
Citation: Khorshidifar M, Nikkhah H, Ramezani A, Entezari M, Daftarian N, Norouzi H, Shahriari M, Radfar M, Nourinia R, Karimi S. Incidence and risk factors of retinopathy of prematurity and utility of the national screening criteria in a tertiary center in Iran. Int J Ophthalmol 2019;12(8):1330-1336. DOI:10.18240/ijo.2019.08.15
·Investigation·
Incidence and risk factors of retinopathy of prematurity and utility of the national screening criteria in a tertiary center in Iran
Milad Khorshidifar1,2, Homayoun Nikkhah1,3, Alireza Ramezani1,4, Morteza Entezari3, Narsis Daftarian2,3, Hamid Norouzi2,3, Mansoor Shahiari2,3, Mitra Radfar2, Ramin Norinia3, Saeed Karimi1,3
1Clinical Research Development Unit of Torfe Medical Center, Shahid Beheshti University of Medical Sciences, Tehran 1149847514, Iran
2Department of Ophthalmology, Imam Hossein Hospital, Shahid Beheshti University of Medical Sciences, Tehran 1617763141, Iran
3Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran 16666, Iran
4Ophthalmic Epidemiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran 16666, Iran
Correspondence to: Homayoun Nikkhah. Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, No.23, Boostan 9 St., Paidarfard St., Pasdaran Ave., Tehran 16666, Iran. H.nikkhah52@gmail.com
Received:
Abstract
AIM: To determine the incidence and risk factors of retinopathy of prematurity (ROP) and the sensitivity of current screening criteria in a tertiary eye center in Tehran, Iran.
METHODS: In a cross-sectional observational study, neonates weighing ≤2000 grams at birth or born <34wk gestational age (GA) and all other infants at risk of ROP admitted to the neonatal intensive care unit (NICU) or referred to our ROP clinic were investigated. The incidence of ROP and severe ROP (i.e. patients needing treatment) were determined. The associations between risk factors and the development and severity of ROP were assessed. We also examined the sensitivity of the current national screening guideline in Iran.
RESULTS: Among 207 infants, the incidence of ROP and severe
ROP was 33.3% and 11.1%, respectively. Mean GA and birth weight (BW) were
significantly lower in ROP vs non-ROP infants (29±2wk vs 33±3wk, P<0.001;
1274±
CONCLUSION: ROP incidence is relatively high in Iran. Identifying ROP risk factors results in more accurate screening and reduces the risk of irreversible vision loss. The ROP screening criteria utilized in Iran are efficient at the present time.
KEYWORDS: retinopathy of prematurity; incidence; risk factors; screening; Iran
DOI:10.18240/ijo.2019.08.15
Citation: Khorshidifar M, Nikkhah H, Ramezani A, Entezari M, Daftarian N, Norouzi H, Shahriari M, Radfar M, Nourinia R, Karimi S. Incidence and risk factors of retinopathy of prematurity and utility of the national screening criteria in a tertiary center in Iran. Int J Ophthalmol 2019;12(8):1330-1336
INTRODUCTION
Retinopathy of prematurity (ROP) in its advanced stages is a vasoproliferative retinal disease that affects premature infants[1]. Low gestational age (GA), low birth weight (BW) and high concentration oxygen therapy are the major ROP risk factors[2]. Regardless, some authors have reported ROP in cases without oxygen therapy[3-5]. In addition to the major risk factors, other factors such as anemia[6], insulin-like growth factor-1 (IGF-1)[7], mean platelet volume (MPV)[8], thrombocytopenia[9], bilirubin level, gender[10], multiple gestation[11], intraventricular hemorrhage (IVH)[12] and blood transfusion[13] were considered to be effective in incidence and progression of ROP.
According to
the World Health Organization (WHO), ROP is an important cause of avoidable
childhood blindness in developing countries[14].
