
·Clinical Research·
Evaluation of suturless scleral fixation with posterior chamber foldable intraocular lens implantation
Ahmed M. Bedda1, Hesham F. ElGoweini1, Ahmed M. Abdelhadi1, Amr M. Elhady2
1Department of Ophthalmology, Alexandria University, Alexandria 11511, Egypt
2Department of Ophthalmology, the General Ophthalmology Hospital, Alexandria 21515, Egypt
Correspondence to: Ahmed M. Abdelhadi. 24 Fawzy Moaz Street, Safwa 5, Entrance 2, Semoha Alexandria 11511, Egypt. aabdelhadi2010@gmail.com
Received:
Abstract
AIM: To assess the sutureless scleral fixation technique for posterior chamber foldable intraocular lens (PCIOL) implantation in aphakic eyes with insufficient or no capsular support.
METHODS: A technique for sutureless intrascleral fixation of the haptics of a standard 3-piece PCIOL was used which ensures sutureless fixation by permanent incarceration of the haptics in a scleral tunnel parallel to the limbus. All patients were evaluated for preoperative status [visual acuity, refractive error, K readings, intraocular pressure (IOP) measurement, slit lamp examination, fundus examination and optical biometry], postoperative status and complications. Ultrasound biomicroscopy (UBM) was done for 10 cases to evaluate optic tilt.
RESULTS: The study evaluated 42 eyes of 42 patients. The follow-up period was 6mo. Improvement of best corrected visual acuity (BCVA) one line occurred in 10 cases (23.8%) and loss of one line in 3 cases (7.1%). Intraoperative complications included: haptic kink in 4 cases (9.5%), haptic breakage in 1 case (2.4%), haptic dislocation in 1 case (2.4%), haptic slippage in 3 cases (7.1%), IOL dislocation in 1 case (2.4%) and sclerotomy related bleeding in 1 case (2.4%). Postoperative complications included: transient mild vitreous hemorrhage in 3 cases (7.1%), choroidal detachment in 1 case (2.4%), cystoid macular edema (CME) in 1 case (2.4%), optic capture in 1 case (2.4%), subconjunctival haptic in 2 cases (4.8%), ocular hypotony in 4 cases (9.5%) and ocular hypertension in 1 case (2.4%). There were no cases of retinal detachment or endophthalmitis. UBM showed optic tilt in 3 cases (30%).
CONCLUSION: Fixation of three-piece foldable IOL haptics in scleral tunnel parallel to the limbus- provided axial stability and proper centration of the IOL with minimal or no tilt in most cases and a low complication rate during the follow up period which lasted 6mo.
KEYWORDS: aphakia correction; sutureless scleral fixation; foldable three-piece intraocular lens
DOI:10.18240/ijo.2019.08.08
Citation: Bedda AM, ElGoweini HF, Abdelhadi AM, Elhady AM. Evaluation of suturless scleral fixation with posterior chamber foldable intraocular lens implantation. Int J Ophthalmol 2019;12(8):1283-1289
INTRODUCTION
Cataract surgery has become one of the most commonly performed intraocular surgeries, with constantly improving results. Planned extracapsular cataract extraction gradually became more widespread; it was then further refined by phacoemulsification[1]. The presence of weak zoonules makes implantation of an intraocular lens (IOL) into the capsular bag or in the sulcus difficult or even impossible because the success of either of these positions depends on remaining capsular fragments for adequate support. Alternative strategies must be used, like scleral suturing techniques and anterior chamber foldable intraocular lens (ACIOL), with varying results[2].
For the last two decades, suturing the IOL haptics to the scleral wall have presented us with good alternative in cases with absent or deficient capsular bag support. However, this technique has its own set of risks. From the most important concerns are the doubtful long-term stability and structural integrity of the suture materials used in such surgeries. In addition, scleral suturing requires complex operative technical skills, which may be dependent on personal surgeon’s skills[3].
