
Citation: Lin ZL, Lin DR, Chen JJ, Li J, Li XY, Wang LS, Liu ZZ, Cao QZ, Chen C, Zhu Y, Chen WR, Liu YZ, Lin HT. Increased prevalence of parent ratings of ADHD symptoms among children with bilateral congenital cataracts. Int J Ophthalmol 2019;12(8):1323-1329. DOI:10.18240/ijo.2019.08.14
·Investigation·
Increased prevalence of parent ratings of ADHD symptoms among children with bilateral congenital cataracts
Zhuo-Ling Lin1, Duo-Ru Lin1, Jing-Jing Chen1, Jing Li1, Xiao-Yan Li1, Li-Sha Wang1, Zhen-Zhen Liu1, Qian-Zhong Cao1, Chuan Chen1,2, Yi Zhu1,2, Wei-Rong Chen1, Yi-Zhi Liu1, Hao-Tian Lin1
1State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510060, Guangdong Province, China
2Department of Molecular and Cellular Pharmacology, University of Miami Miller School of Medicine, Miami, Florida 33136, USA
Co-first authors: Zhuo-Ling Lin and Duo-Ru Lin
Correspondence to: Hao-Tian Lin. State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Xianlie South Road #54, Guangzhou 510060, Guangdong Province, China. gddlht@aliyun.com
Received:
Abstract
AIM: To investigate the behavioral and psychological disorders and the prevalence of parent ratings of attention deficit hyperactivity disorder (ADHD) symptoms among children with bilateral congenital cataracts (CCs).
METHODS: This cross-sectional study investigated children with bilateral CC aged 3-8y (CC group) using Conners’ Parent Rating Scale-48 (CPRS-48) from July to December 2016. The abnormal rates of psychological symptoms in CC children and normal vision (NV) children were compared using the Chi-square test. The scores of CC children were compared with those of NV children and the Chinese urban norm using the independent samples t-test and one-sample t-test, respectively.
RESULTS: A total of 262 valid questionnaires were collected. The ratio of CC children to NV children was 119:143. The overall rate of psychological symptoms in CC children was 2.28 times higher than that in NV children (46.22% vs 20.28%, Pearson’s χ2=20.062; P<0.001). CC children showed higher scores for conduct problems, learning problems, impulsiveness/hyperactivity, anxiety, and hyperactivity index than NV children and the Chinese urban norm, particularly between the ages of 3 and 5y. Furthermore, male children aged between 6 and 8y showed a higher impulsive/hyperactive score than females of the same age (t=6.083, P<0.001).
CONCLUSION: Children with bilateral CCs have a higher rate of ADHD symptoms than children with NV. This study provides clinical evidence that screening for psychological symptoms and particularly for ADHD symptoms in children with bilateral CC are recommended for an early diagnosis and timely treatment.
KEYWORDS: attention deficit hyperactivity disorder; congenital cataract; Conners’ Parent Rating Scale-48
DOI:10.18240/ijo.2019.08.14
Citation: Lin ZL, Lin DR, Chen JJ, Li J, Li XY, Wang LS, Liu ZZ, Cao QZ, Chen C, Zhu Y, Chen WR, Liu YZ, Lin HT. Increased prevalence of parent ratings of ADHD symptoms among children with bilateral congenital cataracts. Int J Ophthalmol 2019;12(8):1323-1329
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is one of the most common neurobehavioral disorders of childhood[1]. Factors such as genetics[2], neurophysiology[3], perinatal conditions[4], and the environment[5] have been linked to ADHD. Furthermore, visual skills, such as stereopsis and formed perception, are also reported strongly correlated with mental development[6]. However, it is unclear that whether the visual disorder increases the rate of psychological symptoms. Recently, we found that patients with age-related cataracts presented decreased brain functions and altered grey matter volumes in visual and cognitive-related areas[7], indicating a close relationship of damages between vision and mental health. A national study of 75 171 children in the United States also showed that the prevalence of ADHD in children with visual impairments was 1.9 times higher than in those with normal vision (NV)[1].
