the mean age of patients with Ca-ex-PA
was 54.3y (range from 26 to 76). Eight patients (53.3%) were female and seven
patients (46.7%) were male. Among 15 cases of Ca-ex-PAs, there were 6
myoepithelial carcinomas, 4 adenocarcinomas, 3 epithelial-myoepithelial carcinomas,
2 squamous cell carcinomas.
Histologically, PA is benign
neoplasm consisting of ductal cells (DCs) and myoepithelial cells (MECs) which
are in a chondromyxoid stroma. All specimens of PA had a pseudocapsule of
variably thick and were composed of lumens formed with double-layered cellular
walls as well as myoepitheliomatous cells of spindle shape. The DCs are
generally cuboidal epithelium cells lining a tubule. And that the MECs are
generally spindle, oval, or polygonal with punctate nuclei chromatin which has
no nucleolus or only has a minute one. The outer layer MECs in the ductular
structures feathered into the stroma. The malignant components of the Ca-ex-PAs
are myoepithelial carcinomas, adenocarcinomas, epithelial-myoepithelial
carcinomas, squamous cell carcinomas respectively (Figure 1).
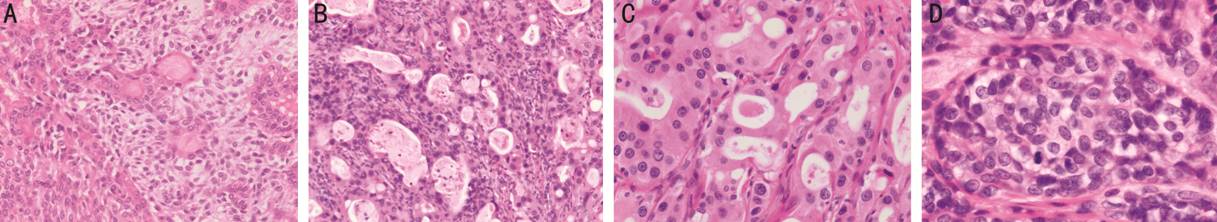
Figure 1 Histopathology of lacrimal
gland Ca-ex-PA and PA A: PA is composed of MECs and DCs in
a chondromyxoid stroma (HE×200); B: Epithelial-myoepithelial carcinoma displays
DCs and MECs with atypical hyperchromatic nuclei (HE×200); C: Adenocarcinoma
consist of cuboidal cells which have large hyperchromatic nuclei with prominent
nucleoli (HE×200); D: Myoepithelial carcinoma is composed of clear tumour cells
arranged in small lobules and sheets with hyperchromatic nuclei and mitosis
(HE×400).
Immunohistochemically, in the Pas,
the DCs displayed strong and diffuse positivity to cytokeratin. And the
myoepithelial component showed positive to vimentin and few positive to
pan-cytokeratin. While in Ca-ex-PAs, pan-cytokeratin was positive in all cases,
and vimentin was positive in myoepithelial carcinomas (Figure 2).
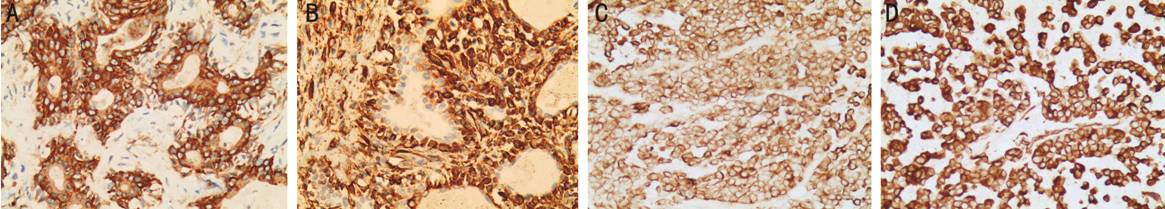
Figure 2 Immunohistochemical
staining results of PA and myoepithelial carcinoma (×200) A: DCs in PA displayed strong and
diffuse positivity for pan-cytokeratin; B: The myoepithelial of PA were
positive to vimentin; C: The tumor cells of myoepithelial carcinoma showed
positive to pan-cytokeratin; D: The tumor cells of myoepithelial carcinoma
showed also positive to vimentin.
