
Citation: Luo ZW, Wang HT, Wang N, Sheng
WW, Jin M, Lu Y, Bai YJ, Zou SQ, Pang YL, Xu H, Zhang X. Establishment
of an adult zebrafish model of retinal neurodegeneration induced by NMDA.
Int J Ophthalmol 2019;12(8):1250-1261. DOI:10.18240/ijo.2019.08.04
·Basic Research·
Establishment of an adult zebrafish model of retinal
neurodegeneration induced by NMDA
Zhi-Wen Luo1,2,
Han-Tsing Wang3,4, Ning Wang1,2, Wei-Wei Sheng1,2,
Ming Jin1, Ye Lu1, Yi-Jiang Bai1,2, Su-Qi Zou3,4,
Yu-Lian Pang1, Hong Xu3,4, Xu Zhang1,4
1Affiliated Eye Hospital of Nanchang
University; Jiangxi Research Institute of Ophthalmology & Visual Science,
Nanchang 330006, Jiangxi Province, China
2Queen Mary School of Nanchang
University, Nanchang 330031, Jiangxi Province, China
3Institute of Life Science, Nanchang
University, Nanchang 330031, Jiangxi Province, China
4Jiangxi Provincial Collaborative
Innovation Center for Cardiovascular, Digestive and Neuropsychiatric Diseases,
Nanchang 330031, Jiangxi Province, China
Correspondence to: Xu Zhang. Affiliated Eye Hospital
of Nanchang University, 463 Bayi Road, Nanchang 330006, Jiangxi Province,
China. xuzhang19@163.com; Hong Xu. Institute of Life Science, Nanchang
University, Nanchang 330031, Jiangxi Province, China. xuhong@ncu.edu.cn
Received:
Abstract
AIM: To establish a model of
retinal neurodegeneration induced by N-Methyl-D-aspartic acid (NMDA) in adult
zebrafish.
METHODS: We compared the effects of three
different NMDA delivery methods on retinal neurodegeneration in adult
zebrafish: immersion (I.M.), intravitreal injection (I.V.), and intraperitoneal
injection (I.P.), and examined retinal pathology and degeneration by
hematoxylin and eosin and TUNEL staining in the treated zebrafish. Effects of
the NMDA receptor antagonist MK-801 and the natural product resveratrol on
NMDA-induced retinal neurodegeneration were also assessed.
RESULTS: The thickened inner retina was
seen in histology with 100 μmol/L NMDA by I.M. administration. Significant apoptosis in the retinal
ganglion cell layer and retinal thickness reduction occurred in 0.5 mol/L NMDA
I.P. administration group.Seizure-like behavioral changes, but no retinal
histological alteration occurred in 16 mg/kg NMDA I.P. administration group.
Resveratrol and MK-801 prevented NMDA-induced retinal neurodegeneration in the
zebrafish.
CONCLUSION: Among the three drug
administration methods, I.V. injection of NMDA is the most suitable for
establishment of an acute retinal damage model in zebrafish. I.M. with NMDA is
likely the best for use as a chronic retinal damage model. I.P. treatment with
NMDA causes brain damage. Resveratrol and MK801 may be a clinically valuable
treatment for retinal neurodegeneration.
KEYWORDS: zebrafish; NMDA; administration
method; retinal ganglion cell; glaucomatous animal model; resveratrol
DOI:10.18240/ijo.2019.08.04
Citation: Luo ZW, Wang HT, Wang N, Sheng WW, Jin M, Lu Y, Bai YJ,
Zou SQ, Pang YL, Xu H, Zhang X. Establishment of an adult
zebrafish model of retinal neurodegeneration induced by NMDA. Int
J Ophthalmol 2019;12(8):1250-1261
INTRODUCTION
Glaucoma is
the second leading cause of blindness worldwide, with over 60 million people
(and over 10 million in China) suffering from this disease[1].
Pathologically, glaucoma is characterized by the progressive loss of retinal
ganglion cells (RGC) and their axons, leading to visual field defects and optic
nerve atrophy[2-5]. An elevated
intraocular N-Methyl-D-aspartic acid (NMDA) concentration plays an important
role in retinal ganglion cell loss[6]. Currently,
glaucoma drug discovery is focused on visual nerve protection, and the
discovery of any treatments that can prevent retinal ganglion cell death would
have a major clinical impact[7]. Many glaucoma
models have been developed in rats, rabbits, rhesus macaques, and dogs. Some of
these glaucoma models have used direct injection of NMDA to the vitreous
chamber of rat eyes, which leads to death of retinal ganglion cells[8-10]. Compared with mammals, less
glaucoma studies have utilized zebrafish despite its usefulness in eye
research.
Zebrafish
has become increasing popular as a vertebrate model for developmental biology
and genetics research over the past 20y[11-12]. There are many reasons for its prevalence.
Maintaining zebrafish is relatively easy and they can quickly and effortlessly
be bred in large numbers. Moreover, many zebrafish genes have been highly
conserved throughout animal evolution, with 70%-80% of its genes having
homologs in humans[13]. These advantages have
facilitated the adoption of zebrafish as a valuable preclinical drug screening
system in pharmaceutical research[14-15].