Congenital cataracts (CCs) is a typical visual disorder and the leading cause of childhood blindness, which occurs before or during the critical behavioral and psychological developmental stages[8]. The complications of astigmatic refractive error and convergence insufficiency among CC children[9-10] are commonly associated with neurodevelopmental disorders[11-14]. These findings are consistent with the observations in our clinical practice of treating more than 6000 CC children at the Zhongshan Ophthalmic Center (ZOC). Our group observed that CC patients were more anxious or hyperactive during ocular examinations compared with healthy children. However, the rate of psychological symptoms and the risk of ADHD in children with bilateral CC remains unclear due to unexplored. Therefore, in this study, we conducted a cross-sectional, face-to-face investigation to evaluate the behavioral and psychological symptoms among bilateral CC children using the Conners’ Parent Rating Scale (CPRS) questionnaire, an assessment tool for screening ADHD that obtains parental reports of childhood behavioral problems in research and clinical settings[15-17]. The findings of this study would help ophthalmologists understand the psychological symptoms of CC children to provide better medical care for both physical and psychological health.
SUBJECTS AND METHODS
Ethical Approval This study was registered at clinicaltrials.gov (NCT 03692728) and approved by the Human Research Ethics Committee at the ZOC. Written informed consent was obtained from the legal guardian of each participant. All procedures performed in this study were consistent with the tenets of the Declaration of Helsinki and were approved by the Ethical Review Committee of the ZOC at Sun Yat-sen University (ERC-ZOC-SYSU).
Participants This cross-sectional study included bilateral CC children and children with NV aged 3-8y who presented to the ZOC between July and December 2016. All included CC patients were registered members of the Childhood Cataract Program of the Chinese Ministry of Health (CCPMOH), a national platform focused on childhood cataract research[10]. The diagnosis of CC was independently confirmed by two experienced pediatric ophthalmologists (Lin HT and Chen WR) based on a comprehensive evaluation of the onset age (within one year after birth), morphological features of lens opacity, family history, and detailed medical records.
Patients
complicated with systemic manifestations, such as Lowe syndrome, Marfan
syndrome, and Down syndrome, were excluded. Patients with a self-reported
family history of ADHD or brain and neuronal deficits were also excluded. Best
corrected visual acuity (BCVA) in the better eye, complicated strabismus and
nystagmus, surgical age, and surgical procedure were reviewed. Postoperative
refractive error correction and postoperative complications, including ocular
hypertension (OH, >
CPRS Questionnaire and Survey Method Due to the close contact between children and parents, this study investigated the risks of ADHD of both CC patients and NV children using the CPRS-48 questionnaire (CPRS-48, Chinese revision of 1978)[20]. The CPRS-48 contains 5 evaluating subscales, including the conduct problems, learning problems, psychosomatic, impulsiveness/hyperactivity, and anxiety subscales. A total of 48 items are included in the questionnaire, and each item is scored from 0 to 3. The final scores of the 5 subscales are defined as the mean scores of the corresponding items: conduct problems (2+8+14+19+20+21+22+23+27+33+34+39)/12; learning problems (10+25+31+37)/4; psychosomatic (32+41+43+44+48)/5; impulsiveness/hyperactivity (4+5+11+13)/4; anxiety (12+16+24+47)/4; and the hyperactivity index (4+7+11+13+14+25+31+33+37+38)/10. Because childhood behaviors may be influenced by regional customs, cultural backgrounds, and economic conditions, the norm of the CPRS-48 may vary in different countries[21-22]. Therefore, the scores of the CC children were also compared to those of the Chinese urban norm. The Chinese urban norm, with a homogeneity reliability of 0.932 and a split-half reliability of 0.900, was established based on the findings obtained from 3576 Chinese children aged 3-18y from 14 representative cities[23]. Any item with a score two standard deviations away from the mean of the Chinese urban norm (mean+2SD) indicates a psychological symptom, and the overall abnormal rate (the proportion of children with psychological symptoms) on the CPRS-48 was calculated as an indicator of childhood psychological disorders.
