
Citation: Zou XL, Yu YZ, Yu HH, Wang GF, Pi RB, Xu Z, Zhang C,
Zhou WJ, Li DD, Chen XG, Zou YP. Protective effects of lipoic acid-niacin
dimers against blue light-induced oxidative damage to retinal pigment
epithelium cells. Int J Ophthalmol 2019;12(8):1262-1271. DOI:10.18240/ijo.2019.08.05
·Basic Research·
Protective
effects of lipoic acid-niacin dimers against blue light-induced oxidative
damage to retinal pigment epithelium cells
Xiu-Lan Zou1, Yong-Zhen Yu1,
Hong-Hua Yu2, Guan-Feng Wang3, Rong-Biao Pi4,
Zhe Xu5, Chu Zhang1, Wen-Jie Zhou1, Dan-Dan Li6,
Xuan-Ge Chen1, Yu-Ping Zou1
1Department of Ophthalmology, General
Hospital of Southern Theatre Command of PLA, Guangzhou 510010, Guangdong
Province, China
2Department of Ophthalmology,
Guangdong Provincial People’s Hospital, Guangzhou 510000, Guangdong Province,
China
3Department of Ophthalmology, Third
Affiliated Hospital of Guangzhou Medical University, Guangzhou 510000,
Guangdong Province, China
4School of Pharmaceutical Sciences,
Sun Yat-sen University, Guangzhou 510000, Guangdong Province, China
5Department of Ophthalmology, the
First Affiliated Hospital, Guangdong Pharmaceutical University, Guangzhou
510000, Guangdong Province, China
6Zhongshan Ophthalmic Centre of Sun
Yat-sen University, Guangzhou 510000, Guangdong Province, China
Co-first authors: Xiu-Lan Zou and Yong-Zhen Yu
Correspondence to: Yu-Ping Zou. Department of
Ophthalmology, General Hospital of Southern Theatre Command of PLA, Guangzhou
510010, Guangdong Province, China. gzzouyuping@163.com
Received: 2018-06-17
Accepted: 2019-05-15
Abstract
AIM: To evaluate the protective effects of lipoic acid-niacin (N2L) dimers against blue light (BL)-induced
oxidative damage to human retinal pigment epithelium (hRPE) cells in vitro.
METHODS:
hRPE
cells were divided into a control group (CG), a BL group, an N2L plus BL irradiation group, an α-lipoic acid (ALA) plus BL
group, an ALA-only group, and an N2L-only
group. hRPE cellular viability was detected by performing
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) bromide assays, and
apoptosis was evaluated by annexin-V-PE/7-AAD staining followed by flow
cytometry. Ultrastructural changes in subcellular organelles were observed by
transmission electron microscopy. Reactive oxygen species formation was assayed
by flow cytometry. The expression levels of the apoptosis-related proteins
BCL-2 associated X protein (BAX), B-cell leukmia/lymphoma 2 (BCL-2), and
caspase-3 were quantified by Western blot analysis.
RESULTS: BL exposure with a light density of 4±0.5 mW/cm2
exceeding 6h caused hRPE toxicity, whereas treatment with a high dose of N2L (100 mol/L) or ALA (150 mol/L) maintained
cell viability at control levels. BL exposure caused vacuole-like degeneration,
mitochondrial swelling, and reduced microvillus formation; however, a high dose
of N2L or ALA maintained the
ultrastructure of hRPE cells and their organelles. High doses of N2L and ALA also protected hRPE cells from
BL-induced apoptosis, which was confirmed by Western blot analysis: BCL-2
expression significantly increased, while BAX and caspase-3 expression slightly
decreased compared to the CG.
CONCLUSION: High-dose N2L treatment (>100 mol/L) can reduce
oxidative damage in degenerating hRPE cells exposed to BL with an efficacy
similar to ALA.
KEYWORDS: lipoic acid-niacin dimers; retinal
pigment epithelium cell; lipoic acid; oxidative stress; reactive oxygen
species; apoptosis
DOI:10.18240/ijo.2019.08.05
Citation:
Zou XL, Yu YZ, Yu HH, Wang GF, Pi RB, Xu Z, Zhang C, Zhou WJ, Li DD, Chen XG,
Zou YP. Protective effects of lipoic acid-niacin dimers against blue
light-induced oxidative damage to retinal pigment epithelium cells. Int J
Ophthalmol 2019;12(8):1262-1271
INTRODUCTION
Among all eye diseases, age-related
macular degeneration (AMD) is the main contributor to irreversible vision loss
in elderly people, especially in the developing world[1].
Although the pathological mechanism of AMD has not yet been clearly elucidated,
some evidence points to a critical role of oxidative stress in AMD progression.
Human retinal pigment epithelial (hRPE) cells and photoreceptor cells are
exposed to an environment of high oxygen pressure and high metabolism, and are
therefore continuously subjected to oxidative stress[2].
Moreover, accumulating evidence indicates that mitochondria are the subcellular
targets of oxidative stress in hRPE cells and are closely associated with the
hRPE cell aging process[3]. Accordingly, many
recent studies have focused on investigating the relationships between hRPE
cells and oxidative stress. Sreekumar et al[4] showed
that humanin protected hRPE cells against oxidative stress-induced cell death
and restored mitochondrial function. Lambros and Plafker[5]
provided evidence indicating that nuclear factor-E2 related factor 2 (Nrf2),
the master antioxidant transcription factor, played a significant role in hRPE
cells during aging and the onset of AMD. Moreover, Liu et al[6] found that hydroxytyrosol was a mitochondria-targeting
antioxidant nutrient that could protect against age-related hRPE degeneration.
Despite this insight, it remains an arduous task to develop an effective
strategy to protect hRPE cells from oxidative stress-induced damage to slow
down or prevent AMD progression.
Alpha-lipoic acid (ALA) and nicotinic acid (niacin) are
widely recognized as potent antioxidants that also exert neuroprotective effects.
ALA functions in energy and metabolism, playing a primary role as a
mitochondrial redox sensor, and is thus a powerful antioxidant[7] that has been shown to be effective for anti-aging,
slowing the progression of vision loss, and improving diabetes retinopathy and
its complications. However, the application of ALA is limited because it is
only found in minute quantities in tissues and, thus, only a small amount of
ALA can be absorbed from food. In addition to its antioxidant property, niacin
is an important drug for reducing blood lipid levels, including low-density
lipoprotein-cholesterols, very low-density lipoprotein-cholesterols, and
lipoprotein(a), while raising high-density lipoprotein cholesterol when administered
at a pharmacological dose[8]. However, like ALA,
its clinical application is currently limited owing to adverse side effects
such as flushing owing to blood vessel dilation, nausea or diarrhea,
urethritis, and low lipid solubility[9].
Nevertheless, one study demonstrated that the pharmaceutical activity of niacin
could be strengthened and its side effects weakened when combined with other
medications[10].
