
Citation: Wang L, Shi KP, Li H, Huang H, Wu
WB, Cai CS, Zhang XT, Zhu XB. Activation of the TRAAK two-pore domain potassium
channels in rd1 mice protects photoreceptor cells from apoptosis. Int J
Ophthalmol 2019;12(8):1243-1249. DOI:10.18240/ijo.2019.08.03
·Basic Research·
Activation of the TRAAK two-pore domain potassium
channels in rd1 mice protects photoreceptor cells from apoptosis
Lei Wang, Kang-Pei Shi, Han Li,
Hao Huang, Wen-Bin Wu, Chu-Sheng Cai, Xiao-Tong Zhang, Xiao-Bo Zhu
State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun
Yat-sen University, Guangzhou 510060, Guangdong Province, China
Correspondence to: Xiao-Bo Zhu. State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic
Center, Sun Yat-sen University 54S Xianlie Road, Guangzhou 510060, Guangdong
Province, China. zhuxbo@mail.sysu.edu.cn
Received:
Abstract
AIM: To investigate the expression
of TWIK-related arachidonic acid-stimulated K+ channel (TRAAK) in
retinal degeneration mice (rd1) and further evaluate how TRAAK affect
photoreceptor cell apoptosis.
METHODS: The rd1 mice were distributed
into blank (no treatment), control (1.4% DMSO, intraperitoneal injection) and
riluzole groups (4 mg/kg·d, intraperitoneal injection) from postnatal 7d to 10,
14 and 18d; C57 group (no treatment), as age-matched wild-type control. The
thickness of the outer nuclear layer (ONL) of retina was detected by paraffin
section hematoxylin and eosin staining. The expression of TRAAK and the
apoptosis of the ONL cells were detected by immunostaining, Western blotting,
and real-time polymerase chain reaction.
RESULTS: The channel agonist riluzole
activated TRAAK and delayed the apoptosis of photoreceptor cells in ONL layer
of rd1 mice. Both at mRNA and protein levels, after riluzole treatment, TRAAK
expression was significantly upregulated, when compared with the control and
blank group. Then we detected a series of apoptosis related mRNA and protein.
The anti-apoptotic factor Bcl-2 downregulated and the pro-apoptotic factors Bax
and cleaved-caspase-3 upregulated significantly.
CONCLUSION: Riluzole elevates the
expression of TRAAK and inhibits the development of apoptosis. Activation of
TRAAK may have some potential effects to put off photoreceptor apoptosis.
KEYWORDS: TRAAK;
riluzole; photoreceptor cell; apoptosis
DOI:10.18240/ijo.2019.08.03
Citation: Wang L, Shi KP, Li H, Huang H, Wu WB, Cai CS, Zhang XT,
Zhu XB. Activation of the TRAAK two-pore domain potassium channels in rd1 mice protects
photoreceptor cells from apoptosis. Int J Ophthalmol
2019;12(8):1243-1249
INTRODUCTION
Retinitis pigmentosa (RP) is a large class of retinal disease caused by a
group of inherited gene mutations that cause photoreceptor cell death[1]. The main manifestations are night blindness,
progressive visual field damage, fundus pigmentation, abnormal or wave-free
retinal electroretinogram, and central vision loss[2].
Retinal metabolism is active, and photoreceptor cells are at a high oxygen
level for a long time, which may result in their own oxidative stress[3]. Bcl-2 and caspase-3 are two important members in
regulating cell apoptosis[4]. Activation of Bcl-2-related
family members and changes in mitochondrial permeability and membrane potential
may occur when cells are stimulated by extracellular apoptosis signals or
related factors, such as increases in reactive oxygen species (ROS) and widely
changes of oxidative stress injury. All of the alteration lead to cascade
activation of the caspase-3 family, which inevitably leads to cell apoptosis[5]. During the occurrence and development of RP, oxidative
stress injury always occurs, which induced attentional apoptotic effect in
photoreceptor cells.
The TREK-TRAAK channel belongs to two-pore potassium ion channels family.
This member contains TREK-1, TREK-2, and TRAAK[6].