The proliferation index of Ki-67 for
the PAs was obviously lower with an average of 3.2%±1.3% (range of 1% to 6%).
The average C-myc positivity in the PAs was 4.6%±1.5% (range of 2% to 8%). The
proliferation index of Ki
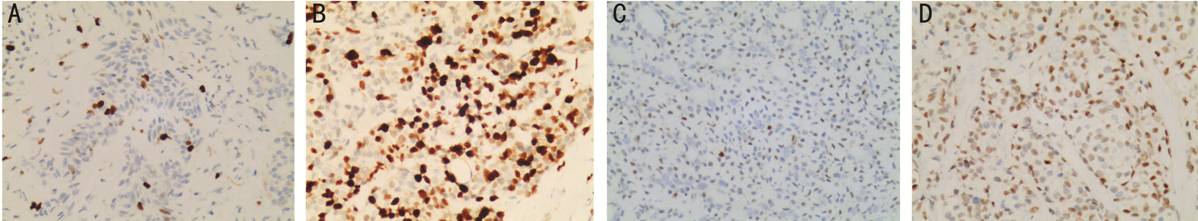
Figure 3 Immunohistochemical staining results of PA and
Ca-ex-PA (×200) A: A few of Ki-67 positive cells in
PA; B: A lot of Ki-67 positive cells in Ca-ex-PA; C: A few of C-myc positive
cells in PA; D: A lot of C-myc positive cells in Ca-ex-PA.
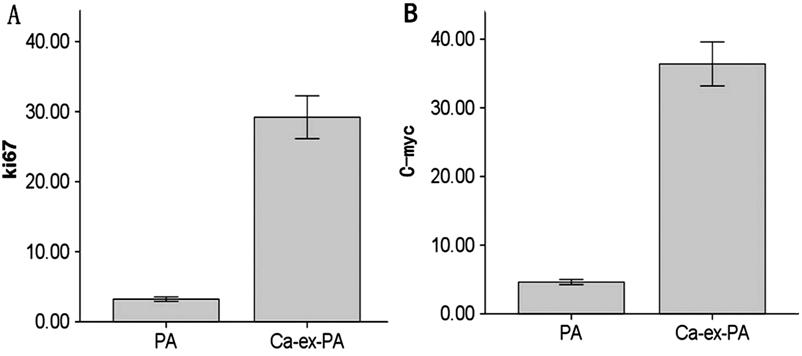
Figure 4 Immunohistochemical results
of Ca-ex-Pas and PAs A: Compared with PA, Ca-ex-PAs
showed higher Ki67 expression (P<0.01); B: Compared with PA,
Ca-ex-PAs showed higher C-myc expression (P<0.01). Bars indicate
standard deviation. PA, n=64; Ca-ex-PA, n=15.
DISCUSSION
Lacrimal PA is a kind of benign
tumor with an ability to transform into Ca-ex-PA. Clinically, patients with PA
generally present with a history of slowly increasing bulbar displacement
painlessly. Patients with Ca-ex-PA usually present with rapidly increasing
bulbar displacement with a poor prognosis that has a median survival of 3y[8-9].
Histologically, PA is a benign
neoplasm consisting of DCs and spindle or polygonal MECs in a chondromyxoid
stroma with a pseudoencapsule. The lumens usually contained eosinophilic,
amorphous secretory material which was positive for Alcian blue and periodic
acid Schiff. There is basophilic mucoid material around the ductlike units[8]. PA had double-layered, epitheliumlined glandular
structures which have small to expanding lumens and the MECs of the outer layer
of ductular structures feathering into the stroma[8].
In PA, both myoepithelial and
luminal cells could transform into malignancy. But in most cases, the
malignancy seems to occure from luminal cells[10].