A third epidemic of ROP has occurred in developing countries such as Iran[15]. The improvement of neonatal care in Iran has
increased the survival rate of premature infants and consequently, the
incidence of ROP. However, there are few studies which have evaluated the
incidence and risk factors of ROP in Iran[16-17]. Additionally, the ROP screening criteria in Iran was
determined to be different from developed countries. The American Academy of
Pediatrics (AAP) screening guidelines for ROP recommends screening for infants
with BW≤
The aim of the current study was to determine the overall incidence of ROP and severe ROP at a tertiary eye care center, to investigate a wide range of risk factors regarding ROP development, and to test the sensitivity of Iranian screening criteria for ROP[20].
SUBJECTS AND METHODS
Ethical Approval The study was approved by the Ethics Committee of our institute (IRB code: Ir. Sbmu.msp.rec.1396.519) and performed in agreement with the ethical principles in the Declaration of Helsinki. Written informed consent was obtained from the parents.
This was a
cross-sectional observational study performed from August 2016 through August
2017 at a tertiary eye care center. Preterm infants with BW≤
Eye Examination Methods All examinations were done by four experienced retina specialists. Pupils were fully dilated using tropicamide 0.5% and phenylephrine 5% eye drops. After topical anesthesia, each infant was examined with an indirect ophthalmoscope using a lid speculum, a scleral depressor and a +20 D or +30 D lens.
Data Collection Infant information and risk factors were collected including delivery method (cesarean section versus vaginal delivery), GA, BW, gender, Apgar scores at 1min, hemoglobin and bilirubin levels, septicemia, IVH, MPV, platelet count, IGF-1 level, blood transfusion, neonatal intensive care unit (NICU) admission period (days), gestational diabetes mellitus (GDM), necrotizing enterocolitis (NEC), patent ductus arteriosus (PDA), acute respiratory distress syndrome (ARDS) and use of surfactant. Furthermore, maternal information was gathered including maternal age, multiple gestation pregnancies, history of pre-eclampsia, eclampsia and hemolysis, elevated liver enzyme levels, and low platelet levels (HELLP) syndrome. The results of fundus examination were also recorded (i.e. stage, zone, plus disease and necessity for therapy).
Statistical Analysis Data were analyzed using SPSS software (SPSS 24.0, SPSS Inc, Chicago, IL, USA). To describe data, we used mean, standard deviation, median, range, frequency and percentage. Simple log binomial regression was used to find the relative risk of different risk factors on incidence and severity of ROP. In addition, multiple log binomial regression was employed to compute the adjusted risk ratio as well as the simultaneous association of presumed risk factors and ROP incidence and severity. To assess the diagnostic power of the defined criteria, we used sensitivity, specificity, positive and negative predictive value as well as positive and negative likelihood ratio. All the estimated measures were accompanied by corresponding 95% confidence intervals (CI). We used the Poisson method to calculate the 95%CI of incidences. P value less than 0.05 was considered statistically significant.
RESULTS
In total, 216 infants were screened for ROP. Of them, 9 subjects were excluded due to incomplete data related to ROP exams. Therefore, the data of 207 infants were used for statistical analyses; 100 (48.3%) female and 107 (51.7%) male (Table 1). Of all cases, 91 (44.8%) received surfactant, 90 (43.9%) received blood transfusions, 50 (24.4%) developed sepsis, 35 (17.0%) presented with PDA, 6 (2.9%) developed IVH and 2 (1.0%) developed NEC. Other characteristics and associated systemic findings are listed in Table 2.