Activation of TRAAK generates background potassium leakage currents that are
participated in regulating excitability of cells and the resting membrane
potential[7-10]. TRAAK is widely
expresses in the central nervous system. Activation of TRAAK by polyunsaturated
fatty acids in brain tissue has a protective effect on neuronal death induced
by cerebral ischemia[11]. In addition, TRAAK also
widely expresses in the mouse retina[12]. Our
previous studies have shown that apoptosis of A-RPE19 and human-RPE (hRPE)
cells induced by t-BH can be inhibited by TRAAK agonists by activating of TRAAK
in vitro[13-14]. In
this study, the channel agonist riluzole was used to activate this channel in
the retina of a RP mouse model (rd1 mouse) to observe the changes in apoptosis
in the retina-related cell layer and the expression of apoptosis related factors.
MATERIALS AND METHODS
Ethical Approval All of the animal experiments
conformed to the relevant regulations of the ARVO Center on Animal Feeding,
Ophthalmology and Visual Research and ethics committee of the Zhongshan Eye
Center (ethics code: 2015-131).
Animals The rd1 mice (FVB/rd1) and C57BL/6
mice were purchased respectively from Vital River Laboratory Animal Technology
Co. Ltd. (Beijing, China) and Southern Medical University (Guangzhou, China).
All animals were kept in the SPF laboratory of Zhongshan Ophthalmic Animal
Center and were fed with clean food and water (
Frozen Sections Fresh eyeballs were rapidly
enucleated and placed in liquid nitrogen to snap frozen. Then the tissue was
preserved at
Immunostaining The immunofluorescence staining
assay of sections was described previously[17].
First, the tissue sections were deparaffinized and rehydrated, then, at room
temperature, the sections were incubated with 1% BSA (MP Biomedicals, USA) and
0.5% Triton X-100 (MP Biomedicals, USA) for 30 min. After that, primary
antibodies against TRAAK (1:100; Alomone labs, Israel) in 1% BSA were incubated
at
H&E Staining for Paraffin Sections
The eyeballs
of rd1 and C57BL/6 mice were paraffin embedded. The 5 μm thickness of tissue
sections were sliced for stained with hematoxylin and eosin (H&E). Then
sections were H&E, and images were acquired using a digital imaging system
(Olympus, Japan). The outer nuclear layer (ONL) thickness were analyzed and
calculated at 200 μm far away from optic nerve head.
TUNEL Assays The above sections were
double-stained with a TUNEL kit (Roche, Switzerland) and
RT-PCR Total retina tissue RNA was
extracted with Trizol reagent (MP Biomedicals, USA). Reverse transcription
procedures according to HiScript® II Q RT SuperMix kit (Vazyme,
China) manufacture’s protocol. And then quantitative RT-PCR was conducted via
a ChamQ SYBR Color qPCR Master Mix kit (Vazyme, China) following the
manufacture’s protocol. The PCR cycle conditions for the reaction were
Protein Extraction and Western Blotting Mouse retinal tissues were
homogenized 100:
Statistical Analysis Data were presented with the
means±standard deviation (SD). One-way ANOVA was used to assess differences
between experimental and control group. Each experiment was repeated 3 times,
as indicated “n=
RESULTS
Localization and Expression of TRAAK on Mouse Retinas TRAAK is represented by red
fluorescence and is widely expressed on the retina in rd1 mice and C57BL/6 mice
(Figure
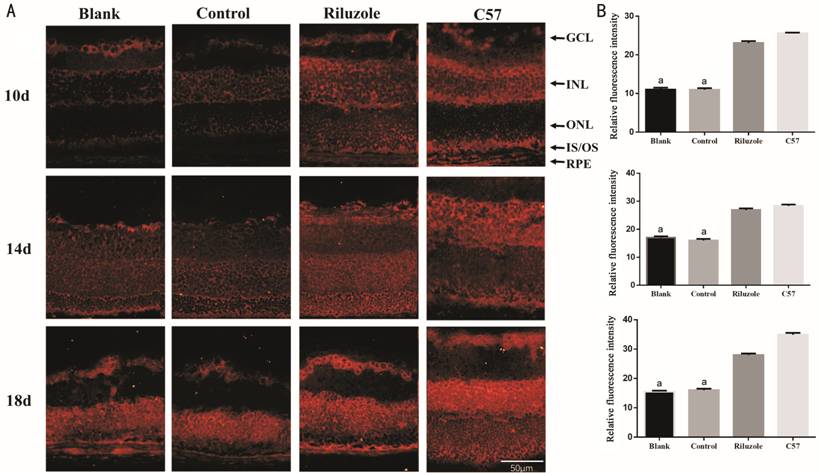
Figure 1 Immunofluorescence analysis demonstrated the expression of TRAAK
on the retina A: The expression and distribution
of TRAAK on the retina of mice at 10, 14, and 18d, TRAAK presented red
fluorescence; B: Relative fluorescence intensity of TRAAK K2P on the retina of
mice at 10, 14, and 18d. GCL: Ganglion cells layer; INL: Inner nuclear layer;
ONL: Outer nuclear layer; IS/OS: Inner and outer segments; RPE: Retinal pigment
epithelial. The bar graphs show the means±SD (n=3). aP<0.01
vs riluzole group. Magnification 200×.