The novel complex formed between
niacin and ALA, designated as the lipoic acid-niacin dimers (N2L), was developed in Dr. Pi RB. Pi’s
laboratory at the School of Pharmaceutical Sciences, Sun Yat-sen University,
under the chemical name of 5-[1,2-dithiolan-3-yl]-N-(2-{[(3-pyridyl)formyl]amino}ethyl)
pentanamide[11-12]. Unlike ALA
and niacin alone, N2L is a
multi-functional medication that exhibits stronger anti-oxidative and
anti-inflammatory properties and superior blood-lipid regulation. This complex
further enables higher drug bioavailability, with easier crossing of the
blood-brain barrier and faster drug metabolism. In addition, the side effects
of niacin and ALA mentioned above are reduced in N2L[11-12].
Recently, Pi et al[12] evaluated the
potential of N2L as a
neuroprotective drug. They reported that N2L was not toxic to hippocampal mouse neurons, but efficiently
protected neurons against cell toxicity due to L-glutamate, and they demonstrated the superior property of
the niacin-like unit in the context of N2L.
In this study, we focused on the
potential benefits of the stronger anti-oxidative property of N2L in protecting hRPE cells from oxidative
damage. We previously reported that N2L
could protect hRPE cells from apoptosis and cell death induced by acrolein,
suggesting that N2L could
protect cells from the injury caused by oxidative stress[13].
In another study, we found that N2L
could protect RPE-19 cells by up-regulating expression of the anti-apoptotic
factor B-cell leukmiay lymphoma 2 (BCL-2) and inhibiting expression of the
pro-apoptotic factor BCL-2 associated xprotein (BAX)[14].
The primary purpose of this study
was to explore the potential effects of N2L in protecting hRPE cells from oxidative damage
induced by blue light (BL) in vitro, which were compared to the
effects of ALA. These results could serve as a valuable basis for developing N2L as an alternative therapeutic drug for
preventing and treating AMD.
MATERIALS AND METHODS
Ethical Approval The experiments performed in this
study complied with the tenets of the Declaration of Helsinki: all work was
performed after approval from the Organ Procurement Organization and the
Hospital Ethics Committees, and all donors signed informed consent forms.
Tissue Samples and Cell Culture hRPE cells were obtained from
freshly isolated donor eyes from patients died by accident (i.e.,
immediately post-operation) provided by the eye bank of the Department of
Ophthalmology, General Hospital of Southern Theatre Command of PLA.
Each freshly isolated eye was soaked
for 15min in 20 mL antibiotics (100 IU/mL penicillin and 100 μg/mL
streptomycin; Gibco, USA) and D-Hanks medium (Gibco, USA), after which the
retinal neuroepithelium layer was peeled away by carefully removing the
anterior segment by cutting it out from the limbus. Then, the upside down,
hanging eye cup was separated from the retinal neuroepithelium layer and the
vitreous body. After placing the outer wall of the eye containing the RPE layer
and sclera in the eye cup and washing with D-Hanks medium, 3.0 mL 1% trypsin
(Gibco, USA) was added, and the specimen was incubated in a humidified 5% CO2
atmosphere at 37℃ for 1h. Subsequently, 3 mL 10% fetal bovine serum
(FBS; Gibco, USA) was added, and the hRPE cells were collected in 15-mL
centrifuge tubes, and centrifuged twice at 1100 rpm for 8min after washing the
cells with phosphate-buffered saline (PBS). Then, the cells were identified by
immunofluorescence with the RPE65 biomarker[15],
and the cells were plated in culture dishes and cultured in Dulbecco’s modified
Eagle’s medium-low glucose (DMEM-LG; Gibco, USA) supplemented with 10% FBS and
antibiotics, and incubated at 37℃ for 72h. Finally, the
growth of the hRPE cells was observed by IX70 Inverted Microscope (OLYMPUS,
Japan). After reaching confluence, the cells were detached in
trypsin-ethylenediaminetetraacetic acid (EDTA; Gibco, USA) solution. hRPE cells
in logarithmic growth phase at passages 4-6 were used for the experiments.
Establishment of the BL-Induced hRPE
Cell Damage Model A humidified 5% CO2
incubator was converted into a BL incubator box. Non-reflective paper was
adhered to the inside walls of the box, and five light-emitting diodes
producing BL (Yuelong Factory, Zhongshan, Guangdong Province, China) were fixed
on the inside top of the incubator, ensuring that the lights were well
distributed within the incubator. The distance between the BL, and cell culture
plane was adjusted so that the BL density was 4±0.5 mW/cm2 with FL-1D BL irradiation (Photoelectric
Instrument Factory of Beijing Normal University). The wave length of the BL was
approximately 450 nm. In previous experiments, we found that the hRPE cells
were clearly injured when exposed to 4±0.5 mW/cm2 BL for at least
6h.
Experimental Groups The hRPE cells were cultured in 10%
FBS-supplemented DMEM-LG until to 50% confluency, then the hRPE cells were
grouped into the control group (CG), cultured in cultured in serum-free DMEM
without any treatment (i.e., no N2L or ALA); the BL-irradiation group, exposed to BL
and cultured in cultured in serum-free DMEM without N2L and ALA; the N2L plus BL-irradiation group, exposed to BL and
cultured in serum-free medium with 50, 100, 150, or 200 μmol/L N2L; the ALA plus BL-irradiation group,
exposed to BL and cultured in serum-free medium with 50, 100, 150, and 200
μmol/L ALA. N2L and ALA
(School of Pharmacy, Sun Yat-sen University, China) were dissolved in DMEM-LG
without FBS (Serum starvation therapy) and the drug concentration was adjusted
to the indicated concentration in the culture medium.
As the hRPE cells were divided into
CG, the BL group, the N2L
plus BL-irradiation group and the ALA plus BL-irradiation group, all the cells
were cultured for a further 24h (drug incubation time) in medium containing different
concentrations of ALA or N2L
(0-200 μmol/L ) until the cells reached confluence almost at the same time.
Finally, the treatment hRPE cells groups were exposed to BL at 4±0.5 mW/cm2
for 0-24h in different experiment groups (including CG).
Cell Viability In order to find out whether N2L has toxicity, and the significant
protected concentrations of N2L
and ALA on hRPE cells, the grouped RPE cells were exposed to BL for 24h by
comparing cell viability; then the hRPE cells at a certain drug concentration
were exposed to BL for 3, 6, 12, and 24h, to study the relationship between
cell viability and exposure time; finally study the relationship between hRPE
cell viability and drug-treatment time (6, 12, or 24h) before BL illumination
with N2L or ALA at
a certain concentration. As grouped and after treatment, hRPE cell viabilities
were measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
(MTT) bromide assay (Gibco, USA). Briefly, the cells were plated in triplicate
wells of 96-well microplates at a density of 5×104 cells per well;
three wells also served as the CG. All hRPE cells were cultured according to
the experimental groups, CG were covered with tinfoil when the 96-well
microplates exposed to BL. Twenty microliters of MTT (5 mg/mL) solution was
added to each microwell, and the cells were incubated in a 5% CO2
incubator for 4h at 37℃. After carefully removing the medium from each
well, 150 μL dimethyl sulfoxide (Sigma, St. Louis, MO, USA) was added, and the
96-well microplates were placed on a table concentrator for 10min to allow time
for sufficient mixing. The optical density was read on a microplate reader at
490 nm.