All questionnaire investigations were performed by the same researcher (Lin ZL). Questionnaires were completed by parents or guardians who lived with the patients and thoroughly knew their behavioral and psychological conditions. The questionnaires that met the following conditions were considered invalid and excluded from further analysis: 1) More than one-third of the items were left blank; 2) All items were given the same scores or were marked with obvious regularity.
Statistical Analysis All measurements were analyzed using the Statistical Package for the Social Sciences (SPSS ver. 19.0; SPSS, Inc., Chicago, IL, USA). The normal distributions of the CPRS-48 subscale scores were tested using the Kolmogorov-Smirnov test. Differences in age and sex between the CC and NV groups were compared using the independent samples t-test and Chi-square test, respectively. Comparisons of abnormal rates between the CC and NV groups were performed using the Chi-square test. Scores on all subscales recorded for the CC group were compared with those of the NV group and the Chinese urban norm using the independent samples t-test and one-sample t-test, respectively. The level of significance was P<0.05.
RESULTS
Participant Recruitment and Demographics A total of 291 questionnaires were issued, of which 262 were valid and included in this study. The remaining 29 questionnaires were excluded for the following reasons: 4 for limited Chinese proficiency, 3 for Marfan syndrome, 3 for blank responses, and 19 for invalid questionnaires (Figure 1). The mean age of all included children was 5.33±1.65y, and the ratio of CC patients to NV children was 119:143. Basic information and the visual acuity of the two groups are shown in Table 1. No significant differences in age and sex were found between the groups. The NV group exhibited better BCVA compared to the CC group (logMAR 0.10±0.16 vs 0.59±0.53, t=10.642, P<0.001).
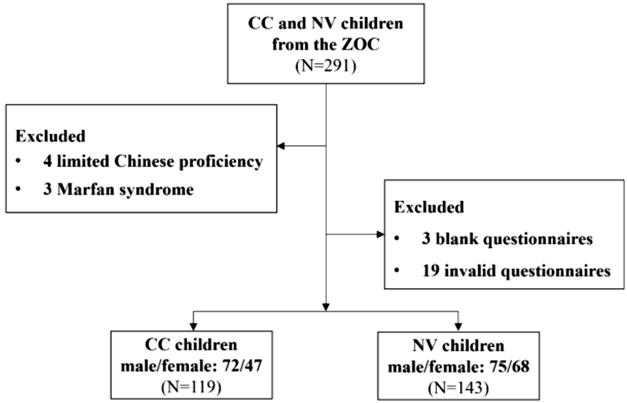
Figure 1 Flowchart of the selection and grouping of the children CC: Congenital cataract; ZOC: Zhongshan Ophthalmic Center; NV: Normal vision.
Table 1 Comparisons of basic information and visual acuity between the CC patients and NV children
Parameters |
CC group |
NV group |
t |
P |
n (%) |
119 (45.42) |
143 (45.54) |
- |
- |
Male:Female |
72:47 |
75:68 |
0.428 |
|
Age (3-5y) |
3.92±0.84 |
4.05±0.82 |
-0.870 |
0.386 |
Age (6-8y) |
6.81±0.76 |
6.96±0.89 |
-1.061 |
0.291 |
BCVA (3-5y, logMAR) |
0.77±0.40 |
0.15±0.10 |
11.777 |
<0.001b |
BCVA (6-8y, logMAR) |
0.42±0.39 |
0.07±0.18 |
6.417 |
<0.001b |
CC: Congenital cataract; NV: Normal vision; BCVA: Best corrected visual acuity; aPearson’s Chi-square test; bStatistically significant as determined by the independent samples t-test (P<0.05).