Importantly,
the visual system of zebrafish is highly similar to the human visual system but
develops in just 5d after birth, which is much faster than most other animal
models[16-17]. Moreover, the
central nervous system of zebrafish has similar structural properties with the
mammalian system (including the fore-, mid-, and hind-brain, diencephalon,
telencephalon, and cerebellum), and the noradrenergic, serotonergic, GABAergic,
and histaminergic signaling systems are also highly similar[11,18]. This has made zebrafish a valuable model for
studying brain diseases. Establishment of a better glaucoma model in zebrafish
would be valuable not only for understanding more about its molecular pathology
but may also be a useful model system to screen for drugs that can protect the
visual nerves damaged in glaucoma. Both directions will help to produce
superior clinical treatments for glaucoma.
NMDA, an
analog of L-glutamate and an important excitatory neurotransmitter in the
mammalian central nervous system, has been used in many neuronal diseases
model, such as Alzheimer’s disease, Huntington disease, Parkinson’s disease,
epilepsy, and glaucoma[19-24].
NMDA-induced cellular excitotoxicity can eventually cause cell death[25-26]. The primary administration
methods for drugs in adult zebrafish that we utilized are immersion (I.M.),
intravitreal (I.V.) injection, and intraperitoneal (I.P.) injection. In
previous studies, I.M. allowed for drug absorption directly through zebrafish
skin from the aqueous environment[27], while I.P.
drug injection was used in zebrafish to cause seizure-like behavior[28]. I.V. has been used to inject ouabain into the eyes
of zebrafish to cause retinal damage[29].
Although additional methods, such as intraperitoneal perfusion, have been used
in some studies, they have been excluded from this study.
In this study, we investigated two important issues related to the
establishment of a glaucomatous zebrafish model for drug screening: whether the
three NMDA delivery methods can cause retinal damage in adult zebrafish and if
resveratrol or MK-801 can provide protection against this retinal
neurodegeneration. MK-801, a noncompetitive antagonist of NMDA receptors, was
included to serve as a positive control as it should directly prevent
NMDA-induced excitotoxicity. Resveratrol, a natural product found in many
plants, was studied given its many documented health-promoting effects,
including its ability to increase lifespan[30]
and prevent age-related diseases such as inflammation[31],
neurodegeneration[32-33],
epilepsy[34], heart disease, metabolic disorders,
and autoimmune diseases[35]. Moreover, we have
previously found resveratrol to exert positive effects in models of retinal
neurodegeneration[36].
MATERIALS AND METHODS
Ethical Approval The study was approved by the
Ethical Review Committee of Nanchang University (Nanchang, China). We confirm
adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision
Research.
Animals Adult male and female wild-type
zebrafish (Danio Rerio, AB strain) were obtained from the China
Zebrafish Resource Center (Wuhan, China). All adult zebrafish were raised in a
zebrafish breeding system (HAISHENG Biotech, China) at
Drug Preparations NMDA (M3262, Sigma, USA) was
dissolved in phosphate-buffered saline (PBS; I.M.: 100 μmol/L; I.P.: 8, 12 and
16 mg/kg; I.V.: 0.1, 0.3 and 0.5 mol/L; Figure 1). MK-801 (M107, Sigma, USA)
was dissolved in 50% ethanol/50% PBS (I.P.: 3 mg/kg, I.V.: 0.05 mol/L).
Resveratrol (R5010, Sigma, USA) was dissolved in 100% ethanol and kept in the
dark while stored and throughout the entire experiment (50 mg/L). MS-222
(A5040, Sigma, USA) was dissolved in distilled water (
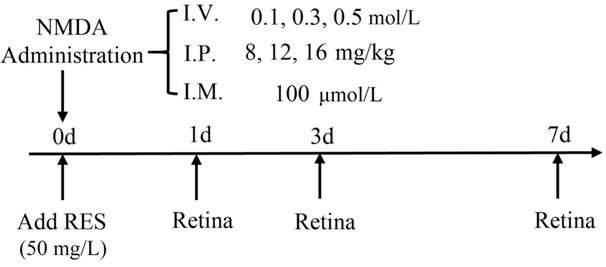
Figure 1 Experimental protocols I.V.: Intravitreal injection; I.P.:
Intraperitoneal injection; I.M.: Immersion.
Intravitreal Injection Prior to I.V., zebrafish were
anaesthetized with MS-222. The approximate volume of the vitreous cavity was calculated to be
approximately 200-500 nL by measurements taken with digital calipers as
described previously[37]. In a preliminary
experiment to determine a suitable injection volume we found 100 nL PBS would
not cause any retinal damage or behavioral change. Subsequently, 100 nL freshly
prepared NMDA solutions were delivered into the right eye of treated zebrafish
through a small incision between the vitreous body and the retina made by a
very thin acupuncture needle pin (
Intraperitoneal Injection Adult zebrafish were first
anaesthetized with MS-222 and then injected intraperitoneally (in the middle of
the abdomen as with rodents) with NMDA (dosages were selected by comparison
with those typically used in rodents[28]).
Treatment times were 1, 3, and 7d. Injections of 10 μL drug (NMDA or MK-801) or
1×PBS (control) were then performed in one side of the fish.