Transmission Electron Microscopy
Experiments Different hRPE cell treatment groups
(including drug levels of 50-200 μm N2L
and ALA with 24h drug incubation time) were exposed to BL for 6h. Transmission
electron microscopy (TEM; HITACHI H-7650, Japan) was used to evaluate changes
in the ultrastructure of hRPE cells after the various treatments. The
cells were treated in 100-mm culture dishes, after which the medium was
removed. The grouped cells were collected in 1.5-mL centrifuge tubes and
centrifuged at 3500 rpm for 25min. The medium was removed, and the cells were
fixed in 0.8 mL of 2.5% glutaraldehyde for more than 2h at 4℃,
followed by sequential dehydration, soaking, embedding, sectioning in an
ultramicrotome, and lead/uranium double-staining. Finally, the ultrastructures
of hRPE cells were observed and photographs were acquired by TEM.
Cell Apoptosis Different hRPE cell treatment groups
(including drug levels of 50-200 μm N2L
and ALA with 24h drug incubation time) were exposed to BL for 6h. The apoptosis
rate of hRPE cells was detected with an Annexin-V-PE/7-AAD Cell Apoptosis Kit
(Merck Millipore, Germany) according to the manufacturer instructions. Briefly,
the cells of each group were plated in 6-well plates at 1×105 cells
per well and treated with the indicated factors. The cells were then digested
with 0.25% trypsin-EDTA and gathered into 15-mL centrifuge tubes. After
centrifugation at 1000 rpm for 5min and resuspending the cells, 100 μL of each
cell suspension was added to a 96-well microplate and 100 μL Annexin-V-P
E/7-AAD solution was added to each well. The samples were stained for 20min at
room temperature in the dark and detected with a Guava flow cytometry system.
Measurement of Reactive Oxygen
Species Production in hRPE Cells Different hRPE cell treatment groups
(including drug levels of 50-200 μm N2L
and ALA with 24h drug incubation time) were exposed to BL from 0.5h to 6h. To
detect the extent of oxidative damage induced by BL and the influence of the
respective treatments on this damage, the amount of reactive oxygen species
(ROS) produced in hRPE cells was evaluated based on the fluorescence intensity
of the fluorescent probe 2’,
7’-dichlorofluorescein-diacetate
(DCFH-DA; Sigma, USA). The cells were incubated with 1 μL of 10 μmol/L DCFH-DA
for 30min in the dark at 37℃, and then the plates
were washed twice with serum-free DMEM-LG and then twice with PBS, and
resuspended in DMEM-LG at 2×104 cells/mL. The cells were analyzed
within 30min by flow cytometry using excitation and emission wavelengths of 488
nm and 525 nm, respectively. The results are expressed as the fluorescence
intensity of DCFH.
Expression of Apoptosis-Related
Proteins Different hRPE cell treatment groups
(including drug levels of 50-200 μmol/L N2L and ALA with 24h drug incubation time) were exposed
to BL for 6h. hRPE cells were homogenized in lysis buffer (30 mmol/L Tris-HCl,
10 mmol/L EGTA, 5 mmol/L EDTA, 1% Triton X-100, 250 mmol/L sucrose) containing
1 mmol/L NaF, 1 mmol/L phenylmethylsulfonyl fluoride, 15 mg/mL aprotinin, 5
mg/mL leupeptin, 5 mg/mL pepstatin, and 1 mmol/L Na3VO4.
The homogenate was sonicated three times for 2s each and centrifuged at 16 000 g for 15min at 4℃. The
protein concentration in the cells was detected with a BCA protein assay kit
(Beyotime, China). Then, the protein was collected and stored at -80℃.
For each sample, an equal amount of protein was loaded onto a 12% sodium
dodecyl sulfate gel and run for 2-3h, after which the proteins were transferred
to polyvinylidene fluoride membranes with an electrophoresis apparatus run at
200 mA for 1h. The membranes were incubated overnight at 4℃
with primary antibodies against β-actin (1:1000; Beyotime, China), cleaved
caspase-3 [1:200; Cell Signaling Technology (CST), USA], BAX (1:200; CST, USA),
and BCL-2 (1:200; CST, USA). The membranes were then washed three times with
PBS (10 min/wash) and incubated for 1h at 37℃ with
a secondary goat anti-rabbit IgG horseradish peroxidase (HRP)-conjugated
antibody (1:3000; CST, USA) or a secondary goat anti-mouse IgG HRP-conjugated
antibody (1:3000; CST, USA). The membranes were processed using the CEL-plus
kit (Merck Millipore, Germany) to visualize the immune-blotting signals, after
which the membranes were washed and the bands were analyzed with Image J
software.
Statistical Analysis Data are expressed as mean±standard
deviation, and were processed with IBM SPSS 20.0 software (USA). Comparisons of
data among groups were evaluated by analysis of variance, followed by the
Dunnett-T3 test. A P value <0.05 was considered
statistically significant.
RESULTS
N2L Protects hRPE Cells from BL-Induced Damage N2L was not toxic to hRPE cells at the tested
concentrations, similar to ALA. The cell viability decreased significantly to
61.10%±3.69% after BL irradiation compared to the CG reference level (set to
100%; Figure 1A). However,
the addition of 100 μmol/L N2L
and 150 μmol/L ALA significantly protected the hRPE cells from subsequent
BL-induced damage (BL irradiation for 24h; Figure 1B). Furthermore, the cell
viability decreased in proportion to the time of exposure to BL irradiation. At
3h of irradiation, the cell viability in the BL group decreased slightly,
whereas the cell viability decreased significantly after irradiation for 24h,
even with 100 μmol/L N2L
and 150 μmol/L ALA treatment (Figure 1C).
In addition, the cell viability increased in proportion to the incubation time
with the drug. At 24h, no differences were found in the viabilities between
cells treated with 100 μmol/L N2L
or 150 μmol/L ALA, versus the CG (Figure 1D).
