
Citation: Alpay A. Posterior vitreous detachment rate following intravitreal dexamethasone injection. Int J Ophthalmol 2019;12(8):1298-1303. DOI:10.18240/ijo.2019.08.10
·Clinical Research·
Posterior vitreous detachment rate following intravitreal dexamethasone injection
Atilla Alpay
Department of Ophthalmology, the School of Medicine, Zonguldak Bülent Ecevit University, Zonguldak 67600, Turkey
Correspondence to: Atilla Alpay. Zonguldak Bülent Ecevit Üniversitesi Tip Fakültesi, Göz Hastaliklari Anabilim Dali, Kozlu, Zonguldak 67600, Turkey. atillaalpay@hotmail.com
Received:
Abstract
AIM: To determine whether intravitreal dexamethasone (DEX) implant induces posterior vitreous detachment or not.
METHODS: We retrospectively reviewed 810 eyes of 405 patients who underwent intravitreal DEX implantation due to macular edema caused by diabetic and retinal venous occlusion in our clinic. The eyes having no injection were determined as the control group. The examination findings of the patients before the injection and 3mo after the injection and optical coherence tomography (OCT) images were scanned. The pre-injection OCT findings and OCT findings of the patients having no posterior vitreous detachment (PVD) and determined to have partial PVD were compared.
RESULTS: The separation in vitreoretinal adhesion and total PVD development of DEX-injected 56/208 (26.9%) eyes were statistically greater in comparison with the 12/129 (9.3%) eyes that had not been injected (P=0.001). PVD development was observed more in the patients that were younger, had larger macula thickness and lower visual acuity.
CONCLUSION: It can be stated that intravitreal DEX implant induces PVD development. Prospective, controlled studies are required in order to determine prognosis of vitreoretinal disease in PVD-developed patients and in non-PVD-developed patients.
KEYWORDS: dexamethasone implant; intravitreal injection; vitreoretinal adhesion; diabetic macular edema; retinal vein occlusion; posterior vitreous detchment
DOI:10.18240/ijo.2019.08.10
Citation: Alpay A. Posterior vitreous detachment rate following intravitreal dexamethasone injection. Int J Ophthalmol 2019;12(8):1298-1303
INTRODUCTION
Today, intravitreal corticosteroids and vascular endothelial growth factor inhibitors (anti-VEGF) are widely used for the treatment of macular edema due to diabetic retinopathy and retinal vein occlusion. One of the unintended effects of intravitreal injections is posterior vitreous detachment (PVD). The posterior vitreous is more tightly adherent to the retina surface, especially the vitreous base, optic disc, retinal vessels and fovea[1]. This attachment decreases with increasing age. The attachments in perifoveal area cannot be separated during the development of PVD and they can cause vitreomacular traction (VMT) and various macula diseases if they become persistent[2-8]. Persistent vitreomacular adhesion (VMA) is observed more frequently, in the eyes having diabetic retinopathy and age-related macular degeneration (AMD) compared to the healthy eyes[9-12] and vitreoretinal traction associated pathologies can regress with natural or surgical separation[12-14]. Intravitreal ocriplasmin or microplasmin injections were implemented for eliminating the VMA dependent complications by creating total PVD and successful results were obtained[15-17]. It was observed that triamcinolone and anti-VEGFs’ intravitreal injections accelerate PVD development[18]. Dexamethasone (DEX) intravitreal implant 0.7 mg (Ozurdex®, Allergan, Inc., Irvine, CA, USA) has been approved for use in the treatment of macular edema caused by diabetes, and retinal vein occlusion. We could not find any studies examining whether PVD ratio increases after intravitreal DEX implant in the literature. Intravitreal DEX implant is different from anti-VEGF drugs in terms of its structure. While intravitreal anti-VEGFs are injected by 30 gauge injectors, DEX implant is injected by 22 gauge injector and sometimes vitreous loss from injection area is observed[19]. Additionally, the implant, which is a rigid object staying in vitreous for 3-4mo, can contribute to PVD development by implementing perpetual traction to vitreous through eye movements. Therefore, we examined whether PVD develops after the injection of DEX implant into eye and if so, what its level is.