Similarly, the use of iris fixated lenses in cases where there is insufficient capsular support bring about many concerns; they are not the best choice with the lack of an intact iris diaphragm. They can possibly result in iris chafing, secondary intraocular inflammation with subsequent cystoid macular edema (CME)[4].
Recently, sutureless intrascleral posterior chamber IOL haptic fixation was suggested as an alternative implantation of an IOL in the bag in complicated cases. The sutureless technique entails permanent incarceration of the lens haptics inside the scleral tunnels. This haptic fixation technique into pre-prepared scleral tunnels combines the control of a closed-eye system surgery with the post-surgery axial stability of the IOL. Reported complications of transscleral fixation of posterior chamber foldable intraocular lens (PCIOLs) include liability to either suture erosion or suture-knot exposure, and possible dislocations caused by a broken suture, all of which can be avoided by trying the sutureless technique[5].
SUBJECTS AND METHODS
Ethical Approval An interventional prospective case series was carried on 30 aphakic eyes. The author followed the tenets of the Helsinki Declaration. The assigned investigator informed all patients about the risks and benefits of the surgery, and a formal consent was obtained in writing after thorough explanation of the procedure. The study was reviewed and subsequently approved by the Scientific and the Ethics Committee at the Faculty of Medicine, Alexandria University Ophthalmology Department.
Inclusion criteria included aphakia with insufficient or no capsular support.
Exclusion criteria included aphakia with adequate capsular support, increased intraocular pressure (IOP), previous glaucoma surgery, scleral diseases and causes of poor vision like amblyopia, macular scar, macular hole, choroidal neovascular membrane, etc).
Full clinical history was taken from all patients including their age, complaint, history of ocular trauma or disease, date of previous cataract surgery, optical correction in case of aphakia and relevant systemic diseases e.g. hypertension, diabetes mellitus etc. Complete ophthalmic examination includes preoperative and postoperative refraction, corrected visual acuity (later transformed to logMAR for statistical analysis), K readings using auto-keratometry, Slit lamp examination before and after pupillary dilatation, IOP measurement using Goldman applanation tonometry, fundus examination (biomicroscopy & indirect ophthalmoscopy) and special ophthalmic investigations e.g. Biometry (aphakic mode) were all carried out. Both intraoperative and postoperative complications were recorded.
Ultrasound
Biomicroscopy Ultrasound biomicroscopy (UBM) was
performed in Alexandria University for 10 cases after completing the six months
of follow up to assess IOL tilt using a 35 MHz probe. After topical anesthesia,
a suitable cup was chosen and placed in its position. A small amount of a
coupling solution (methyl-cellulose) was used to seal the base of the cup then
the cup was filled with balanced salt solution. Cross-sectional images were
obtained on both the vertical axis and horizontal axes. The IOL optic tilt was
identified using the technique described by Loya et al[6] The optic was imaged on the UBM and the distance to the posterior surfaces of
the overlying iris was reported. Optic tilt was determined as follows: First,
drawing a line along the posterior iris surface, marking the iris as the plane
of reference for the optic position. Consequently, no tilt of the optic was
reported when the reference line along the iris and the IOL optic axis was
parallel (Figure 1). IOL optic position was measured using the caliper tool in
the UBM system. The distance between the posterior iris plan and the IOL optic
was measured in 4 positions, namely the superior (12 o’clock), inferior (6
o’clock), nasal (3 o’clock in the right eye and 9 o’clock in left eye), and
temporal (9 o’clock in right eye and 3 o’clock in left eye). Tilt was measured
in millimeters. The difference between the superior and inferior distances was
named vertical tilt. The difference between the nasal and temporal distances
was named, horizontal tilt. When the difference between the 2 edges of the lens
from the iris plane was more than
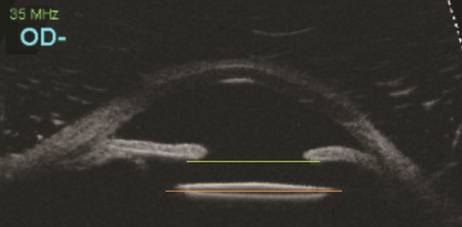
Figure 1 Reference lines along the iris and the IOL optic axis were parallel.