The prevalence of strabismus (esotropia or exotropia) and nystagmus in all CC patients was 62.18% (74/119) and 56.30% (67/119), respectively. A total of 104 children (87.39%) underwent cataract surgery. Among these patients, 50% (52/104) children underwent cataract extraction (CE) and intraocular lens (IOL) implantation in one surgery, 40.38% (42/104) underwent CE and IOL implantation in two separate surgeries, and the remaining 9.62% (10/104) were left aphakic. The mean age of the postoperative patients at the primary surgery was 31.09±25.53mo, and the mean age at the secondary IOL implantation was 51.51±17.00mo. All refractive errors of the postoperative patients were corrected by frame glasses. The rates of postoperative OH and laser-treated VAO were 15.13% (18/119) and 15.97% (19/119), respectively.
Comparison of the Overall Rates of Psychological Symptoms The overall rate of psychological symptoms in the CC patients was 2.28 times higher than that of the NV children (46.22% vs 20.28%, Pearson’s χ2=20.062, P<0.001). The rates of psychological symptoms in the CC patients and NV children were further compared in a subgroup analysis based on age and sex (Table 2). Between the ages of 3 and 5, female CC patients showed significantly higher rates of psychological symptoms than female NV children (50% vs 12.9%, Pearson’s χ2=9.025, P=0.006), while between the ages of 6 and 8, male CC patients showed significantly higher rates of psychological symptoms than male NV children (65.71% vs 31.82%, Pearson’s χ2=8.995, P=0.003).
Table 2 Comparisons of the overall rates of psychological symptoms between the CC patients and NV children
Age |
Sex |
CC group (%) |
NV group (%) |
χ2 |
P |
3-5y |
M |
29.73 (11/37) |
16.13 (5/31) |
1.734 |
0.254 |
F |
50 (12/24) |
12.90 (4/31) |
9.025 |
|
|
6-8y |
M |
65.71 (23/35) |
31.82 (14/44) |
8.995 |
|
F |
39.13 (9/23) |
16.22 (6/37) |
3.972 |
0.067 |
|
Overall |
46.22 (55/119) |
20.28 (29/143) |
20.062 |
<
|
|
CC: Congenital cataract; NV: Normal vision. aStatistically significant as determined by Pearson’s Chi-square test (P<0.05).
Comparisons of the CPRS-48 Subscale Scores The CPRS-48 subscale scores of the CC children were compared with those of the NV group and the Chinese urban norm (Table 3). In bilateral CC children aged 3-5 years old, the subscale scores for conduct problems, learning problems, impulsiveness/hyperactivity, anxiety, and the hyperactivity index were significantly higher than those of the NV group and the Chinese urban norm. In bilateral CC children aged 6-8 years old, the subscale scores for impulsiveness/hyperactivity in both sexes and hyperactivity index in females were significantly higher than those of the NV group and the Chinese urban norm; the subscale scores for conduct problems, learning problems, and anxiety were significantly higher than the Chinese urban norm. We also found that bilateral CC boys aged 6-8 years old showed higher scores on the impulsiveness/hyperactivity subscale than girls of the same age (t=6.083, P<0.001).
Table 3 Comparisons of the CPRS-48 subscale scores among CC patients, NV children, and the Chinese urban norm
Age |
Sex |
Group |
n |
Conduct problems |
Learning problems |
Psychosomatic |
Impulsiveness/hyperactivity |
Anxiety |
Hyperactivity index |
3-5y |
M |
CC |
37 |
0.79±
|
1.05±
|
0.18±0.25 |
1.20±
|
0.61±
|
1.08±
|
NV |
31 |
0.44±0.45 |
0.46±0.47 |
0.10±0.19 |
0.63±0.74 |
0.27±0.31 |
0.53±0.54 |
||
Norm |
194 |
0.48±0.36 |
0.64±0.53 |
0.15±0.24 |
0.58±0.56 |
0.43±0.37 |
0.55±0.43 |
||
F |
CC |
24 |
0.67±
|
0.85±
|
0.15±0.22 |
1.10±
|
0.56±0.33b |
0.87±
|
|
NV |
31 |
0.36±0.40 |
0.44±0.51 |
0.14±0.24 |
0.46±0.54 |
0.40±0.43 |
0.42±0.47 |
||
Norm |
157 |
0.39±0.27 |
0.49±0.42 |
0.11±0.20 |
0.47±0.44 |
0.40±0.35 |
0.45±0.35 |
||
6-8y |
M |
CC |
35 |
0.66±0.36b |
1.11±0.59b |
0.19±0.36 |
1.98±
|
0.51±0.50b |
0.88±0.49 |
NV |
44 |
0.59±0.33 |
0.93±0.51 |
0.19±0.33 |
0.77±0.53 |
0.44±0.31 |
0.74±0.38 |
||
Norm |
407 |
0.41±0.32 |
0.61±0.49 |
0.17±0.26 |
0.58±0.47 |
0.34±0.38 |
0.78±0.56 |
||
F |
CC |
23 |
0.58±0.33b |
0.96±0.49b |
0.15±0.16 |
0.84±
|
0.45±0.40b |
0.74±
|
|
NV |
37 |
0.46±0.22 |
0.72±0.43 |
0.15±0.27 |
0.59±0.39 |
0.46±0.41 |
0.54±0.31 |
||
Norm |
383 |
0.34±0.29 |
0.51±0.46 |