Table 1 Baseline characteristics of the study population mean±SD; median (range)
Parameters |
Total |
No ROP |
ROP |
||
Total |
Mild |
Severe |
|||
Gestational age (wk) |
32±3; 32 (24 to 41) |
33±3; 33 (26 to 41) |
29±2; 28 (24 to 35) |
30±2; 29 (26 to 35) |
28±2; 28 (24 to 32) |
Mother age (y) |
31±5; 31 (18 to 48) |
31±5; 31 (20 to 48) |
31±6; 31 (18 to 41) |
30±5; 30 (18 to 41) |
32±6; 33 (18 to 40) |
Birth weight (g) |
1701±610; 1650 (530 to 3700) |
1916±550; 1860 (750 to 3700) |
1274±489; 1200 (530 to 3200) |
1393±542; 1245 (530 to 3200) |
1036±221; 1050 (700 to 1480) |
Bilirubin (mg/dL) |
10±2.9; 10 (2 to 22.2) |
10.2±3; 10 (2 to 22.2) |
9.7±2.4; 9.7 (4 to 15) |
9.9±2.6; 9.6 (4 to 15) |
9.3±2.2; 10 (4 to 12) |
NICU admission (d) |
24±21; 18 (0 to 96) |
16±14; 12 (0 to 90) |
38±24; 31 (4 to 96) |
32±22; 28 (5 to 95) |
49±25; 47 (4 to 96) |
Apgar score |
7±2; 7 (0 to 10) |
7±2; 7 (0 to 10) |
6±2; 6 (1 to 9) |
6±2; 7 (3 to 9) |
4±2; 4 (1 to 8) |
Mean platelet volume (fL) |
10.4±1.2; 10.3 (8.1 to 13.2) |
10.3±1.2; 10.2 (8.1 to 13.2) |
10.5±1.1; 10.3 (8.5 to 12.5) |
10.7±1.1; 10.7 (9.2 to 12.3) |
10.5±1.1; 10.3 (8.5 to 12.5) |
Platelet count (×103/µL) |
254±114; 228 (22 to 646) |
256±103; 238 (22 to 646) |
250±133; 200 (40 to 581) |
258±154; 200 (40 to 581) |
241±104; 204 (54 to 450) |
IGF-1 (nmol/L) |
108.6±408.3; 31 (15 to 2518) |
132.7±477.6; 32 (15 to 2518) |
43.6±40.4; 30.5 (15 to 152) |
38±24.3; 31 (18 to 65) |
46±47.2; 30 (15 to 152) |
Hemoglobin (mg/dL) |
13±3; 13.3 (7.1 to 20.2) |
13.7±2.8; 14 (7.1 to 20.2) |
11.7±2.8; 11.1 (7.8 to 18) |
12.6±2.7; 12.7 (8.1 to 18) |
10.5±2.5; 9.7 (7.8 to 15.6) |
ROP: Retinopathy of prematurity; NICU: Neonatal intensive care unit; IGF-1: Insulin-like growth factor-1.
Table 2 The incidence of ROP and severe ROP based on potential risk factors n (%)
Parameters |
Total |
No ROP |
ROP |
||
Total |
Mild |
Severe |
|||
Mother age (y) |
|||||
18-25 |
29 (14.4) |
18 (62.1) |
11 (37.9) |
8 (27.6) |
3 (10.3) |
26-30 |
63 (31.3) |
43 (68.3) |
20 (31.7) |
16 (25.4) |
4 (6.3) |
31-35 |
69 (34.3) |
43 (62.3) |
26 (37.7) |
15 (21.7) |
11 (15.9) |
36+ |
40 (19.9) |
29 (72.5) |
11 (27.5) |
6 (15.0) |
5 (12.5) |
Gender |
|||||
Female |
100 (48.3) |
66 (66.0) |
34 (34.0) |
23 (23.0) |
11 (11.0) |
Male |
107 (51.7) |
72 (67.3) |
35 (32.7) |
23 (21.5) |
12 (11.2) |
Blood transfusion |
|||||
No |
115 (56.1) |
99 (86.1) |
16 (13.9) |
15 (13.0) |
1 (0.9) |
Yes |
90 (43.9) |
39 (43.3) |