Surgical
Technique Localized conjunctival peritomy was
done. After inserting an infusion cannula or anterior chamber maintainer to maintain
a pressurized globe and preventing the globe from collapsing during all steps
of the surgery, 2 sclerotomies were made with 23-gauge MVR about
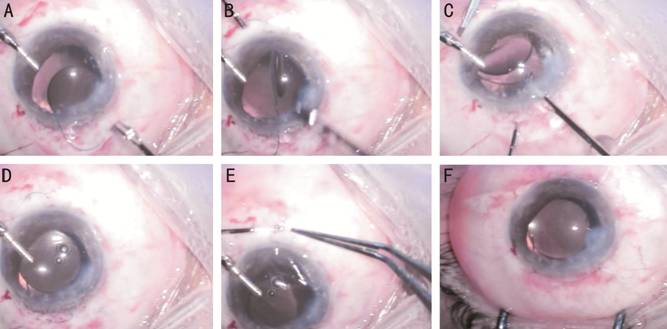
Figure 2 Surgical technique A: Introduction of IOL; B: Externalization of inferior haptic; C: Handshake technique; D: Externalization of both superior haptic; E: Introducing of the haptic in scleral tunnel; F: Closure of conjunctiva and stromal hydration of corneal wounds.
RESULTS
The mean preoperative best corrected visual acuity (BCVA; logMAR) was 0.44±0.15 (range: 0.80-0.18). The mean postoperative BCVA was 0.74±0.22 (range: 1.30-0.48), 0.59±0.21 (range: 1.30-0.30), 0.46±0.19 (range: 1.0-0.18), 0.43±0.140 (range: 0.80-0.18) and 0.42±0.13 (range: 0.60-0.18). Distribution of cases according to level of BCVA showed in Table 1 and Figure 3.
Table 1 Comparison between preoperative and postoperative BCVA
Parameters |
Preoperative |
Postoperative |
||||
1d |
1wk |
1mo |
3mo |
6mo |
||
BCVA (logMAR) |
||||||
Min-max |
0.80-0.18 |
1.30-0.48 |
1.30-0.30 |
1.0-0.18 |
0.80-0.18 |
0.60-0.18 |
Mean±SD |
0.44±0.15 |
0.74±0.22 |
0.59±0.21 |
0.46±0.19 |
0.43±0.14 |
0.42±0.13 |
F (P) |
51.383 (<0.001) |
|||||
Pa |
<0.001 |
<0.001 |
1.000 |
1.000 |
0.813 |
|
F: F test (ANOVA) with repeated measures. aSignificance between groups were done using stands for adjusted Bonferroni P-value for ANOVA with repeated measures for comparing between the pre-operative values with each other.

Figure 3 Comparison between preoperative and postoperative BCVA (logMAR).
Analysis cases according to visual improvement we found that in 5 cases BCVA improved one line, 2 cases showed loss of one line and in 23 there were no change from the preoperative level (Table 2).
Table 2 Changes of BCVA by the end of follow up
BCVA (logMAR) |
By the end of follow up |
Percentage |
Improvement one line |
5 |
16.7 |
Lost one line |
2 |
6.6 |
Similar to preoperative |
23 |
76.7 |
BCVA: Best corrected visual acuity.
Intraoperative complications were mainly related to IOL haptic manipulation. Kinking of haptic occurred in three cases, breakage occurred in one case, slippage occurred in two cases and haptic dislocation occurred in one case. IOL posterior dislocation occurred in one case. Intraocular bleeding occurred in one case during sclerotomy (Table 3, Figure 4).