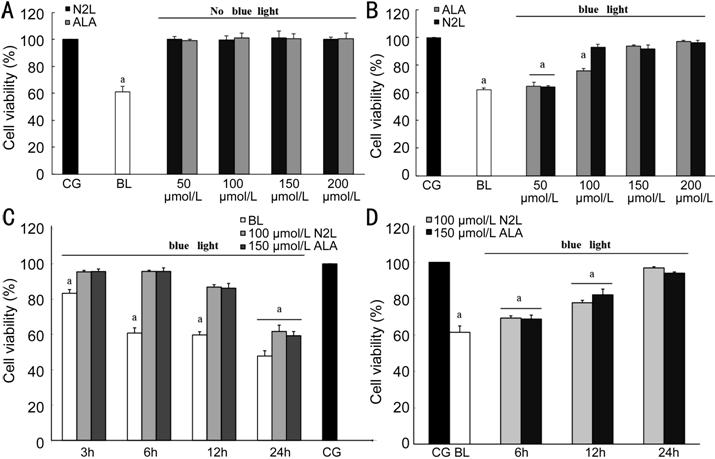
Figure 1 Cell viabilities tested by
the MTT assay A: No effects on hRPE cell viability
were observed with N2L and ALA at
any concentration tested, compared to the CG; B: Effects of different levels of
N2L, ALA, and BL alone on cell
viability after BL irradiation for 24h compared to the CG; C: Influence of BL
on the cell viability over time (3 , 6, 12, or 24h), and the protective effects
of 100 μmol/L N2L and 150
μmol/L ALA against this damage; D: hRPE cell viability in relation to
drug-treatment time (6, 12, or 24h) before BL illumination. All values are
relative to the CG cell viability set to 100%. Data are shown as means±standard
deviation (n=3). aP<0.05 versus control. CG:
Control group; BL: Blue light group; N2L:
N2L group alone; ALA: ALA group
alone.
TEM Observations of the hRPE Cell
Ultrastructure The structures of cells in the CG
were clearly visualized, revealing several cells with slender microvilli,
melanin granules, and abundant cytoplasmic organelles. The chromatin was
distributed homogeneously in the nucleus (Figure 2A, 2A1).
In contrast, obvious damage was clearly induced by exposure to BL. For example,
the cell microvilli decreased in number or disappeared completely. The cell
bodies were diminished or distorted. The mitochondria were enlarged in the
cytoplasm, and the mitochondrial crest clearance became wider. Chromatin
pyknosis was observed in the cell nucleus (Figure 2B, 2B1). Compared to these
untreated cells, the BL-induced damage was far less obvious for hRPE cells
pre-treated with 50 μmol/L ALA (Figure 2C, 2C1)
and 50 μmol/L N2L (Figure 2G, 2G1).
Although the cell microvilli also decreased or detached from the cell membrane,
minimal vacuole-like denaturation was found in the cytoplasm. Similar to the BL
group, the mitochondria were swollen and the cell nuclei were more rounded with
some speckled chromatins detected. Cell damage was noticeably reduced in the
presence of increasing concentrations of ALA or N2L. The structure of BL-exposed hRPE cells treated
with 100 μmol/L ALA (Figure 2D, 2D1) was generally normal, with only
slight changes detected. Slender microvilli were abundant, the mitochondria and
other organelles were swollen or only slightly enlarged, and the chromatin was
homogeneously distributed in the nucleus. The cells showed similar normal
structures in groups treated with higher concentrations of ALA (150 μmol/L,
Figure 2E, 2E1; 200 μmol/L, Figure 2F,
2F1) or N2L (100 μmol/L, Figure 2H, 2H1; 150 μmol/L,
Figure 2I, 2I1, 200 μmol/L, Figure 2J, 2J1). In these cases, slender microvilli
were abundant and the mitochondria were not swollen or enlarged. Moreover,
melanin granules and other organelles were clearly seen, and the chromatin was
homogeneously distributed in the nucleus.
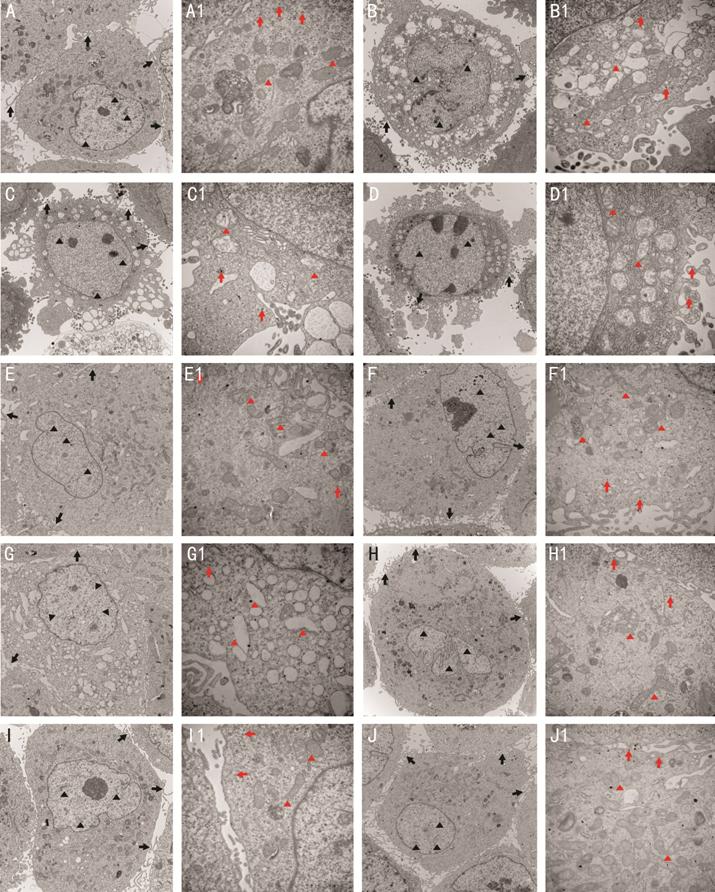
Figure 2 Ultrastructural changes in
hRPE cells treated with different concentrations of N2L and ALA after BL exposure, as determined by
TEM A, A1: CG; B, B1: BL group; C, C1:
50 μmol/L ALA with BL; D, D1: 100 μmol/L ALA with BL; E, E1: 150 μmol/L ALA
with BL; F, F1: 200 μmol/L ALA with BL; G, G1: 50 μmol/L N2L with BL; H, H1: 100 μmol/L N2L with BL; I, I1: 150 μmol/L N2L with BL; J, J1: 200 μmol/L N2L with BL. Black triangles pointed to
chromatin, black arrows pointed to slender microvilli, red triangles pointed to
mitochondria, and red arrows pointed to melanin granules. A-J: ×8000
magnification; A1-J1: ×25 000 magnification. CG: Control group; BL: Blue light
group; N2L: N2L plus BL-irradiation group; ALA: ALA plus
BL-irradiation group.
hRPE Cell Apoptosis The percentage of apoptotic hRPE
cells in the different groups was detected by flow cytometry, which revealed
that a high dose N2L or ALA
could inhibit apoptosis (Figure 3). By comparing the rates of hRPE cell
apoptosis between the CG with cell apoptosis of 7.57%±0.84% and the BL group
with cell apoptosis of 25.4%±3.13%, we found that apoptosis increased
significantly in the BL group (P<0.05). As shown, a high dose of N2L (150 μmol/L) or ALA (150 μmol/L)
significantly inhibited BL-induced hRPE cell apoptosis, compared to that in the
BL group (all P<0.05, compared with the CG. While there is no
statistical significance was observed between 100 μmol/L N2L with cell apoptosis of 6.77%±0.55% and 100
μmol/L ALA with cell apoptosis of 11.17%±0.9% compared with the CG.