Riluzole Reduced the Apoptosis of Photoreceptor Cells The riluzole group was more elevated
compared with control and blank group in the thickness of ONL at the three time
points; there existed no significant difference between control and blank group
(Figure 2). In the ONL of the riluzole group at the 3 time points, fewer
TUNEL-positive (TUNEL+) cells was detected. Like previous result, no
significant differences in the number of TUNEL+ cells between control and blank
groups, and the C57 group had almost no TUNEL+ cells (Figure
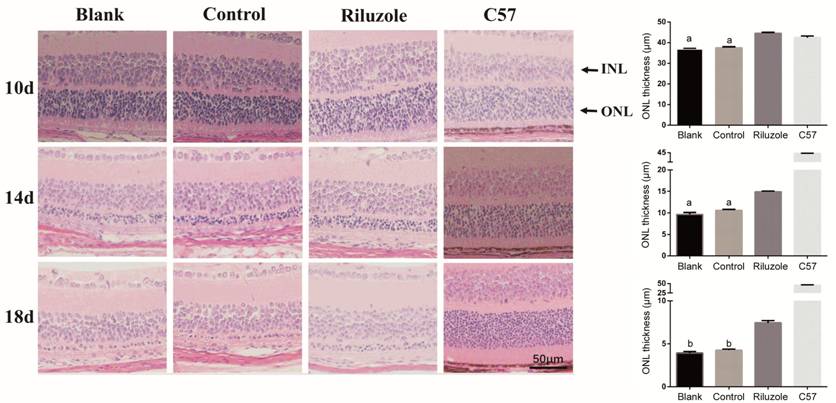
Figure 2 H&E staining in paraffin section detected the thickness of ONL
in all groups At 10, 14, and 18d, the thickness of the
ONL in all groups were measured. aP<0.05 vs
riluzole group; the bar graphs show the means±SD (n=4). ONL: Outer
nuclear layer. bP<0.01 vs riluzole group.
Magnification 200×.
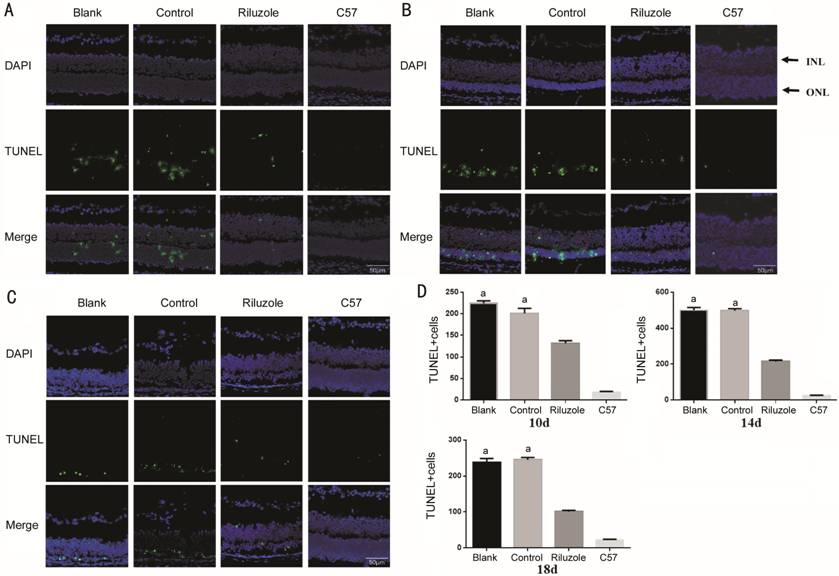
Figure 3 TUNEL staining revealed the distribution of apoptotic cells in the
retina A-C: Apoptosis of cells in all group
at 10, 14, and 18d. The cell nucleus stained with DAPI showed blue