51 (56.7) |
29 (32.2) |
22 (24.4) |
Surfactant |
|||||
No |
112 (55.2) |
95 (84.8) |
17 (15.2) |
17 (15.2) |
0 |
Yes |
91 (44.8) |
42 (46.2) |
49 (53.8) |
26 (28.6) |
23 (25.3) |
IVH |
|||||
No |
199 (97.1) |
136 (68.3) |
63 (31.7) |
42 (21.1) |
21 (10.6) |
Yes |
6 (2.9) |
2 (33.3) |
4 (66.7) |
2 (33.3) |
2 (33.3) |
GDM |
|||||
No |
192 (93.7) |
128 (66.7) |
64 (33.3) |
43 (22.4) |
21 (10.9) |
Yes |
13 (6.3) |
10 (76.9) |
3 (23.1) |
1 (7.7) |
2 (15.4) |
NEC |
|||||
No |
203 (99.0) |
137 (67.5) |
66 (32.5) |
43 (21.2) |
23 (11.3) |
Yes |
2 (1.0) |
1 (50.0) |
1 (50.0) |
1 (50.0) |
0 |
ARDS |
|||||
No |
59 (28.9) |
54 (91.5) |
5 (8.5) |
5 (8.5) |
0 |
Yes |
145 (71.1) |
84 (57.9) |
61 (42.1) |
38 (26.2) |
23 (15.9) |
Sepsis |
|||||
No |
155 (75.6) |
114 (73.5) |
41 (26.5) |
31 (20.0) |
10 (6.5) |
Yes |
50 (24.4) |
24 (48.0) |
26 (52.0) |
13 (26.0) |
13 (26.0) |
Pre-eclampsia, eclampsia or HELLP syndrome |
|||||
No |
165 (80.5) |
115 (69.7) |
50 (30.3) |
36 (21.8) |
14 (8.5) |
Yes |
40 (19.5) |
23 (57.5) |
17 (42.5) |
8 (20.0) |
9 (22.5) |
PDA |
|||||
No |
171 (83.0) |
124 (72.5) |
47 (27.5) |
37 (21.6) |
10 (5.8) |
Yes |
35 (17.0) |
14 (40.0) |
21 (60.0) |
8 (22.9) |
13 (37.1) |
Cesarean section |
|||||
No |
30 (15.5) |
18 (60.0) |
12 (40.0) |
8 (26.7) |
4 (13.3) |
Yes |
164 (84.5) |
111 (67.7) |
53 (32.3) |
35 (21.3) |
18 (11.0) |
Multiple gestation |
|||||
No |
139 (67.1) |
95 (68.3) |
44 (31.7) |
28 (20.1) |
16 (11.5) |
Yes |
68 (32.9) |
43 (63.2) |
25 (36.8) |
18 (26.5) |
7 (10.3) |
ROP: Retinopathy of prematurity; IVF: In vitro fertilization; IVH: Intraventricular hemorrhage; GDM: Gestational diabetes mellitus; NEC: Necrotizing enterocolitis; ARDS: Acute respiratory distress syndrome; PDA: Patent ductus arteriosus.
Totally 69
infants out of 207 (33.3%, 95%CI: 25.9%-42.2%) were recognized to have ROP.
Mean BW and GA in ROP infants were significantly less than no ROP cases
[1274±489 (530-3200) g vs 1916±550 (750-3700) g, P<0.001 and
29±2 (24-35)wk vs 33±3 (26-41)wk, P<0.001, respectively; Table
1]. Those infants with ROP were recognized as follows: stage
Of all ROP cases, 23 (33.3%, 95%CI: 21.1%-50%) received treatment due to severe ROP. Totally 16 cases were treated with laser in both eyes (22.2% of all ROP cases), and 7 infants received a combination of laser and intravitreal bevacizumab (0.625 mg) in the same session in both eyes (10.1% of all ROP cases). All cases received only one session of treatment.