SUBJECTS AND METHODS
Ethical Approval This study was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from the patients.
The medical records of 405 patients who had intravitreal DEX injection for macular edema caused by diabetes and retinal vein occlusion were examined from April 2013 to January 2018 retrospectively.
The eyes which had any intravitreal injection and retinal laser photocoagulation before or 3mo after the injection, eyes with vitreoretinal surgery, complicated cataract surgery, intraocular inflammation, traumata history, and eyes with diagnosed proliferative diabetic retinopathy, macular hole, myopic fundus in fundus examination[20]; and had cataract surgery in the first 3mo after injection were excluded from the study. The other eye of the patients whose only one eye was injected was determined as the control group. For control group inclusion criteria were in addition to the inclusion criteria mentioned above, eyes without diabetic retinopathy, eyes without diabetic maculopathy and eyes with diabetic retinopathy and/or maculopathy but central macular thickness of less than 250 µm.
Intravitreal
injections were performed in a clean injection room, in a horizontal position.
Paraprocaine hydrochloride 0.5% (Alcaine, Alcon-Couvreur, Puurs, Belgium) was
instilled in the eyes of patients to be injected. The eyes were covered by a
sterile drape after orbital area was cleaned with 10% povidone-iodine solution.
Then the eyes were opened by eye speculum and a couple of 5% povidone-iodine
solution was instilled into the conjunctiva. After a minute, povidone-iodine
was washed by sterile saline. The injection area was marked in the superior
temporal area, 3.5
Ophthalmologic examination findings and OCT images before intravitreal DEX injection and 3mo after the injection of the patients included in the study were examined. The eye examination findings, macular thickness, and vitreoretinal relationship in terms of OCT images were recorded and graded[21]. The vitreoretinal relationship was graded as shown in Figure 1.
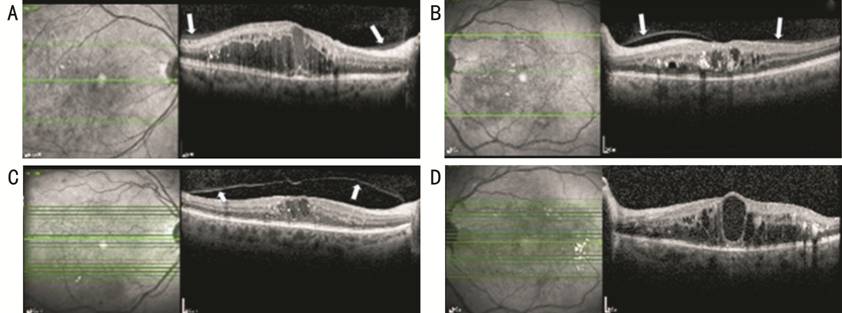
Figure 1 Spectral domain optical coherence tomography images to illustrate the posterior vitreous detachment classification A: Posterior hyaloid has completely attached to the macula and optical disc (stage 0); B: Posterior hyaloid has attached to fovea and optic disc however there was a shallow detachment around fovea (stage 1); C: Posterior hyaloid has separated from macula and only attached to optical disc (stage 2); D: Posterior hyaloid entirely separated from macula and optical disc and could not be seen in posterior hyaloid OCT image (stage 3). Posterior hyaloid membrane was demonstrated with arrow.
The pre-injection and post-injection PVD levels after 3mo were compared in terms of the eyes that had stages 0, 1, 2 PVD. Demographical characteristics of the cases where stage 3 PVD was determined were examined.
Statistical Analysis The statistical analyses of the study were performed using SPSS 19.0 package program (USA). The descriptive statistics of continuous variables were shown via median, minimum and maximum values; categorical variables were shown via frequency and percentage. Shapiro Wilk test was used for testing normality. When making comparison between 2 groups of the variables that did not show normal distribution Mann-Whitney U test was used. Pearson Chi-square test was used for inter-groups comparison of categorical variables. In all statistical comparisons in the study, P<0.05 were accepted as statistically significant.