fluorescence, and the TUNEL+ cells were distributed in the ONL and INL with
green granular fluorescence. D: Quantity of TUNEL+ cells in the ONL of all
groups at 10, 14, and 18d. DAPI:
Riluzole Activated the Expression of TRAAK and Inhibited the Development of
Apoptosis In riluzole group, the mRNA and
protein expression levels of TRAAK were significantly upregulated than those in
the blank and control group at three time points. The mRNA and protein
expression levels of TRAAK between the control and blank group had no
significant differences. Versus with the control and blank groups, the mRNA and
protein expression levels of Bcl
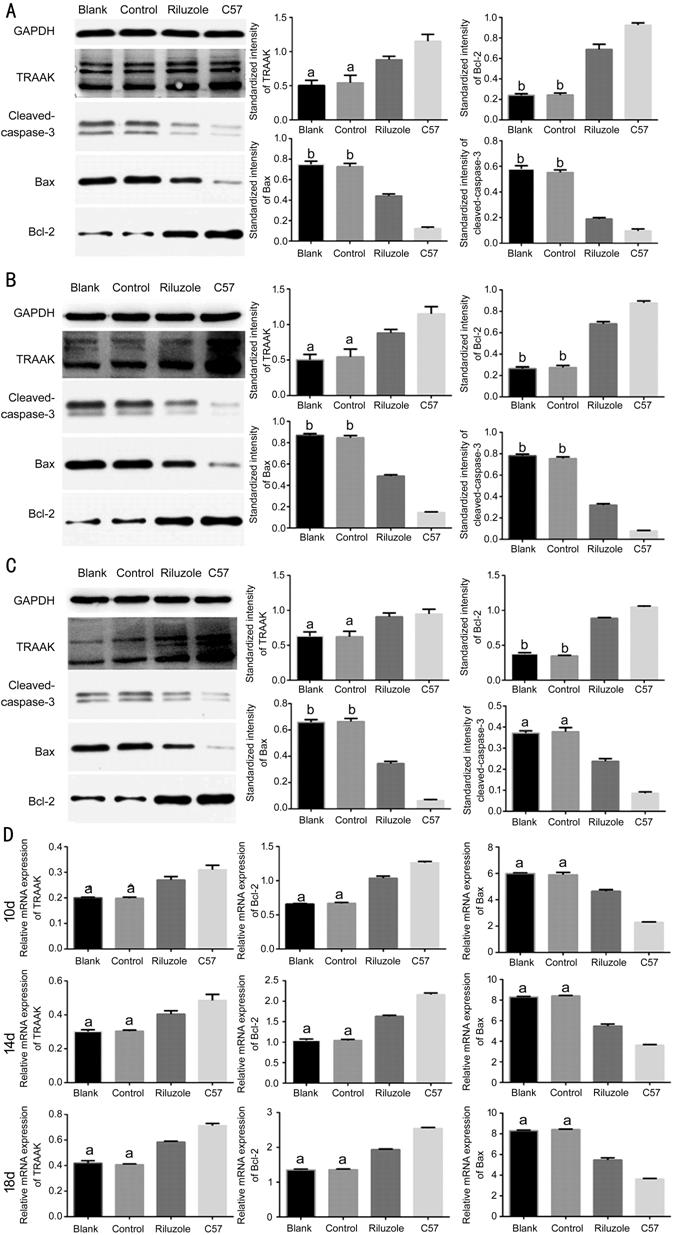
Figure 4 Western blotting and RT-PCR demonstrated the variations of TRAAK,
and apoptosis-related factors expression levels A-C: Western blotting detected
protein expression of TRAAK, Bcl-2, Bax and cleaved caspase-3 at 10, 14, and
18d; D: RT-PCR detected the mRNA expression levels of TRAAK, Bcl-2 and Bax at
10, 14, and 18d. The bar graphs show the means±SD (n=3). aP<0.05
vs riluzole group; bP<0.01 vs riluzole
group.