We
categorized the ROP cases into four groups based on BW and GA separately
(Figure 1). It was demonstrated that both lower BW and lower GA were significantly
associated with increased incidence of ROP (P for trend <0.001). The
highest ROP incidence was found in infants with BW≤
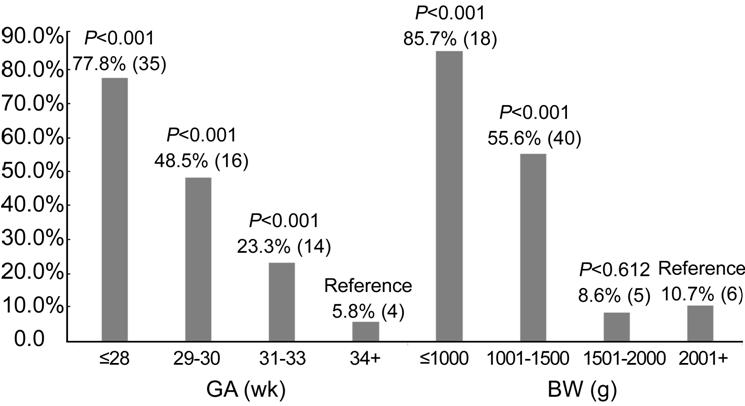
Figure 1 The rate of retinopathy of prematurity based on gestational age and birth weight The columns show the percentage (number) of retinopathy of prematurity cases in each GA and BW categories. P values are in comparison to the reference value. GA: Gestational age; BW: Birth weight.
Univariate and Multivariate Analysis Univariate analysis showed a significant relation between the incidence of ROP and GA, BW, NICU admission period, Apgar score, blood transfusion, surfactant usage, ARDS, sepsis, IVH and PDA. Severe ROP however, was significantly associated with GA, BW, NICU admission period, Apgar score, surfactant usage, ARDS, NEC, PDA as well as pre-eclampsia, eclampsia and HELLP syndrome (Table 3). The risk factors found to be significant in univariate analysis were used for multivariate logistic regression analysis. We utilized the likelihood ratio backward elimination method to obtain the most parsimonious model. It showed that only three independent risk factors including GA [relative risk (RR): 0.788], blood transfusion (RR: 1.888) and BW (RR: 0.857) had significant correlation with overall ROP incidence, while none of the risk factors were found to have independent significant correlation with severe ROP (Table 4).
Table 3 Univariate analysis of risk factors for overall and severe retinopathy of prematurity
Risk Factors |
Overall ROP incidence |
Severe ROP incidence |
||
RR (95%CI) |
P |
RR (95%CI) |
P |
|
Gestational age |
0.747 (0.684 to 0.816) |
<0.001 |
0.804 (0.655 to 0.987) |
0.038 |
Mother age |
0.984 (0.942 to 1.028) |
0.470 |
1.040 (0.964 to 1.121) |
0.314 |
Birth weight |
0.857 (0.826 to 0.889) |
<0.001 |
0.844 (0.767 to 0.929) |
<0.001 |
Bilirubin (Maximum) |
0.948 (0.846 to 1.062) |
0.356 |
0.867 (0.691 to 1.087) |
0.215 |
NICU admission (d) |
1.023 (1.014 to 1.031) |
<0.001 |
1.017 (1.002 to 1.032) |
0.024 |
Apgar score |
0.791 (0.700 to 0.893) |
<0.001 |
0.742 (0.600 to 0.919) |
0.006 |
Platelet volume |
1.139 (0.797 to 1.629) |
0.475 |
0.927 (0.569 to 1.509) |
0.760 |
Platelet count |
1.000 (0.997 to 1.002) |
0.816 |
0.999 (0.996 to 1.003) |
0.737 |
IGF-1 |
0.999 (0.992 to 1.005) |
0.714 |
1.002 (0.984 to 1.020) |
0.869 |
Blood transfusion |
4.073 (2.323 to 7.142) |
<0.001 |
6.902 (0.930 to 1.204) |
0.059 |
Surfactant |
3.548 (2.043 to 6.159) |
<0.001 |
N/A |
<0.001 |
ARDS |
4.964 (1.995 to 2.354) |
<0.001 |
N/A |
<0.001 |
Sepsis |
1.966 (1.203 to 3.213) |
0.007 |
2.050 (0.899 to 4.675) |
0.088 |
Gender |
0.962 (0.689 to 1.343) |
0.820 |
1.060 (0.594 to 1.890) |
0.844 |
IVH |
2.106 (1.031 to 4.303) |
0.041 |
1.500 (0.538 to 4.183) |
0.438 |
GDM |
0.692 (0.305 to 1.570) |
0.379 |
2.032 (0.729 to 5.666) |
0.175 |
Pre-eclampsia, eclampsia or HELLP syndrome |
1.402 (0.950 to 2.070) |
0.088 |
1.891 (1.046 to 3.418) |
0.035 |
PDA |
2.183 (1.517 to 3.141) |
<0.001 |
2.910 (1.624 to 5.212) |
<0.001 |
Cesarean section |
0.808 (0.519 to 1.258) |
0.345 |
1.019 (0.474 to 2.192) |
0.962 |
Multiple gestation |
1.161 (0.821 to 1.643) |
0.398 |
0.770 (0.411 to 1.443) |
0.415 |
ROP: Retinopathy of prematurity; RR: Relative risk; CI: Confidence interval; IGF-1: Insulin-like growth factor-1; ARDS: Acute respiratory distress syndrome; IVH: Intraventricular hemorrhage; GDM: Gestational diabetes mellitus; PDA: Patent ductus arteriosus; N/A: Not available due to no observation in one of the levels.