Immersion For NMDA I.M. treatment, we exposed
the adult zebrafish (n=6 for each small group) to a solution of 100
μmol/L NMDA for different time points (0h, 1, 3, 7d; solution was prepared
under
Cardiac Perfusion, Eye Dissection and Storage Zebrafish were euthanized, set on a
custom operating table, and then a T-shape lesion was cut into the thorax of
each zebrafish. A microinjector was then used to inject 2 mL PBS followed by 2
mL 2% PFA/2% glutaraldehyde (G5882, Sigma, USA) into the heart. Subsequently,
the whole eyes from each zebrafish were harvested, put into 10 mL 1×PBS for
several minutes, and then fixed with 2 mL 2% PFA/2% glutaraldehyde at
Hematoxylin and Eosin Staining and Histological Evaluation Briefly, eyes were dehydrated
stepwise in 70%, 80% (2×), 95% (2×), and 100% (3×) ethanol for 30min per step.
Then they were processed with xylene (3×) for 20min per step and embedded in
paraffin. Next, the embedded eyes were cut with a paraffin slicing machine
(LEICA RM2235) into 4 μm thick sections (horizontal with the optic nerve head).
Each section contained the whole retina from both superior and inferior
hemisphere because the eyes were aligned vertically to the ground and sections
were made along the vertical meridian. Hematoxylin and eosin (H&E) staining
of sections was then performed and their morphology determined by light
microscopy (Optiphot-2, Nikon, Tokyo, Japan). The total retinal ganglion cell
number in the retinal ganglion cell layer (GCL) was manually counted in a
region at the middle of one side of the retina between the center of the optic
nerve head and ending. Thickness measurements were then determined for the
nerve fiber layer (NFL), the GCL and the whole retina by software analysis
(Image-pro Plus 6.0). Pictures were captured using an IX71 camera (OLYMPUS,
Japan).
TUNEL Staining Animals were euthanized with ice
water after treatment with drugs. Retinal horizontal sections were obtained as
described in the histological evaluation step. Following the manufacturer’s
instructions, TUNEL staining was performed to detect apoptotic cells using the
In Situ Apoptosis Detection Kit (11684817910, Roche, Germany). Images of TUNEL
staining were collected with an LSM800 microscope (ZEISS, Gottingen, Germany).
Statistical Analysis Graphpad PRISM 7.00 was used for
data analysis, with values presented as the mean±SEM. Student’s t-test
was used to compare the means of different groups with a P<0.05
considered significant.
RESULTS
NMDA-Induced Inner Retinal Thickening with Immersion Treatment We first assessed the effectiveness
of the I.M. method on the retina. In order to induce retinal neurodegeneration,
the adult zebrafish were immersed into 100 μmol/L NMDA for various times
(0-7d). H&E staining showed that the mean thickness NFL+GCL increased significantly
after 1d and gradually reached approximately 2× larger by 7d compared with the
control group (P=0.0021; Figure
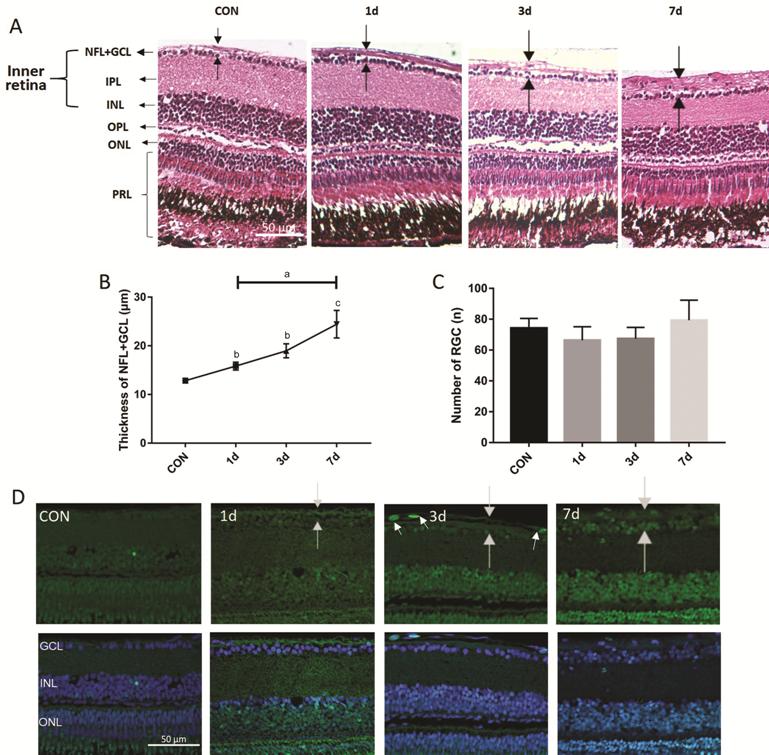
Figure 2 Histology of NMDA-induced thicker NFL (time gradient) A-D: The zebrafish were treated by I.M.
in NMDA and divided into 4 distinct groups with different treatment times. A:
H&E staining shows representative paraffin sections (4 μm) from a wild-type
control retina immersed in distilled water along with retinas treated with 100
μmol/L NMDA for 1, 3, and 7d. The dark arrow points out the thickness of each
layer. B: The thickness of the NFL+GCL (y-axis) plotted against the NMDA
treatment time; C: The number of GCL (y-axis) plotted against the NMDA
treatment time. Error bars represent standard error of the mean (±SEM); n=6.
(unpaired t-test, aP<0.05, bP<0.01,
and cP<0.001 compared with control); D: TUNEL staining of
NMDA treatment time points and control retinas. White arrows point to apoptotic
cells. NFL: Nerve fiber layer; GCL: Ganglion cell layer; IPL: Inner plexiform
layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL: Outer nuclear
layer; PRL: Photoreceptor layer. Magnification is 40×. Scale bar, 50 µm.