Table 3 Distribution of the studied cases according to intra-operative complications
Intra-operative complications |
n (%) |
Haptic kink |
3 (10.0) |
Haptic breakage |
1 (3.3) |
Haptic dislocation |
1 (3.3) |
Haptic slippage |
2 (6.7) |
IOL dislocation |
1 (3.3) |
Sclerotomy related bleeding |
1 (3.3) |
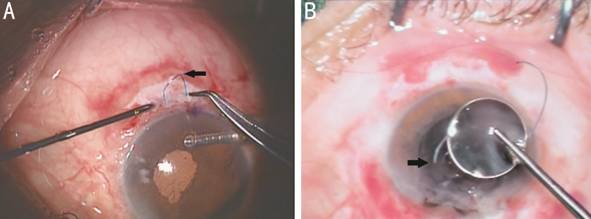
Figure 4 Intra operative complications related to IOL haptic manipulation A: Kinking of the inferior haptic during the pulling on it; B: Breakage of one haptic of the IOL, which necessitated replacement of the IOL.
Temporary
corneal edema occurred in 9 cases at early postoperative period. Uveitis with
anterior chamber cells +2 occurred in 7 cases and anterior chamber cells +3
occurred in 2 cases. Ocular hypertension (defined as IOP≥
Table 4 Distribution of the studied cases according to postoperative complications
Parameters |
n (%) |
Postoperative complications |
|
Temporal corneal edema |
9 (30) |
Uveitis |
|
AC cells +2 |
7 (23.3) |
AC cells +3 |
2 (6.7) |
IOP |
|
Ocular hypotony |
2 (6.7) |
Ocular hypertension |
1 (3.3) |
Transient mild vitreous hemorrhage |
3 (10.0) |
Choroidal detachment |
1 (3.3) |
CME |
1 (3.3) |
Optic capture |
1 (3.3) |
Subconjunctival haptic |
2 (6.7) |
AC: Anterior chamber; CME: Cystoid macular edema.
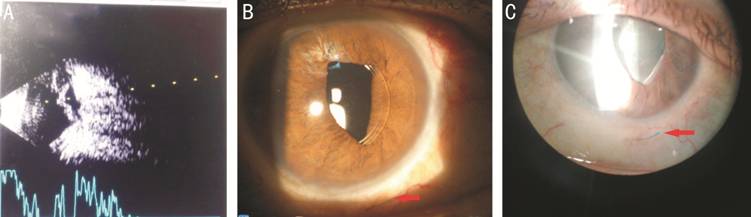
Figure 5 Postoperative complications A: Combined B&A scan US showing choroidal detachment complicating one case which resolved completely within 1wk with medical therapy; B: Optic capture and subconjunctival haptic evident by the red arrow; C: Subconjunctival haptic, shown by the red arrow in a case evident after 3wk from surgery.
Intraocular
Lens Optic Position The mean distance of the IOL optic
position from the iris pigment epithelium plane were 0.82±
Table 5 Descriptive of the studied cases according to UBM
Parameters |
Data |
Tilt, n (%) |
|
Absent (<
|
7 (70.0) |
Present (>
|
3 (30.0) |
Vertical tilt |
1 (10.0) |
Horizontal tilt |
2 (20.0) |
Superior |
|
Min-max |
0.73-0.86 |
Mean±SD |
0.82±0.04 |
Inferior |
|
Min-max |
0.76-1.35 |
Mean±SD |
0.87±0.17 |
Nasal |
|
Min-max |
0.74-1.25 |
Mean±SD |
0.89±0.18 |
Temporal |
|
Min-max |
0.58-0.87 |
Mean±SD |
0.80±0.09 |
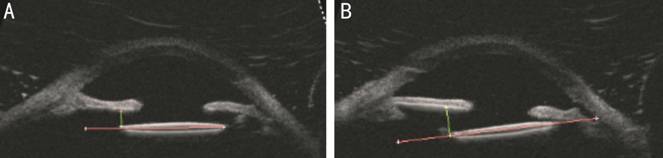
Figure 6
Ultrasound biomicroscopy A: No optic tilt (difference between
distances of the two optic edges from posterior iris surface <
DISCUSSION
In the present study we used a technique similar to Scharioth et al[7] except that 23-gauge forceps and 23-gauge microvitreoretinal (MVR) were used instead of 25-gauge forceps and 24-gauge cannula respectively. Scharioth et al[7] presented the data for 63 patients (30 females, 33 males) with a mean age for the study population of 64y (range 15 to 87y).