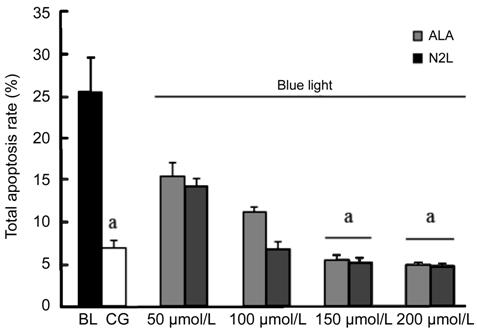
Figure 3 Apoptosis rates detected by
flow cytometry after annexin V-PE/7-AAD fluorescent staining hRPE cells were incubated in DMEM-LG
FBS-free medium and not otherwise treated (CG group), exposed to BL (BL group),
or treated with increasing concentrations of N2L or ALA (50, 100, 150, and 200 μmol/L) prior to BL
exposure. Data are shown as means±standard deviation (n=3). aP<0.05,
versus the GC group. CG: Control group; BL: Blue light group; N2L: N2L plus BL-irradiation group; ALA: ALA plus
BL-irradiation group.
Reactive Oxygen Species
Production To assess the mitochondrial function
in hRPE cells, we detected the contents of intracellular ROS by flow cytometry
after staining with the fluorescent probe DCFH-DA (10 μmol/L; Figure 4).
Pre-treatment with 100 μmol/L N2L
or 150 μmol/L ALA resulted in a time-independent inhibition of ROS production
after exposure to BL for various durations [0 (CG), 0.5, 1, 2, 3, 4, or 5h].
After BL illumination for 0.5h, a slight reduction in the ROS fluorescence was
noted in the groups treated with 100 μmol/L N2L or 150 μmol/L ALA, but the difference compared to
the BL group was not statistically significant. However, with irradiation for
1h or more, the intracellular ROS contents were significantly reduced in the
100 μmol/L N2L- and 150
μmol/L ALA-treatment groups.
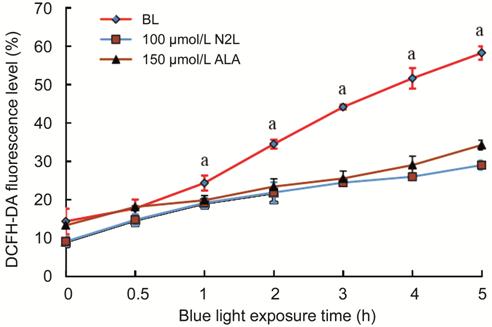
Figure 4 Intracellular ROS levels
measured by flow cytometry after staining with the fluorescent probe
DCFH-DA Data are shown as the means±standard
deviation (n=3). aP<0.05 versus BL
group (0-h group). CG: Control group; BL: Blue light group; N2L: N2L plus BL-irradiation group; ALA: ALA plus
BL-irradiation group.
Expression of Apoptosis-Related
Proteins To examine changes in the expression
of apoptosis-related proteins due to BL, we quantified the protein-expression
levels of the pro-apoptotic proteins BAX and caspase-3, and the anti-apoptotic
protein BCL-2 by Western blot analysis. The apoptotic ratio of protein BAX,
BCL-2 and caspase-3 in BL
groups, ALA groups and N2L
groups were compared to the CG in each classification. As shown in Figure 5,
compared to the CG with protein apoptotic ratio of BAX was 0.1%±0.01%, of
caspase-3 was 0.25%±0.04%, the BL group showed significantly up-regulated
expression of both BAX with protein apoptotic ratio was 0.23%±0.03% and
caspase-3 with protein apoptotic ratio was 0.57%±0.06% (P<0.05), but
down-regulated expression of BCL-2 in
BL group with protein apoptotic ratio was 0.21%±0.14% compared to CG with
protein apoptotic ratio was 0.32%±0.38% (P<0.05). In contrast,
treatment with 100 μmol/L N2L
and 150 μmol/L ALA significantly up-regulated BCL-2 expression, with apoptotic
ratio was 0.49%±0.05% in N2L
group, apoptotic ratio was 0.51%±0.07% in ALA group, which compared to CG with
apoptotic ratio was 0.32±0.38% (P<0.05), and slightly down-regulated
BAX and caspase-3 expression, with BAX apoptotic ratio was 0.04%±0.001% in N2L group. BAX apoptotic ratio was 0.06%±0.01%
in ALA group, which compared to CG with BAX apoptotic ratio was 0.1%±0.01%; and
with caspase-3 apoptotic ratio was 0.17%±0.05% in N2L group, caspase-3 apoptotic ratio was 0.26%±0.05% in
ALA group, which compared to GC with caspase-3 apoptotic ratio was 0.25%±0.04%,
although these differences were not statistically significant.
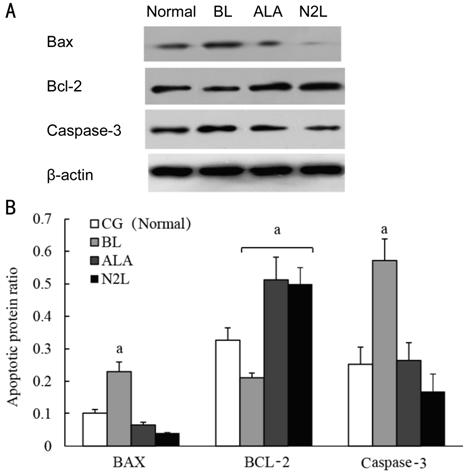
Figure 5 Effects of 100 μmol/L N2L and 150 μmol/L ALA on the protein
expression levels of BAX, caspase-3, and BCL-2 A: hRPE cells were grouped into the CG, the BL group,
and BL groups treated with 100 μmol/L N2L or 150 μmol/L ALA, and incubated accordingly for
24h. The cells were then exposed to 4±0.5 mW/cm2 BL for 6h. The
expression levels of apoptosis-related proteins were assessed via
Western blotting. B: Quantitative analysis of the expression of
apoptosis-related proteins in the different groups of hRPE cells. Data are
shown as the mean±standard deviation (n=3). aP<0.05
versus control group (CG). CG: Control group; BL: Blue light group; N2L: N2L plus BL-irradiation group; ALA: ALA plus BL-irradiation
group.
DISCUSSION
N2L is a newly derived compound that shows superior
properties compared to its constituent components, including a potent
anti-oxidative effect, a neuroprotective effect, and higher bioavailability.