NMDA-Induced Thicker NFL, Retinal Thickness Reduction, and Retinal Ganglion
Cell Apoptosis with Intravitreal Injection
We tested
different drug concentrations with a treatment time of 1d to determine the
optimal intravitreal dose of each drug. The 100 nL solution was microinjected
into each eye of all zebrafish treatment groups. Preliminary experiments
indicated that a suitable dose range of NMDA was 0-0.5 mol/L, from which we
found the most effective concentration to be 0.5 mol/L (0.6 mol/L was found to
be lethal for zebrafish, causing seizure-like symptoms and death in just
several minutes). We also determined that 1d was a suitable time point to
induce significant retinal damage. Therefore, 100 nL 0.5mol/L NMDA injection
for 1d was used for subsequent testing.
We found that the NFL+GCL thickness increased while the retinal thickness
was decreased significantly after NMDA treatment (retinal thickness slightly
increased in the 0.1 mol/L NMDA-treated group but decreased considerably in the
0.3 and 0.5 mol/L NMDA-treated groups, P<0.0001; Figure
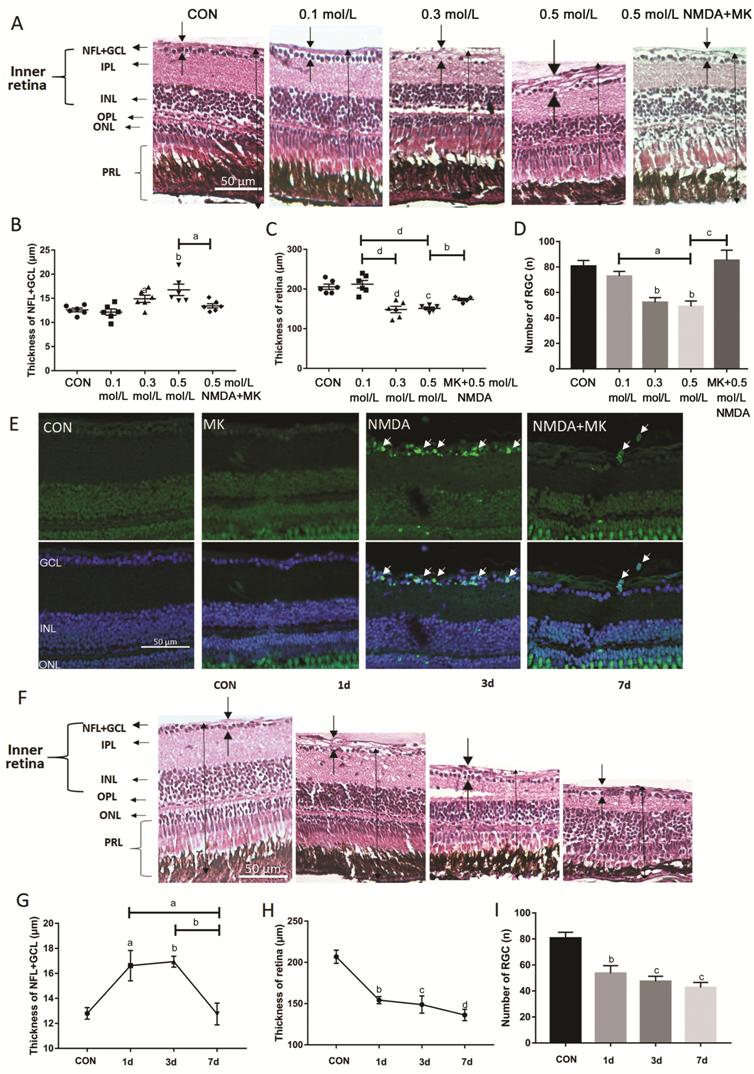
Figure 3 NMDA-induced alterations in retinal histology, thickness and
retinal ganglion cell apoptosis A-D: The zebrafish were treated by
I.V. of NMDA at different doses for 1d. A: H&E staining of paraffin
sections (4 μm) from a control retina treated with 100 nL PBS and retinas
treated with 0.1, 0.3, 0.5 mol/L NMDA, and 0.05 mol/L MK-801+0.5 mol/L NMDA.
The dark arrows show the thickness of each layer. B: The thickness of the
NFL+GCL from different treatment groups; C: The thickness of retinas from
different treatment groups; D: The retinal ganglion cell number of retinas from
different treatment groups. Error bars represent standard error of the mean
(±SEM); n=6 (unpaired t-test, aP<0.05, bP<0.01,
cP<0.001, and dP<0.0001 compared with
control); E: TUNEL staining of representative control retinas and retinas
treated with 50 mmol/L MK-801, 0.5 mol/L NMDA, and MK-801+NMDA. The white
arrows point to apoptosis cells. F: H&E staining of paraffin sections (4
μm) from retinas treated intravitreally with 1× PBS (control) or with 0.5 mol/L
NMDA for various times; G: The thickness of the NFL+GCL from different
treatment groups; H: The thickness of retinas from different treatment groups;
I: The retinal ganglion cell number from retinas of different treatment groups.
Error bars represent standard error of the mean (±SEM); n=6 (unpaired t-test,
aP<0.05, bP<0.01, cP<0.001,
and dP<0.0001 compared with control). Original
magnification is 40×. Scale bar, 50 µm.