Once carcinoma has arised, it could present with multiple tumor phenotypes. The
most common malignant component in Ca-ex-PA is adenocarcinoma. And the other
malignant components are myoepithelial carcinoma, adenoid cystic carcinoma,
epithelial-myoepithelial carcinoma, squamous cell carcinoma, and clear cell
carcinoma, adenosquamous carcinoma, acinic cell carcinoma[11-12]. Katabi et al[13]
reported that the salivary duct carcinoma and myoepithelial carcinomas are the
most common subtypes of Ca-ex-PA. In our series of 15 cases of Ca-ex-PA there
are 6 myoepithelial carcinomas, 4 adenocarcinomas, 3 epithelial-myoepithelial
carcinomas, and 2 squamous cell carcinomas respectively. So in our study the
most common malignant component in Ca-ex-PA is myoepithelial carcinoma. Maybe
the types of lacrimal glandular Ca-ex-PAs are different with salivary
Ca-ex-PAs, which needs to be further studied.
Sometimes it is difficult to
differentiate between Ca-ex-PA and PA. So we need Immunohistochemical stain to
help us to make a correct diagnosis. In this study, the DCs in the PA areas
displayed strong and diffuse positive for pan-cytokeratin and negativefor
vimentin. The myoepithelial components of PA were positive for vimentin and
negative for pan-cytokeratin. These results were similar to the research
reported by Sedassari et al[5].
Myoepithelial carcinoma of lacrimal gland is rare, and there are only a few
cases reported in the literature[14-15].
In our study the myoepithelial carcinoma displayed strong and diffuse positive
to both pan-cytokeratin and vimentin which is similar to the case reported by
Larbcharoensub et al[16].
Ki-67 is a marker showing DNA
synthesis before mitosis. Numerous studies have demonstrated that malignancies
usually have high ki-67 expression related to high cellular proliferation. This
antibody recognizes a nuclear protein that is involved in the premitotic phases
(G1, S, G2 and M) in the cell cycle. This nuclear protein can be used to
estimate the growth status by showing the positive cells from all other present
cells (Ki-67 proliferation index, or PI)[17-18]. Our results manifested an obviously low Ki-67
proliferation index in PAs (average 3.2%±1.3%, range of 2.1% to 5.2%), Whereas
Ca-ex-PAs demonstrated much higher Ki-67 proliferation index (average
29.2%±5.5%; range of 20% to 35%). Whereas the immunohistochemistry expression
results of Ki
C-myc is a key protein in cell cycle
regulation. Encoded by MYC gene, a nuclear phosphoprotein can serve as a factor
of DNA-binding which will activate or repress the transcription of a great
quantity of genes such as P27, P21 and P15, that makes contribution to cell
cycle progression in the phase of early and mid-G1[4,20-21]. Research shows that C-myc not
only functions as a transcription factor that enhances many downstream genes to
translate but also relate to regulating many cellular processes such as
chromate instructure, mRNA translation, DNA replication and biogenesis of
ribosomes[22-23]. MYC
overexpressed in head and neck squamous cell carcinomas[24]
and in gastric carcinomas[25]. Several researches
show that C-myc overexpressed in salivary PA[6-7,26]. Our research found that the
average C-myc positivity in Ca-ex-PAs was much higher than that in PAs, which
helps to make a correct diagnosis in some confused situations.
In a conclusion, the DCs in the PA
displayed positive for pan-cytokeratin and negative for vimentin. The
myoepithelial component in the PA displayed positive for vimentin and negative
for pan-cytokeratin. While the myoepithelial carcinoma showed positive to both
pan-cytokeratin and vimentin. And the average Ki67 and C-myc positivity in
Ca-ex-PAs was much higher than those in PAs. So the set of these four
antibodies could help to provide clues in the differential diagnosis between
Ca-ex-PA and PA of the lacrimal gland.
ACKNOWLEDGEMENTS
Foundation: Supported by the National Natural
Science Foundation of China (No.30371515).
Conflicts of Interest: Zhang P, None; Tang LJ, None; Gao
HH, None; Zhang WX, None; Lin JX, None Yang HS, None.
REFERENCES