However, few studies have been conducted with this novel complex to evaluate
its potential for drug development. Thus, we conducted the first study to
evaluate the protective effects of N2L
on BL-induced oxidative damage in hRPE cells. Previous findings demonstrated
that ALA could protect RPE cells, ganglion cells, and Müller cells from injury,
and against neuropathic diseases in animal models of AMD, diabetic retinopathy
or optic neuropathy[16]. Zhao et al[17] provided the first demonstration that ALA could
protect the rat retina from light-induced damage and degeneration. Other
studies showed that ALA was more readily accessible when its concentration
reached 100-400 μmol/L in vegetables, fruits, or other foods[18-19]. Therefore, we used ALA as a reference to evaluate
the protective effect of N2L
in hRPE cells.
Several studies have demonstrated
that ultraviolet or BL can injure the RPE or retinal ganglion cells, mainly via
apoptosis or a cell death mechanism owing to oxidative stress. When BL reached
3-7 mW/cm2 after irradiation for 3-24h, clear damage to the RPE or
ganglion cells was observed, and the degree of cell damage depended on the BL
density and irradiation time[20-22].
We previously found that the RPE was obviously damaged when exposed to BL at
4±0.5 mW/cm2 for 3h, with the most evident damage detected after 6h
of irradiation[23-24].
Therefore, we selected 4±0.5 mW/cm2 as the parameters for BL
treatment with hRPE cells in the experiments in this study, and 6h light
irradiation time were selected in majority experiments.
As previously studies has showed and
mentioned before, N2L could
protect hRPE cells from apoptosis and cell death induced by acrolein[14], and N2L
could protect RPE-19 cells by up-regulating expression of the anti-apoptotic
factor BCL-2 and inhibiting expression of the pro-apoptotic factor BAX[15]. In the study we found that 150 μmol/L ALA or 100
μmol/L N2L treatment
alone exerted no cytotoxic effects on hRPE cells by comparing cell viability,
and thus these concentrations were used for subsequent experiments. At these
levels, both drugs showed protective effects against the BL-induced damage in
relation to both the incubation and BL-exposure times.
Organelles such as mitochondria,
lipofuscins, and lysosomes play a critical role in the hRPE cell-damage
mechanism induced by BL[25-26].
The mitochondrion is the only organelle that provides energy to living cells
and functions as the site of ROS generation (via the electron transport
respiratory chain and ATP synthesis) upon BL stimulation in RPE cells[22,27]. Moreover,
N-retinylidene-N-retiny-thanolenrine (A2E) is an autofluorescent component of
lipofuscins in the RPE, which can itself produce ROS when it absorbs BL,
thereby inducing lipid peroxidation and causing mitochondria to generate even
more ROS. These ROS (along with A2E) may damage the mitochondria, lysosomes, or
DNA in hRPE cells[25,28]. In
turn, ROS formation triggers the apoptosis cascade through the mitochondrial
pathway, and A2E can specifically target cytochrome C oxidase to activate the
apoptosis pathway[20,26].
Sparrow et al[25,28]
found that the oxidative stress reaction could decrease the mitochondrial
membrane potential and open the mitochondrial permeability transition pore,
thereby releasing mitochondrial cytochrome C to promote apoptosis in RPE cells.
Accordingly, we assessed the effects
of BL, N2L, and ALA
on the ultrastructural changes of hRPE cells, especially in the mitochondria,
and detected the total apoptosis rate and degree of ROS production. We found
that BL decreased or eliminated the cell microvilli, resulted in swelling of
the mitochondria, and led to chromatin pyknosis in the nucleus of hRPE cells.
However, treatment with 100 μmol/L N2L
or 150 μmol/L ALA could maintain the integrity of the cell ultrastructure under
BL exposure, demonstrating the protective effect of N2L at certain extent. The most obvious changes were
noted in the mitochondria, and both N2L
and ALA suppressed the marked elevation in apoptosis after BL irradiation in a
dose-dependent manner.
Liang and Godley[3]
demonstrated that the ROS contents increased significantly after RPE cells were
exposed to 2.8 mW/cm2 BL from 1h to 6h, which was accompanied by mitochondria
injury and a positive relationship observed between ROS production and the
BL-irradiation time. We also found time-dependent effects for N2L and ALA, where the ROS contents decreased
only slightly after 1h of illumination, but with more significant inhibitory
effects detected after 2h of irradiation.
Several studies have already
demonstrated a close relationship between ALA with mitochondrial ROS
production, and both the reduced dihydrolipoic and oxidized forms of ALA quenched
the formation of hydroxyl radicals (OH-), singlet oxygen (O2-),
and hydrogen peroxide (H2O2). As a natural antioxidant,
ALA is a mitochondrial coenzyme that can reverse the age-associated decline in
mitochondria function[19]. ALA can also reduce
the oxidized forms of several important antioxidants, including vitamin C,
glutathione, and coenzyme Q10[19,29].
Recently, Li et al[30] demonstrated that
ALA could protect lens epithelial cells from apoptosis and activate
anti-oxidative enzymes. Li et al[16]
further explained that ALA can protect RPE cells from oxidative stress-induced
injury through the protein kinase B (PKB)/BAX signaling pathway. Our data
suggest that N2L may play a
similar protective role with respect to ROS formation and oxidative stress.
Many researchers also have
emphasized that apoptosis is the main mechanism of oxidative damage[27] and that the mitochondria are crucial in mediating
apoptosis. Ji et al[31] found that ALA
could protect ganglion cells from apoptosis by a mitochondrial pathway after
light insults. The caspase family executes and participates in apoptotic
activities in various cell types, and caspase-3 is the key trigger of the
apoptosis cascade in hRPE cells[32-33].
The pro-apoptotic protein BAX and the anti-apoptotic protein BCL-2 are also
crucial regulator of BL-induced apoptosis in hRPE cells[28,34]. Indeed, we inferred that N2L and ALA could prevent BL-induced apoptosis by
re-balancing the expression of BAX, BCL-2, and caspase-3. The BCL-2 protein
family is localized in the outer and inner mitochondrial membranes, along with
other anti-apoptotic proteins such as BCL-X and BCL-w. Under normal
circumstances, BCL-2 resides in the outer mitochondrial membrane can prevent
calcium (Ca2+) influx[26,35].
Therefore, the mitochondrial membrane potential can be stabilized and thus
inhibit the mitochondria from releasing apoptosis-related cytokines such as
cytochrome C, apoptosis-inducing factor, and pro-caspase-3[20]
so as to prevent an apoptosis cascade. Once hRPE cells are exposed to BL, ROS
production is induced and apoptosis is mediated by the responses of the
mitochondria[27]. These changes can result in the
swelling of cells and/or the mitochondria to release apoptotic cytokines
accompanied by activating apoptotic-inducing factor and pro-caspase-3. Thus,
elevated caspase-3 expression is a clear signal of activated cell apoptosis.