DISCUSSION
The rd1 mouse model is a successful and representative animal model for RP.
There are morphological changes mainly in the retinal ONL layer, with gradual
thinning over time that leads to the complete disappearance of the ONL. At
approximately 8d, the outer segment starts to die, followed by the death of the
inner segment and photoreceptor cells, which peaks around 14d and is nearly
complete by 21d[18]. In light of this sequence,
this study evaluated the occurrence and development of photoreceptor apoptosis
from the perspective of morphology and molecular change mechanisms at the early
(10d), peak (14d) and later (18d) stages of mouse apoptosis.
Cell shrinkage usually happens at early phase of apoptosis and always
accompanies with decreasing in intracellular potassium concentration regulating
by TRAAK[19]. Hence, we speculate reversing
potassium outflow at the early stage of apoptosis may alter the progression of
apoptosis. Riluzole is a first-line drug for the treatment of lateral sclerosis
of spinal cord and has a certain effect on cerebral ischemia, anxiety and other
diseases[20-21]. The effect of
riluzole on TRAAK is rapid, reversible, dose-dependent and, unlike TREK-1 or
TREK-2, it is a sustained response[22]. A study
reported that riluzole protected nerve cell from apoptosis and improved the
recovery of retinal function in rats with ischemic injury [23].
Intraperitoneal injection and topical treatments of riluzole can delay the
damage of retinal ganglion cells in the glaucoma model of rats with high
intraocular pressure[24]. These protective
effects may be partially own to activation of TRAAK. In this study, we used
riluzole to activate TRAAK in rd1 mice to explore whether there is the same
protective effect against apoptosis and to determine the possible mechanism.
The distribution and expression of TRAAK in mice retinas are shown in
Figure 1. It can be seen that TRAAK is expressed in almost all retinal layers
of the two kinds of mice, mainly in the ganglion cells, ONL, inner nuclear
layer and retinal pigment epithelial, which is basically consistent with
previous research[25]. TRAAK is widely involved
in the physiological activities of the retina, but its specific mechanism of
action on the retina remains unclear. In this study, the average fluorescence
intensity of TRAAK in ONL in the riluzole group were higher than that in the
control and blank groups at all time points. The thickness of the ONL in the
riluzole group was thicker than that in the control and blank groups. In
riluzole group, we found the quantity of TUNEL-positive cells in the ONL of the
were decreased. These results demonstrated that riluzole downregulated the ONL
photoreceptor cells’ apoptosis by upregulating the expression of the TRAAK in
the retina of rd1 mice. To further explore the possible relationship between
the expression of TRAAK and apoptosis, Western blot and RT-PCR assays were
performed. In the riluzole group, the protein expression of TRAAK was
increased, and the mRNA expression showed the same trend. The increase of this
expression was consistent with the change in the previous relative fluorescence
intensity of TRAAK. The expression of Bax and cleaved-caspase-3 were decreased
in the riluzole groups at the level of transcription and translation, and
expression of Bcl-2 was elevated. These changes reversed apoptosis signaling in
normal rd1 mice, indicating that increased expression of the TRAAK had a
protective effect against apoptosis, demonstrating that this channel was
involved in early and late regulation of apoptosis.
This study had certain limitations. We have manifested riluzole, as a TRAAK
channel agonist can protect and even reverse the photoreceptor apoptosis in
vitro and vivo. However, the specific mechanism about TRAAK channel
regulating apoptosis needs further research. Besides, due to the experimental
conditions and other factors, our experiment did not detect the changes of
mouse visual function, such as electroretinogram or electrooculogram. Moreover,
relative electrophysiological technique to future studies should be included to
evaluate TRAAK effect sufficiently. In conclusion, our results show that the
use of riluzole increases the expression of the TRAAK in the retina of rd1
mice, delays the apoptosis of photoreceptor cells in the ONL layer of rd1 mice,
and inhibits the development of apoptosis. Therefore, TRAAK has potential
research value in the treatment of RP.
ACKNOWLEDGEMENTS
Foundation: Supported by
National Natural Science Foundation of China (No.81271012)
Conflicts of Interest: Wang L, None; Shi KP, None; Li H, None;
Huang H, None; Wu WB, None; Cai CS, None; Zhang XT,
None; Zhu XB, None.
REFERENCES