Agarwal et al[8], used technique in 10 eyes of 10 patients similar to our technique but created a scleral flap to bury the haptics under it, 22-gauge needle and 25-gauge forceps were used in their study. Also, fibrin glue was used to glue the haptics in the sclera tunnel, to seal the sclera flap and to close the conjunctiva.
Kumar et al[9] studied two hundred eight eyes of 185 patients. The mean age for his patients was 49.7±23y (range 7 to 85y). The patients included were 100 males & 85 females. In their study, 23 patients (12.4%) had bilateral foldable glued-IOL surgeries performed in two different settings. The main reason for implanting glued IOLs were intraoperative capsular loss that resulted inadequate capsular support and sub-luxated lens.
Narang[10] used the technique in 50 eyes of 45 patients. Sclerotomies with a 20-gauge needle was made beneath previously prepared partial-thickness scleral flaps fashioned 180 degrees opposite each other.
Saleh et
al[11] presented his data for eight eyes (5
men & 3 women) with traumatic cataract and inadequate capsular support. The
mean age was 55.1±23.3y. The technique used was a little different from our
study. Needle guided externalization of the haptics was used. A 23-gauge
cannula was introduced at
Ohta et al[12] studied 44 eyes of 40 patients. They performed a Y-shaped incision in the sclera. This eliminated the need to raise a large lamellar scleral flap or to have to use fibrin glue because the haptic can be fixed both inside the tunnel and in the groove. Sclerotomy is done parallel to the iris plane with a 24-gauge angled MVR knife, and a scleral tunnel was made parallel to the limbus at the branching point of the Y-shaped incision.
The techniques of the previous studies were a little different from the technique adopted in the current study. Almost all studies have a similar basic principle of sutureless intrascleral haptics fixation. Different size of sclerotomy, different size of forceps, and method of haptic externalization and whether to use sclera flap or not were the main difference points.
The use of small-diameter scleral tunnels for the IOL haptic fixation reduces the surgical manipulation and trauma to the underlying sclera. This small-diameter scleral tunnels can provide what we call leak-free closure. Scleral tunnels fashioned must be uniform and narrow to avoid postoperative IOL tilt. It is less time consuming because it stabilizes the IOL to the globe wall without the need for difficult suturing procedures. Posterior chamber IOL tilt and decentration can be minimized by accurate placement of the haptics in the scleral tunnel above the ciliary sulcus.
The implanted 3-piece PCIOL has a haptic designed to occupy the full diameter of the ciliary sulcus. So, with this technique the haptic is placed in its normal curved configuration without traction, secured in the prepared sclera tunnel, consequently, there is greater stability of the IOL than in suture scleral fixated IOLs which tends to move with time. Moreover, the use of foldable IOLs especially with the aid of injector would significantly decrease surgical induced astigmatism.
Intraoperative centration of the IOL is made possible with this procedure due to adjustments of the final intrascleral position of the haptics. A crucial difference between both the sutureless technique and the traditional method of suture fixation of the IOL to the scleral wall is that, postoperative re-positioning of the IOL is difficult after suture fixation. With intrascleral fixation, re-positioning is easily performed by reopening the incision in both the conjunctiva & sclera and adjusting the position of the IOL haptic.
Agrawal et al[8] used scleral flap to cover part of the haptic to prevent haptic exposure. It also covers the sclerotomy site to lessen postoperative complication like hypotony and endophthalmitis. Moreover, fibrin glue was utilized to seal the haptics in the sclera tunnels and to seal the sclerotomy site with the overlying sclera flaps so that there is no trabeculotomy sort of opening left and reduces any chance of endophthalmitis.