RESULTS
A total of 572 eyes of 287 patients including 168 females and 119 males were included in the study. The mean age of the patients was 66.6±8 (range: 24-90y). Results from Shapiro Wilk normality test of age data showed abnormal data distribution. Both eyes of 74 patients and only one eye of 213 patients were injected. The number of eyes determined as control group was 211. No difference was found in terms of age and gender between the patients in injection and control groups (P=0.591, P=0.541). The demographical characteristics of patients were illustrated in Table 1.
Table 1 Demographical characteristics of patients
Items |
Patients |
Eyes (n=572) |
|
Study group |
Control group |
||
Total (n) |
287 |
361 |
211 |
Age (y), mean±SD |
66.7±9 |
66.6±8 |
66.9±9 |
Gender (n) |
|||
Male |
119 |
148 |
88 |
Female |
168 |
213 |
123 |
Diagnosis (n) |
|||
Diabetic macula edema |
183 |
256 |
109 |
Retinal vein occlusion |
104 |
105 |
102 |
When the patients’ OCTs before DEX implant injection were analyzed, 208 eyes in the study group and 129 eyes in the control group was found to have a vitreoretinal relationship at stages 0, 1, 2. Stage 3 vitreoretinal relationship was observed in 153 of the eyes in the study group and 82 of the eyes in the control group (Table 2).
Table 2 Vitreoretinal attachment stages before intravitreal Dexamethasone implant injection n (%)
Stages |
Study group |
Control group |
Total |
Stage 0 |
55 (15.2) |
48 (22.7) |
103 (18.0) |
Stage 1 |
91 (25.2) |
43 (20.4) |
134 (23.4) |
Stage 2 |
62 (17.2) |
38 (18) |
100 (17.4) |
Stage 3 |
153 (42.4) |
82 (38.9) |
235 (41.0) |
Patients (total n) |
361 |
211 |
572 |
An increase in vitreoretinal relationship stages was observed with increasing mean age. While mean age of the patients in stage 0 was 60.6±1, the mean age of the patients in stage 3 was as 69.8±8 (Figure 2).
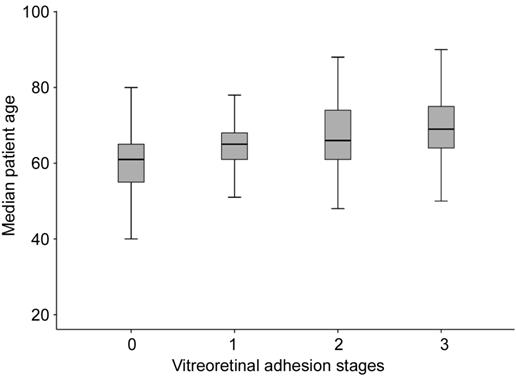
Figure 2 The relationship between mean age of patients and vitreoretinal adhesion stages.
The duration of follow-up for the patients was at least 3mo. In the end of 3mo, a progress in vitreoretinal adhesion stage was observed in 56 (26.9%) of 208 eyes in the study group and 12 (9.3%) of 129 eyes in the control group. When the variance in stages was compared, the difference between two groups was found statistically significant (P=0.001). The highest progression was determined in the shifts from stage 1 to stage 2 and secondly, from stage 2 to stage 3. Stage 3 PVD developed in 22 (14.4%) eyes in the study group while progression to stage 3 PVD occurred in 4 (3.1%) eyes in control group in the same period. Stage shifts in study and control groups were presented in Table 3.