0.15±0.23 |
0.41±0.45 |
0.32±0.32 |
0.40±0.34 |
CC: Congenital cataract group; NV: Normal vision group; Norm: The Chinese urban norm. aP<0.05, significantly different from the NV group, as determined by an independent samples t-test; bP<0.05, significantly different from the Chinese urban norm, as determined by a one-sample t-test.
DISCUSSION
Behavioral and psychological problems in children with visual problems can be easily ignored by ophthalmologists. Decreased visual acuity is often readily corrected by surgeries or wearing glasses, while the impairments in mental health associated with visual dysfunction can have a profound effect on the patient’s quality of life (QoL)[24]. Recent studies have paid increasing attention to the association between childhood visual problems and psychological disorders, especially ADHD. Kutzbach et al[25] found an increased prevalence of self-reported ADHD among patients with albinism-related vision impairment. Additionally, DeCarlo et al[1] reported a higher prevalence of ADHD among children with visual impairments that were not correctable with glasses or contact lenses than that of children with NV (15.6% vs 8.3%, respectively). However, few studies have investigated the ADHD symptoms and related psychological problems among children with CC, a typical visual impairment that cannot be completely corrected by optical correction. In our study, all CC children were in refractive error states with convergence insufficiency after surgery (90.38% pseudophakia and 9.62% aphakia), and over half of the CC children presented with the complications of strabismus (62.18%) or nystagmus (56.30%). All these ocular complications contribute to the development of childhood behavioral and psychological disorders[1,11-14]. Reimelt et al[11] found that both the refractive error and strabismus were independently associated with ADHD in the single logistic regression models. Furthermore, the average age at primary surgery among the CC children in this study was 31.09mo, which represented a delay of nearly 2y compared to the age reported in developed countries[26]. A long period of binocular form deprivation in very young children may lead to developmental visual disorder and difficulties in acquiring information. Since visual inputs provide the predominant sensory input to the brain, vision problems can profoundly impact attention. Children may not be able to keep their attention focused if they are unable to see clearly[1]. Additionally, CC patients require more executive functioning (the advanced cognitive processes that enable people to organize, plan, pay attention, and manage time and space) to compensate for their visual deficit, leaving less executive function in reserve for the maintenance of an attentional state[1,27].
The mental health of children with CC was evaluated using the QoL questionnaire used in previous studies. For example, Ye et al[28] found that bilateral CC children had lower scores for both psychology and communication factors compared to NV children. The British Congenital Cataract Interest Group (BCCIG) showed that in CC children, the mean psychosocial health score was lower than the physical health score (72.93 vs 80.76)[29]. Additionally, Tailor et al[30] reported that CC children had lower psychosocial Pediatric Quality of Life Inventory (PedsQL) subscores than physical subscores. Furthermore, several studies reported that CC children had low QoL scores comparable to those of children with severe congenital cardiac defects or childhood cancers[31-32]. However, the QoL questionnaires include only one or two psychology-related factors and cannot be used to comprehensively assess all aspects of the mental health status of CC patients. Notably, the CPRS questionnaire used in this study uses a special rating scale to fully analyze children and adolescents’ developmental behavior and mental health by including five behavioral and psychological factors[33]. We showed that compared to NV children and the Chinese urban norm, CC children exhibited higher scores on the conduct problem, learning problem, impulsiveness/hyperactivity, anxiety, and hyperactivity index subscales, indicating the psychological symptoms and a higher risk of developing ADHD.