A time gradient from 0-7d was then set up to find the optimal NMDA
treatment time. Although already significantly perturbed after only 1d of
treatment, we observed a continued degeneration of the retina throughout the
week of NMDA treatment (Figure
No Histologic Change in the Retina after NMDA Intraperitoneal
Injection For I.P. injection, zebrafish were
injected with different NMDA concentrations on one side of the abdomen. For
reference we referred to the concentrations used in other studies and set up a
dose range of 0-24 mg/kg NMDA for 1d treatment. Zebrafish injected with 24
mg/kg NMDA died immediately and were not analyzed further. At the non-lethal
doses of NMDA (8, 12, and 16 mg/kg), zebrafish presented seizure-like behavior,
however, no changes to the retinal histology were observed (Figure
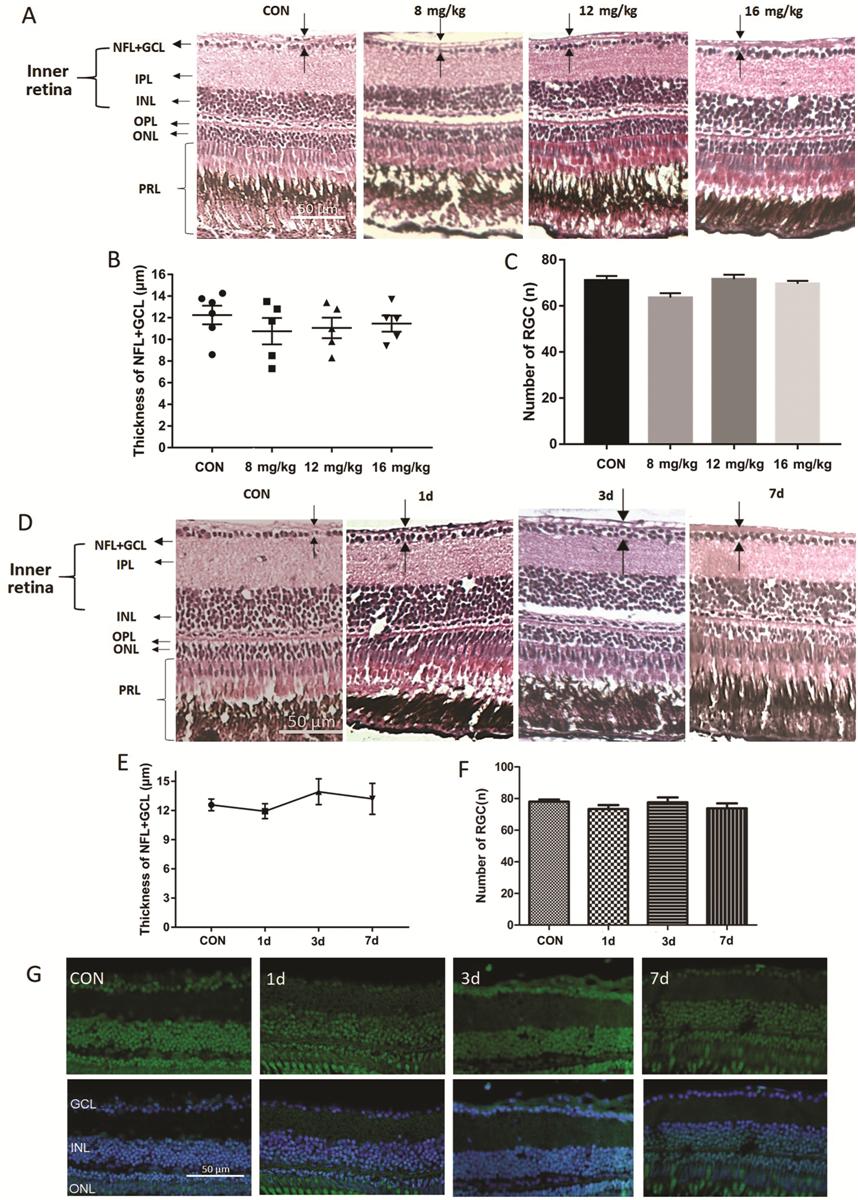
Figure 4 Analysis of intraperitoneal NMDA injection for retinal damage A: H&E staining of paraffin sections
(4 μm) from retinas treated intraperitoneally for one day with 10 μL PBS
(control) or 8, 12, or 16 mg/kg NMDA; B: The thickness of the NFL+GCL of
retinas from each treatment group; C: The retinal ganglion cell number in
retinas from each treatment group. Error bars represent standard error of the
mean (SEM); n=6 (unpaired t-test showed no significant
differences). D: H&E staining of paraffin sections (4 μm) from a control
retina treated intraperitoneally with 10 μL PBS for 1d and retinas treated
intraperitoneally with 16 mg/kg NMDA for 1, 3, and 7d. The dark arrow points
out the thickness of each layer. E: The thickness of the NFL+GCL of retinas
from each time point; F: The retinal ganglion cell number in retinas from each
time point. Error bars represent standard error of the mean (±SEM); n=6.
G: TUNEL staining of retinas from zebrafish treated intraperitoneally with PBS
(control) or with 16 mg/kg NMDA for 1, 3, or 7d. Original magnification is 40×.