Table 3 The number and percentage of the patients whose stages progressed 3mo after DEX implant injection n (%)
Progress in stages |
Study group |
Control group |
P |
Stage 0 |
|||
to 1 |
8/55 (14.5) |
3/48 (6.2) |
0.298 |
to 2 |
1/55 (1.8) |
1/48 (2.1) |
1.000 |
to 3 |
1/55 (1.8) |
1.000 |
|
Stage 1 |
|||
to 2 |
25/91 (27.5) |
4/43 (9.3) |
0.031 |
to 3 |
9/91 (9.9) |
2/43 (4.6) |
0.502 |
Stage 2 |
|||
to 3 |
12/62 (19.3) |
2/38 (5.3) |
0.094 |
Total |
56/208 (26.9) |
12/129 (9.3) |
0.001 |
No statistical significance was found when the shift in vitreoretinal relationship stage was examined in terms of gender, right-left eye, pseudophakic and phakic eyes and the diseases underlying. On the other hand, there was a statistically significant difference when they were assessed in terms of mean age, first visual acuity and macular thickness. While mean age of the patients that had progression in vitreoretinal relationship stage was lower, their macular thickness was greater and visual acuity was observed lower (Table 4).
Table 4 Statistically significant characteristics of the eyes that had progression and non-progression in vitreoretinal relationship stage mean±SD
Items |
Progression in stages |
P |
|
Yes |
No |
||
Age (y) |
62.8±8 |
67.2±9 |
0.001 |
Visual acuity |
0.25±2 |
0.34±3 |
0.015 |
Macular thickness |
541±2 |
424±2 |
0.001 |
The decrease in mean macular thickness was found to be 184.58±280 microns in eyes with PVD seen and 103.28±208 microns in eyes without PVD seen. There was a statistically significant difference between these two groups (P=0.007).
DISCUSSION
Posterior hyaloid membrane having partial adhesion can implement a continuous traction to macula. VMA related traction can cause chronic inflammation, increase in existing macular edema and difficulty in its treatment[22-24]. As a result of chronic inflammation, VEGF and many inflammatory mediators are released. The inflammatory mediators cumulating on the surfaces can cause thickening of posterior hyaloid membrane, becoming harder and more stretched, thereby resulting in more tightly vitreoretinal adhesion than normal and macular edema resistant to the treatment[23,25]. VMT can also contribute to the progression of choroidal neovascularization. With the effect of chronic inflammation in traction area, inflammatory cells released into retinal surface can cause the development of membrane formation in this area and the existence of neovascular membrane. It has been notified that total PVD progression has a protective effect against proliferative diabetic retinopathy development[26]. Hence, total PVD progression can prevent such adverse effects especially in the eyes having partial PVD. PVD can develop spontaneously in healthy people. Some factors can contribute to the development of PVD and one of these factors is aging.
It has been notified that the separations starting between retina and hyaloid in healthy adults after the age of 30 progress to total PVD in 50% of the individuals older than 70y[6]. One of the limitations of our study is that there has not been a control group including normal individuals from the same age group. Therefore, it was compared with the studies conducted before. Weber-Krause and Eckardt[27] stated that PVD ratio increase with aging and partial PVD percentage is 11% and 17% respectively in 65-69 years of age interval in their examination in 703 healthy people. An increase in PVD percentage with aging was also observed in our study. Mean age of our participants was 66.6y. When we look at the total PVD and partial PVD percentages before DEX injection, it was found higher in our study group compared to the healthy individuals. The reason for that can be the fact that we determine partial PVD percentage by OCT more accurately. Moreover, there are some studies notifying that PVD percentage is higher in the eyes having diabetic retinopathy[28-29]. In these studies, total and partial PVD percentage was reported as approximately 50%-67% from the diabetic group over the age of 60y. In our study, partial PVD percentage was found to be 39.9% and the total PVD was found to be 42.1%. Partial PVD percentage in diabetic group was found to be 40%, total PVD 43.6%, partial PVD percentage in vein occlusion group was found to be 36.8%, and the total PVD percentage was found to be 34.9%. Furthermore, PVD percentage in diabetic group was observed higher in comparison with other groups because of the frequent mechanical movements of vitreoretinal interface due to edema. In our study too, the rate of PVD was found to be higher in patients with higher macular thickness and greater decrease in macular thickness after treatment. These findings may suggest that mechanical movement of the macula may increase the development of PVD.