Given that N2L and ALA
significantly increased the expression of anti-apoptotic protein BCL-2, we
inferred N2L and ALA
could make the BCL-2 gathering together in the outer mitochondrial membrane, so
as to enhance the mitochondrial membrane stabilization even under the
circumstance of BL damage. At the same time, N2L and ALA reduced the expression of caspase-3 and BAX
with BL damage, even made no statistical significance, which showed the reduced
tendency, we suspected that the drug concentration decreased the sensitivity,
and further experiments suggested to be taken. Although the result of the
expressions of apoptotic related protein was not exactly as expected, combined
all the other results above, we still inferred N2L could protect the hRPE cell mitochondria from
BL-induced oxidative damage by acting as a mitochondrial cofactor, similar to
ALA, and it maybe inhibit cell apoptosis via the mitochondrial pathway at some
extent.
Overall, our findings inferred that
N2L may protect hRPE cells
against BL-induced oxidative stress, based on analyses of cell viability,
mitochondrial morphological structures, ROS production, and apoptosis, exerting
a similar effect to the well-known antioxidant ALA. We inferred that this
protective effect of N2L
may be mediated by one or more of the following mechanisms: 1) direct
scavenging of ROS; 2) activation of antioxidant defenses; 3) acting as a
cofactor to protect, repair, and stimulate mitochondrial enzymes; and/or 4)
most probably inhibiting apoptosis via the mitochondrial pathway.
However, the protective mechanism remains unclear and should be the focus of
further detailed studies, especially the probably inhibition effect of cell
apoptosis. Besides it would be much more persuasive with sample enlargement in
the experiments. The antioxidant effects of ALA have been studied in several
clinical trials for the treatment of cataracts, glaucoma, diabetic retinopathy,
cardiovascular diseases, and AMD. Given some of the limitations of ALA, our
results inferred that N2L
maybe can be a selective antioxidant therapeutic candidate for treating AMD and
slowing down the progression of the vision impairment caused by AMD, of course
there is still much work to do.
ACKNOWLEDGEMENTS
Foundations: Supported by the Guangzhou Science
and Technology Foundation of Guangdong Province (No.2014J4100035;
No.2014KP000071).
Conflicts of Interest: Zou XL, None; Yu YZ, None; Yu HH,
None; Wang GF, None; Pi RB, None; Xu Z, None; Zhang C,
None; Zhou WJ, None; Li DD, None; Chen XG, None; Zou
YP, None.
REFERENCES
|
1 Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration.
Surv Ophthalmol 1988;32(6):375-413.
https://doi.org/10.1016/0039-6257(88)90052-5
|
|
|
|
2 Tokarz P, Kaarniranta K, Blasiak J. Role of antioxidant enzymes and
small molecular weight antioxidants in the pathogenesis of age-related
macular degeneration (AMD). Biogerontology 2013;14(5):461-482.
https://doi.org/10.1007/s10522-013-9463-2
PMid:24057278 PMCid:PMC3824279
|
|
|
|
|
3 Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage
in human retinal pigment epithelial cells: a possible mechanism for RPE aging
and age-related macular degeneration. Exp Eye Res 2003;76(4):397-403.
https://doi.org/10.1016/S0014-4835(03)00023-X
|
|
|
|
|
4 Sreekumar PG, Ishikawa K, Spee C, Mehta HH, Wan JX, Yen K, Cohen P,
Kannan R, Hinton DR. The mitochondrial-derived peptide humanin protects RPE
cells from oxidative stress, senescence, and mitochondrial dysfunction.
Invest Ophthalmol Vis Sci 2016;57(3):1238-1253.
https://doi.org/10.1167/iovs.15-17053
PMid:26990160 PMCid:PMC4811181
|
|
|
|
|
5 Lambros ML, Plafker SM. Oxidative stress and the nrf2 anti-oxidant
transcription factor in age-related macular degeneration. Adv Exp Med Biol
2016;854:67-72.
https://doi.org/10.1007/978-3-319-17121-0_10
PMid:26427395 PMCid:PMC5757825
|
|
|
|
|
6 Liu ZB, Sun LJ, Zhu L, Jia X, Li XS, Jia HQ, Wang Y, Weber P, Long JG,
Liu JK. Hydroxytyrosol protects retinal pigment epithelial cells from
acrolein-induced oxidative stress and mitochondrial dysfunction. J Neurochem
2007;103(6):2690-2700.
|
|
|
|
|
7 Storm J, Müller S. Lipoic acid metabolism of Plasmodium: a suitable
drug target. Curr Pharm Des 2012;18(24):3480-3489.
https://doi.org/10.2174/138161212801327266
PMid:22607141 PMCid:PMC3426790
|
|
|
|
|
8 Boden WE, Sidhu MS, Toth PP. The therapeutic role of niacin in
dyslipidemia management. J Cardiovasc Pharmacol Ther 2014;19(2): 141-158.
https://doi.org/10.1177/1074248413514481
PMid:24363242
|
|
|
|
|
9 McNamara MJ, Sayanlar JJ, Dooley DJ, Srichai MB, Taylor AJ. A
randomized pilot study on the effect of niacin on pulmonary arterial
pressure. Trials 2015;16:530.
https://doi.org/10.1186/s13063-015-1013-6
PMid:26590128 PMCid:PMC4654874
|
|
|
|
|
10 Ruparelia N, Digby JE, Choudhury RP. Effects of niacin on
atherosclerosis and vascular function. Curr Opin Cardiol 2011;26(1): 66-70.
https://doi.org/10.1097/HCO.0b013e3283410c16
PMid:21045681 PMCid:PMC3145140
|
|
|
|
|
11 Chen X, Gao JW, Jiang YM, Huang P, Xie YH, Pi RB, Zhu SZ, Yao MC.
Determination of newly synthesized lipoic acid-niacin dimer in rat plasma by
UPLC/electrospray ionization tandem mass spectrometry: assay development,
validation and application to a pharmacokinetic study. Biomed Chromatogr
2014;28(2):213-217.
https://doi.org/10.1002/bmc.3006
PMid:23934776
|
|
|
|
|
12 Pi RB, Jiang M, Chao Xj, Liu PQ,Huang Y,Yao MC, He XX. Niacin
derivative: its preparation method and drug component. China, CN
102532114A,[P].2012.07.04
|
|
|
|
|
13 Wang GF, Li WL, Zou XL, Zou YP, Pi RB, Yao M.. Protective effects of
lipoic acid-niacin diad (5a-f) on ARPE-19 cells induced by acrolein. Recent
Advances in Ophthalmology 2013;2(33):106-109.
|
|
|
|
|
14 Zou XL, Wang GF, Li WL, Pi RB, Yu YZ, Zou YP. Anti-apoptosis effect of
lipoic acid-niacin diad on acrolein-induced apoptosis in ARPE-19 cells. The J
Pract Med 2014;30(15):2381-2383.
|
|
|
|
|
15 Zhou WJ, Yu YZ, Xu Z, Zou XL, Li DD, Zou YP.Role of mitochondrial DNA
in human retinal pigment epithelium cells apoptosis induced by blue light.