Scale bar, 50 µm.
Resveratrol Prevented the NMDA-Induced Thickened Inner Retina by
Immersion To assess the effects of resveratrol
on retinal damage, two pairs of treatment and control groups were set up: 100
μmol/L NMDA (treatment groups) versus water (control groups), each one with or
without 50 mg/L resveratrol. All groups were treated for 1d and were kept in
the dark to prevent light-induced degradation of resveratrol. The thickness of
the NFL+GCL was increased in the NMDA-only treated group, whereas it was not
significantly altered in the resveratrol-only group or the NMDA+resveratrol
group (Figure
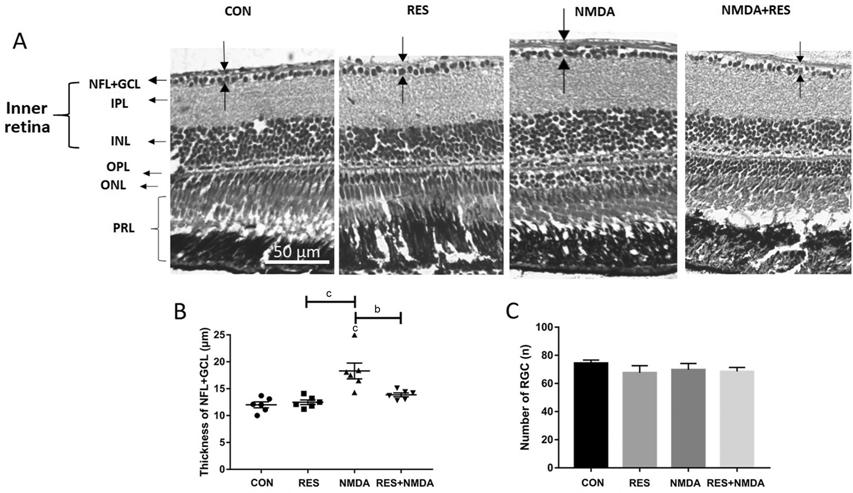
Figure 5 Resveratrol prevents from NMDA-induced thicker NFL A: H&E staining of paraffin
sections (4 μm) from representative retinas treated by I.M. in distilled water
(control), 50 mg/L resveratrol, 100 μmol/L NMDA, or 50 mg/L resveratrol+100
μmol/L NMDA. The dark arrows point out the thickness of each layer. B: The thickness
of the NFL+GCL for each treatment group; C: The retinal ganglion cell number
for each treatment group. Error bars represent standard error of the mean
(±SEM); n=6 (unpaired t-test, bP<0.01 and cP<0.001).
Original magnification is 40×. Scale bar, 50 µm.
Resveratrol Protected from I.V. NMDA-Induced Retinal Damage To further assess resveratrol’s
ability to prevent retinal damage, zebrafish eyes were first injected
intravitreally with 100 nL 0.5 mol/L NMDA, and then the zebrafish were
immediately immersed into 50 mg/L resveratrol for 1d. Whereas NMDA I.V.
treatment caused serious retinal neurodegeneration, and treatment with
resveratrol largely prevented retinal damage caused by NMDA treatment (Figure
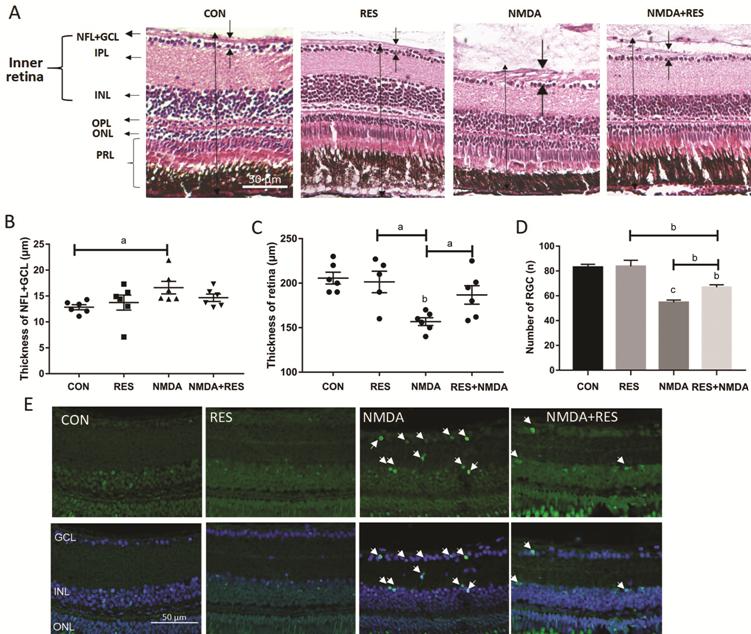
Figure 6 Resveratrol protects against NMDA-induced retinal damage A-E: Zebrafish were treated
intravitreally with NMDA and by I.M. in resveratrol. A: H&E staining of
paraffin sections (4 μm) from retinas treated with I.V. of 100 nL PBS
(control), or 0.5 mol/L NMDA, each plus or minus I.M. in 50 mg/L resveratrol.
The dark arrows point out the thickness of each layer. B: The thickness of the
NFL+GCL from each treatment group; C: The thickness of the retinas from each
treatment group; D: The retinal ganglion cell number from each treatment group.