It has been revealed in many studies that PVD can progress after intravitreal anti-VEGF injections[18,30-32]. Intravitreal DEX implant is commonly used in the treatment of diabetic macula edema, uveitis and retinal vein occlusion causing macula edema[33-35]. We examined whether intravitreal DEX implant contributed to the PVD progression in this study. We determined an increase in partial and total PVD in the 3rd month after DEX implant injection. This increase was found statistically significant compared to the control group. In the first examination, PVD was not seen in 55 of the eyes while partial PVD was visible in 153 of them in our study group. While total PVD was observed in 1 of 55 eyes 3mo after intravitreal DEX injection, progression to partial PVD occurred in 9 of them. Progression in total PVD was observed in 21 of 153 eyes having partial PVD. The percentage of the eyes where progression in PVD stage was observed without observing total PVD is 16.3% of stage 0 PVD eyes, 27.4% of stage 1 PVD. These percentages were statistically significant in comparison with control group. While total PVD progression percentage was observed quite low in the eyes without PVD after single dosage intravitreal DEX implant injection, it was observed that 1/5 of the eyes having partial PVD progressed to total PVD. This makes us think that total PVD progression frequency will increase apparently in perpetual injections.
As we could not find any another study examining PVD percentage after DEX implant intravitreal injection in the literature, we compared with the studies conducted with intravitreal anti-VEGF injection. Geck et al[18] examined PVD percentages in the patients having intravitreal anti-VEGF. The mean ages of patient group were similar to our group in the study where a majority of patient group had AMD. They reported the total PVD as 4.9% after the first injection, 9.8% after the 2nd injection and 27% after the 3rd injection. In our study, total PVD percentage was found as 10.6% (22/208) after single dosage intravitreal DEX implant injection. It was more than 2 times greater than the finding of Geck et al[18] after a single dosage intravitreal anti-VEGF injection. The reason of that might be the continuous mechanic effect DEX implant created by acting in vitreous with the effect of eye movements during 3mo. A second reason can be the fact that posterior vitreous separates more easily in the patients who have diabetic macular edema. A study comparing PVD progression in patients having AMD and diabetic retinopathy could not be found in the literature.
When the eyes where progression in vitreoretinal relationship stage were compared, more progression was observed in the eyes having thicker macula. The reason for that can be the mechanical movement of vitreoretinal interface because of extending macula thickness as it is in our previous theory. Moreover, the mean age of the patients who had progression was found statistically lower. Any opinions regarding the reason of this could not be found. Whether intravitreal injection contributes more to PVD progression in young patients can be explained through prospective studies.
Some other factors influencing PVD progression were reported as eye trauma, previous uveitis attacks, high axial length and cataract surgery[36-37]. The eyes that had trauma and uveitis attack and pseudophakic eyes diagnosed with high myopia and myopic fundus were not included in the study. Axial lengths of the patients were not known as our study is a retrospective one. Fundus examination findings were considered for including the patients because of the fact that refraction values especially of pseudophakic eyes could be deceptive in terms of determining these patients. However, some patients might have gone unnoticed. This is another shortcoming of our study, but we believe that the number of such eyes would not be enough to affect the results of the study.
We used OCT images to determine PVD in our study. OCT seemed to be a useful choice to determine PVD objectively[38]. Even in OCT images taken in medium definition, stage 0 vitreoretinal adhesion can be determined easily. Especially the area where vitreous cortex attached to the side of optic disc appeared in a different tone from the environment. Stages 1 and 2 PVD can be determined even in unclear OCT images. However, we believe that PVD stages were determined objectively via OCT images as we excluded the images when we could not determine at which stage it was.
In conclusion, according to the results of our study, it can be stated that PVD can be induced by intravitreal injection of DEX implant. The effect of total or partial PVD caused by intravitreal injections on macular thickness and visual acuity has not been fully known. In addition, the effect of multiple intravitreal injection of DEX implant on PVD development is not exactly clear. Elimination of these uncertainties by further randomized prospective studies can contribute to the treatment of macular diseases being an important cause of visual loss.
ACKNOWLEDGEMENTS
The author would like to thank Dr. Cagatay Buyukustun for assistance with statistical analysis.
Conflicts of Interest: Alpay A, None.
REFERENCES