Chin J Exp Ophthalmol 2018;4(36):267-272.
|
|
|
|
|
16 Li Y, Shen HW, Shi JM, Tang LS. The effects of alpha lipoic acid in
preventing oxidative stress-induced retinal pigment epithelial cell injury.
Can J Physiol Pharmacol 2014;92(9):765-772.
https://doi.org/10.1139/cjpp-2014-0018
PMid:25179747
|
|
|
|
|
17 Zhao L, Liu ZB, Jia HQ, Feng ZH, Liu JK, Li XS. Lipoamide acts as an
indirect antioxidant by simultaneously stimulating mitochondrial biogenesis
and phase II antioxidant enzyme systems in ARPE-19 cells. PLoS One
2015;10(6):e0128502.
https://doi.org/10.1371/journal.pone.0128502
PMid:26030919 PMCid:PMC4452644
|
|
|
|
|
18 Xia HK, Nan Y, Huang X, Gao J, Pu ML. Effects of tauroursodeoxycholic
acid and alpha-lipoic-acid on the visual response properties of cat retinal
ganglion cells: an in vitro study. Invest Ophthalmol Vis Sci
2015;56(11):6638-6645.
https://doi.org/10.1167/iovs.15-17301
PMid:26469749
|
|
|
|
|
19 Huk-Kolega H, Skibska B, Kleniewska P, Piechota A, Michalski Ł, Goraca
A. Role of lipoic acid in health and disease. Pol Merkur Lekarski
2011;31(183):183-185.
|
|
|
|
|
20 Cai SJ, Yan M, Mao YQ, Zhou Y, Liu GJ. Relationship between blue
light-induced apoptosis and mitochondrial membrane potential and cytochrome C
in cultured human retinal pigment epithelium cells. Zhonghua Yan Ke Za Zhi
2006;42(12):1095-1102.
|
|
|
|
|
21 Chamorro E, Bonnin-Arias C, Pérez-Carrasco MJ, Muñoz de Luna J,
Vázquez D, Sánchez-Ramos C. Effects of light-emitting diode radiations on
human retinal pigment epithelial cells in vitro. Photochem Photobiol
2013;89(2):468-473.
https://doi.org/10.1111/j.1751-1097.2012.01237.x
PMid:22989198
|
|
|
|
|
22 Narimatsu T, Ozawa Y, Miyake S, Kubota S, Hirasawa M, Nagai N,
Shimmura S, Tsubota K. Disruption of cell-cell junctions and induction of
pathological cytokines in the retinal pigment epithelium of light-exposed
mice. Invest Ophthalmol Vis Sci 2013;54(7):4555-4562.
https://doi.org/10.1167/iovs.12-11572
PMid:23761083
|
|
|
|
|
23 Zou XL, Yu YZ,Xu Z, Zhang C, Wang GF, Zou YP. Oxidative damage of
human retinal pigment epithelium cells induced by blue light irradiation and
mitochondria-participated mechanism. Chinese Journal of Experimental
Ophthalmology 2015;2(33):129-134.
|
|
|
|
|
24 Yu YZ, Xu Z, Zou XL, Zhang C, Wang GF, Zou YP. Role of oxidative
stress induced by blue light in human retinal pigment epithelium cells
apoptosis. Recent Advances in Ophthalmology 2015;6(35):520-524.
|
|
|
|
|
25 Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E
mediates blue light-induced damage to retinal pigmented epithelial cells.
Invest Ophthalmol Vis Sci 2000;41(7):1981-1989.
|
|
|
|
|
26 Yu YZ, Zou XL, Zou YP. The relationship between mitochondrial DNA
damage and retinal pigment epithelium cells. Tianjin Med J
2015;43(9):1079-1081.
|
|
|
|
|
27 King A, Gottlieb E, Brooks DG, Murphy MP, Dunaief JL.
Mitochondria-derived reactive oxygen species mediate blue light-induced death
of retinal pigment epithelial cells. Photochem Photobiol 2004;79(5):470-475.
https://doi.org/10.1562/LE-03-17.1
|
|
|
|
|
28 Sparrow JR, Cai B. Blue light-induced apoptosis of A2E-containing RPE:
involvement of caspase-3 and protection by Bcl-2. Invest Ophthalmol Vis Sci
2001;42(6):1356-1362.
|
|
|
|
|
29 Ong SL, Vohra H, Zhang Y, Sutton M, Whitworth JA. The effect of
alpha-lipoic acid on mitochondrial superoxide and glucocorticoid-induced
hypertension. Oxid Med Cell Longev 2013;2013:517045.
https://doi.org/10.1155/2013/517045
PMid:23533693 PMCid:PMC3600316
|
|
|
|
|
30 Li Y, Liu YZ, Shi JM, Jia SB. Alpha lipoic acid protects Lens from
H(2)O(2)-induced cataract by inhibiting apoptosis of lens epithelial cells
and inducing activation of anti-oxidative enzymes. Asian Pac J Trop Med
2013;6(7):548-551.
https://doi.org/10.1016/S1995-7645(13)60094-2
|
|
|
|
|
31 Ji D, Majid AS, Yin ZQ. Α-Lipoic acid attenuates light insults to
neurones. Biol Pharm Bull 2013;36(7):1060-1067.
https://doi.org/10.1248/bpb.b12-00941
PMid:23811555
|
|
|
|
|
32 Migheli A, Cavalla P, Piva R, Giordana MT, Schiffer D. Bcl-2 protein
expression in aged brain and neurodegenerative diseases. Neuroreport
1994;5(15):1906-1908.
https://doi.org/10.1097/00001756-199410000-00016
PMid:7841373
|
|
|
|
|
33 Abu-El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K.
Expression of apoptosis markers in the retinas of human subjects with
diabetes. Invest Ophthalmol Vis Sci 2004;45(8):2760-2766.
https://doi.org/10.1167/iovs.03-1392
PMid:15277502
|
|
|
|
|
34 Wu J, Seregard S, Spångberg B, Oskarsson M, Chen E. Blue light induced
apoptosis in rat retina. Eye (Lond) 1999;13(Pt 4):577-583.
https://doi.org/10.1038/eye.1999.142
PMid:10692935
|
|
|
|
|
35 Khalfaoui T, Basora N, Ouertani-Meddeb A. Apoptotic factors (Bcl-2 and
Bax) and diabetic retinopathy in type 2 diabetes. J Mol Histol
2010;41(2-3):143-152.
https://doi.org/10.1007/s10735-010-9271-9
PMid:20532811
|
|