Error bars represent standard error of the mean (SEM); n=6 (unpaired t-test,
aP<0.05, bP<0.01, and cP<0.0001
compared with control); E: Representative TUNEL staining images of each
treatment group as in A. The white arrows point to apoptotic cells. Original
magnification is 40×. Scale bar, 50 µm.
DISCUSSION
Good preclinical animal models are crucial for successful drug screening.
In this study, we performed three different methods of drug delivery to
establish a retinal neurodegeneration model in adult zebrafish. We found that
I.V. injection of NMDA was the most effective delivery method as it caused
considerable damage to the zebrafish retina in just one day. Additionally, NMDA
administrated by I.M. resulted in a thicker retinal NFL, showing that this
delivery method may also have potential use for an eye-related disease model.
Importantly, we demonstrated that resveratrol and MK-801 both exerted
protective effects in our zebrafish models and significantly reduced
NMDA-induced retinal neurodegeneration caused by two different administration
routes (I.M. and I.V. injection).
Prior zebrafish glaucoma models utilized genetic methods such as gene
knockout to affect the zebrafish eye structure and cause glaucomatous symptoms[38-39]. Genetic disease
models have many advantages for studying the relevant homologous genes and the
underling molecular mechanisms of the disease. However, development of genetic
glaucoma models in zebrafish is slow and costly, which is not ideal for drug
screening due to the large number of fish needed and the slow time necessary to
develop the disease pathology. In contrast, our retinal neurodegeneration
zebrafish model uses drug treatment of wild-type animals to very quickly induce
a glaucoma-like phenotype in a large number of animals, making this system
ideal for drug screening.
NMDA, which is considered to play an important role in the process of
glaucoma, has not been previously used in zebrafish glaucoma research. Although
prior studies have found neuroprotective substances using NMDA treatment in
mice and rats[40-43], this
study is the first to identify neuroprotective compounds using NMDA-induced
neurotoxicity in zebrafish. The NMDA model is convenient and has been widely
used in many glaucomatous animal studies, providing reproducible outcomes with
a simple operation[29].
The I.M. method, which has been used for several eye-related models, such
as low-oxygen water leading to hypoxia-induced retinopathy[44],
has not been used in a glaucomatous zebrafish model. When NMDA is added to the
zebrafish’s water, it is rapidly absorbed by the blood vessels in the skin and the
gills. The compound then diffuses through the systemic circulation system and
reaches the target tissue to bind NMDA receptors and produce a response. In the
process of getting into the retina, NMDA should penetrate two barriers [the
blood-retinal barrier (BRB) and the blood-aqueous barrier (BAB)][45-46]. In a recent study,
the small molecule cadmium chloride (Mr=183.32) was utilized to cause retinal
damage[47], demonstrating the ability of small
molecules to effectively pass through both barriers to cause retinal damage.
Therefore, we hypothesized that the small molecule NMDA (Mr=147.13), a
homolog of L-glutamate which is naturally abundant in the brain and retina[48], should also be able to easily pass through the BRB
and BAB to reach the retina. In our NMDA I.M. experiment we observed a small
number of apoptotic cells in the RNFL as well as inner retinal thickening,
demonstrating that a significant amount of NMDA did make it into the retina
(Figure 3). We believe this phenomenon was the early stage of retinal
neurodegeneration caused by NMDA. Previous studies have shown that inner
retinal thickening was due to swelling of the massive retinal cells and their
axons caused by NMDA-induced excitatory neurotoxicity[49-51]. However, in zebrafish there was no loss of retinal
ganglion cells and only a small amount of apoptosis occurred in the NFL (data
not shown) compared to the analogous rat model where much more apoptosis
occurred[41,52]. We believe
that the apoptotic cells in the NFL are myelin cells because myelin cells exist
in the NFL in lower vertebrates to protect and support the retinal ganglion
cells[53].
According to other studies of established eye disease models, I.M. required
a long time to cause significant symptoms of cellular damage (i.e. apoptotic
retinal cells). For example, I.M. of adult zebrafish in high concentrations of
glucose or cadmium to induce eye damage took 14 and 29d respectively to see
significant levels of retinal apoptosis[47,54]. Furthermore, prior studies have shown that I.M.
often is not as effective as injection to cause high levels of small molecule
accumulation[55]. Therefore, we suspect that the
treatment time and accumulated concentration of NMDA in our I.M. experiment is
likely not sufficient to cause significant damage and induce considerable
retinal cell apoptosis.
Importantly, we found that resveratrol was able to reduce inner retinal
thickening caused by NMDA treatment, indicating that resveratrol possesses
retinal protective properties. These results are consistent with our recent
report that resveratrol increases the expression of Sirtuin genes in the retina
to regulate mitochondria function and produce an anti-excitotoxicity effect[36,56]. Additionally, another recent
report showed that resveratrol delivered via drinking water could
protect rats from the retinal neurodegeneration caused by acute bright light
exposure[57]. These data show that resveratrol
can be effectively delivered into the eye via the circulatory system,
which is in agreement with a prior study showing resveratrol could enter into
the systemic circulation and thereby be delivered to the aqueous humor and
vitreous humor[58]. It should be noted that the
distribution pathway of resveratrol is partly different from that of NMDA, as
resveratrol is not delivered through the BRB like NMDA[58].