In the present study and that by Scharioth et al[7] study, sclera flaps were not used. As the sclera tunnels were made just at the site of the sclerotomy so that the externalized part of the haptic was completely inside the tunnel and no part of which was exposed. There was no need to use sclera flaps to cover the haptics. Moreover, scleral flaps could induce astigmatism after tissue contraction especially if glue was used and also, there the potential for long-term complications e.g. scleral atrophy. Also, fibrin glue was not used, it does not have a tensile strength to keep fixed an IOL haptic, the glue alone does not maintain long term IOL stability, it only acts for a while. What secures the lens in this procedure is a scleral pocket that the tip of the IOL haptic is tucked into. Moreover, the fibrin glue, which is commercially available nowadays is virus inactivated. Although it is checked for viral antigen and antibodies with polymerase chain reaction, there is always the theoretical possibility of viral infections transmission.
Sutureless scleral IOL fixation represents a good alternative for the correction of aphakia with severely damaged capsule especially in cases of iatrogenic iris damage that prevents iris-claw IOL implantation. It restores good vision in aphakic patients with early rehabilitation and a relatively low complication rate. The technique results in clinically well centered and stable IOL with minimal or no tilt in most of the studied cases during the follow up period.
Although long-term data is lacking, all techniques mentioned in previous studies show good visual long-term results without significant side effects[13].
The technique mentioned in the current study have some combined advantages that could make the outcome after a short learning curve reproducible. The use of small gauge in creation of the tunnels make the sclerotomy self sealed, without the need of sutures or glue. This lack of glue makes intraoperative adjustment possible. The high rate of temporary corneal edema is acceptable, as it was present mainly in the early cases, again all cases improved rapidly with topical steroids.
There have been relatively few reports of complications with sutureless scleral fixation, such as IOL decentralization (incidence, 1.97%-5%), CME (1.97%-13.3%), optic capture (2.6%-14.3%), subconjunctival haptic (19%), conjunctival erosion from haptic exposure (12.5%), transient vitreous hemorrhage (6%-18%), and retinal detachment[5,14-17]. In the current study similar complications were met with acceptable percentage, the most frequent being haptic kink in 9.5% of cases. Temporary corneal edema represents the most common postoperative complication, occurring in 11 patients (26.1%). This relatively high rate may be explained by the long duration of the surgery in the first few cases which improved significantly in the last 10 cases, which resulted in less incidence of immediate postoperative inflammation and corneal edema. The next most common complication being ocular hypotony which was diagnosed in 4 eyes (9.5%), followed by ocular hypertension in 3 eyes (7.1%). Transient mild vitreous hemorrhage was identified in 3 eyes (7.1%), this resolved after 3-9d spontaneously. Choroidal detachment, CME and optic capture were noticed each in a single eye (2.4%). This apparent higher rate of complications especially the posterior segment complications may be explained by the choice of cases which might be originally predisposed to these range of problems with any major intraocular surgery.
IOL position is a crucial component for a satisfactory final vision, as malposition that can lead to uncorrectable astigmatism, change in optical higher‑order aberrations, and consequently loss final BCVA.
In the current study, from the 10 cases where UBM was performed, 3 eyes had optic tilt of (≥100 µm); this difference was identified in the vertical axis in one eye (10%) and in the horizontal axis in 2 eyes (20%). An UBM analysis of postoperative glued IOL’s demonstrated microscopic tilt in 17.4% of eyes[6,18].
Limitations of the current study include lack of long term follow up, small sample size which did not allow us to accurately study all the encountered complications and how to avoid them beforehand in future cases.
Also, an obvious drawback of this technique is the inability to check for IOL tilt except postoperatively, which means another journey to the OR to adjust the tilt if found to be significant.
ACKNOWLEDGEMENTS
Authors’ contributions: All authors equally contributed to this study.
Conflicts of Interest: Bedda AM, None, ElGoweini HF, None, Abdelhadi AM, None, Elhady AM, None.
REFERENCES