Notably, compared to NV children, CC patients typically had significantly higher scores on CPRS-48 subscales when they were 3-5 years old. The mean ages at both the primary surgical intervention and secondary IOL implantation of the CC patients were within the first 5 years of life (30.09 and 51.51mo, respectively). Intensive surgical procedures, frequent hospital visits, and eye drop treatments during this period may induce anxiety in bilateral CC children and their families[30]. Moreover, boys are more likely to display impulsive behaviors[34-35]. Abikoff et al[36] demonstrated that boys with ADHD may engage in more rule-breaking and externalizing behaviors than girls. Consistent with these findings, our study showed that school-aged boys with CC (6 to 8 years old) tended to be more impulsive and hyperactive than girls. More attention should be paid to the sex difference in ADHD among bilateral CC patients to better guide diagnosis and treatment.
Unilateral CC patients usually have normal visual acuity in the healthy eye, and their acquisition of visual information is not severely affected. Furthermore, additional management of occlusion or blurring of the unaffected eye in unilateral CC patients may affect motor coordination and emotions[37-38]. Therefore, we included only bilateral CC children in this study to avoid these potential confounding factors. Furthermore, we used the face-to-face interview method to ensure that the parents completely understood each item of the CPRS-48.
The close relationship between CC and ADHD can be supported by the possible common genetic and developmental neurobiological basis. CC is one of the clinical manifestations of the mutations of the paired box homeotic gene-6 (PAX6)[39], which has rich interactions with homeobox protein MEIS-2. The MEIS-2 gene has been nominally associated with the severity of hyperactive-impulsive symptoms in a family-based study of ADHD[40]. Furthermore, a novel syndrome of CC, ADHD, and cognitive impairment linkage to chromosomes 11 disorder have also been revealed in a consanguineous family[41]. However, the analysis of genetic and neurobiological basis between CC and ADHD were not performed in the current study and should be further investigated.
This study also has other limitations. First, parental stress regarding treatment and outcomes for their children was not evaluated. Parental stress and childhood behavior disorders have a bidirectional relationship[42], and we will investigate the effect of parental stress on the development of psychological symptoms in their CC children in our follow-up studies. Second, we used questionnaire investigations in the pediatric ophthalmology clinics to evaluate the risk of ADHD development. Although the CPRS-48 has been widely used to evaluate childhood neurodevelopmental disorders, typically ADHD[43], a definitive diagnosis of ADHD still requires confirmation by pediatric psychologists. Furthermore, the previously reported combination of ocular-motor-cognitive delays[44] may also the factor of increasing prevalence of parent ratings of ADHD symptoms among CC children, which would be further studied in our following research.
In conclusion, we found that bilateral CC children had a higher rate of psychological symptoms than NV children and exhibited a higher prevalence of ADHD symptoms. Timely screening and intervention for ADHD are required in bilateral CC children to prevent dual impairments of both vision and mental health through a joint effort by parents, pediatric ophthalmologists, and psychologists.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81770967; No.91546101); National Key R&D Program (No.2018YFC0116500); the Fundamental Research Funds for the Central Universities (No.18ykpy33; No.16ykjc28); the Youth Pearl River Scholar Funded Scheme (2016-2018); the Fundamental Research Funds of the State Key Laboratory of Ophthalmology (2018-2019).
Conflicts of Interest: Lin ZL, None; Lin DR, None; Chen JJ, None; Li J, None; Li XY, None; Wang LS, None; Liu ZZ, None; Cao QZ, None; Chen C, None; Zhu Y, None; Chen WR, None; Liu YZ, None; Lin HT, None.
REFERENCES