The I.V. injection method, which delivers drug directly into the vitreous
cavity to act on the inner retina, has been widely employed in eye research. In
our study, we showed that I.V. injection of NMDA could cause a massive loss of
retinal ganglion cells and that both MK-801 and resveratrol provided a
protective effect against this treatment, demonstrating this method can be used
to establish a physiologically relevant glaucomatous zebrafish model that could
be successfully employed for drug discovery. This model was established in
reference to similar models in rats by comparing the volume of the vitreous
cavity of rats with that of zebrafish[59] to
estimate a suitable NMDA concentration for zebrafish. However, the equivalent
concentration of NMDA in rats was ineffective for zebrafish, and a relatively
larger dose was necessary in our study. This may be due to drug leakage from
the zebrafish upon being returned to water, which is not a problem with
mammalian models. We also found that 1d was the best time point for our assay,
as it was sufficient to see massive retinal ganglion cell loss, while later at
day 7 the level of retinal ganglion cell loss became much slower, which may be
due to retinal ganglion cell regeneration in zebrafish that begins 3d after
retinal damage[60-61]. Based
on these preliminary studies, I.V. injection of 100 nL 0.5 mol/L NMDA and a
treatment time of 1d were chosen as the optimal parameters to establish our retinal
neurodegeneration zebrafish model for further drug screening.
Administration of kainic acid by I.P. was used successfully in zebrafish to
induce seizure-like behaviors[28,62].
In contrast, I.P. injection of drugs has not been previously used in
glaucomatous models. Since NMDA can be delivered throughout the body including
the eye through systemic circulation, we sought to determine if I.P.
administration of NMDA could also induce retinal damage. However, although NMDA
administration by I.P. was sufficient to induce as much seizure-like behavior
as the previous study, there was no significant change in the histology of the
retina, the number of retinal ganglion cells, or any induction of apoptosis in
the retina of zebrafish treated with NMDA by I.P. This could be due to a lower
level of NMDA reaching the retina versus the brain when administered by I.P.
Alternatively, the level of NMDA needed to cause retinal damage in zebrafish
may be significantly higher than the level necessary to induce seizure-like
behaviors.
Comparing the three drug administration routes, all were able to
effectively deliver treatments to the brain or eye as deduced by their ability
to induce pathological and behavioral alterations. Although I.P. and I.V.
injection of NMDA had a much more rapid effect on zebrafish, I.M. treatment had
a longer lasting effect. We imagine that I.P. and I.V. administration result in
an immediate surge in the NMDA concentration in the blood and target cells to
cause the rapid, acute changes observed, but the NMDA concentration would then
quickly decrease back to baseline levels and may not be sufficient to cause
significant target tissue damage. However, compared with I.V. administration,
I.P. injection of NMDA is less target-specific, and cannot cause accumulation
of a sufficient concentration in the retina but leads to uncontrollable damage
to other organs such as the brain. I.M. administration, on the contrary, could
maintain a relatively stably increased concentration of NMDA in the blood
sufficient to cause long-term NMDA-induced damage, making it more useful as a
model of chronic disease. However, I.M. requires a relatively long time to
cause retinal neurodegeneration, which makes it unsuitable for large-scale drug
screening. At the operational level, I.M is the easiest and most convenient
method of drug administration, whereas I.V. administration is relatively
complicated but most effective for establishing eye disease models in
zebrafish. I.P. administration does not seem suitable for specific models of
eye disease, but it is an easy and effective method for establishing models of
brain disease.
It should be noted that a major flaw of this NMDA-induced neurotoxicity
model is that it focuses on only a single mechanism (glutamate excitotoxicity)
of glaucoma pathobiology. Given the more complex pathogenesis of glaucoma in
humans, these models may not fully represent the glaucoma disease process and
therefore could fail to detect some treatments that would be effective against
glaucoma clinically[61]. Another limitation of
this study is that the NMDA I.M. method did not cause significant damage to the
zebrafish retina, so this model is not sufficient for neuroprotective drug screening
and requires further optimization for it to be a useful model system. Moreover,
even though I.V. administration of NMDA causes the same effect on the zebrafish
as in other animal models, the microinjection procedure can be difficult to
perform, which could hinder its application to drug screening. Therefore,
improvements to each administration procedure are likely required to achieve
the full potential of this model system.
In summary, the data presented in this study demonstrate that intravitreal
NMDA injection is an effective model of retinal neurodegeneration in zebrafish,
while NMDA I.M. may serve as a suitable model of chronic eye diseases. By
comparing the three different drug administration methods, we discovered the
best means to establish effective NMDA-induced models of retinal damage in
zebrafish. These models will be valuable for drug screening campaigns and
studies to identify the underlying mechanisms of glaucomatous retinal
neurodegeneration. Moreover, our study provides further evidence for the
potential utility of resveratrol, a common natural product with a
well-established safety profile, in the treatment of glaucoma.
ACKNOWLEDGEMENTS
Foundations: Supported
by the National Natural Science Foundation of China (No.81271425; No.81260148;
No.31400988; No.81160144; No.31171044); the Jiangxi Provincial Natural Science
Foundation (No.20181ACG70010); the Natural Science Foundation of Jiangxi
(No.20151BBG70243; No.20122BCB23007).
Conflicts of Interest: Luo ZW, None; Wang HT, None; Wang N, None; Sheng
WW, None; Jin M, None; Lu Y, None; Bai YJ, None; Zou
SQ, None; Pang YL, None; Xu H, None; Zhang X, None.
REFERENCES