Table 4 Independent risk factors for retinopathy of prematurity determined by multiple regression analysis
Risk factors |
Overall ROP incidence |
|
RR (95%CI) |
P |
|
Gestational age (1wk) |
0.788 (0.711 to 0.873) |
<0.001 |
Blood transfusion |
1.888 (0.995 to 3.583) |
0.052 |
Birth weight (
|
0.857 (0.813 to 0.903) |
<0.001 |
ROP: Retinopathy of prematurity, RR: Relative risk, CI: Confidence interval.
The
sensitivity for diagnosis of ROP and severe ROP in this study was 95.7% and
100%, respectively, using the Iranian ROP screening criteria (BW≤
Table 5 Sensitivity and specificity of Iran and United States screening criteria to detect retinopathy of prematurity in the present study %
Parameters |
Iran ROP screening criteria |
United States ROP screening criteria |
||
Overall ROP incidence |
Severe ROP incidence |
Overall ROP incidence |
Severe ROP incidence |
|
Sensitivity |
95.7 |
100 |
87 |
100 |
Specificity |
18.8 |
15.8 |
72.5 |
59.2 |
Positive predictive value |
37.1 |
12.9 |
61.2 |
23.5 |
Negative predictive value |
89.7 |
100 |
91.7 |
100 |
ROP: Retinopathy of prematurity.
DISCUSSION
ROP is one of the most common causes of preventable childhood blindness in both developing and developed countries[14]. Iran is undergoing the third epidemic of ROP, hence more studies on the incidence of ROP and its related risk factors is essential.
In our study, the incidence of ROP and severe ROP was 33.3% (69/207) and 11.1% (23/207), respectively. This was in line with other studies conducted in Iran, where the incidence of ROP was 34.5% in Tehran[15], 42.1% in Shiraz[23], 37.2% in the south of Iran[16], and 26.2% in the northeast of Iran[17]. Correspondingly, the incidence of severe ROP was 12.1% in Tehran[15], 9.5% in Shiraz[23], 7.5% in the north of Iran[24], and 8% in the southwest of Iran[25]. The small differences among the incidences of ROP in various regions of the country may be related to different genetics, NICU care, and methods of research. Compared to some other countries, the incidence of ROP in our study was higher than those of the United States (15.58%)[26], Bahrain (20.4%)[27] and China (12.7%)[12], and lower than those of Saudi Arabia (56%)[28] and Canada (40.4%)[29], while the incidence of severe ROP was higher than United States, China (2.3%)[12] and Bahrain (3.8%)[27], and nearly the same as Saudi Arabia (9%)[28] and Canada (9.2%)[29].
In our
study, 85.7% of the infants with BW≤
In the present study, univariate analysis showed a significant relationship between overall and severe ROP incidence and lower BW and GA. Both BW and GA have been identified as the main risk factors for the incidence of ROP by almost all previous studies[27,34-35]. We noted that NICU admission period, ARDS, surfactant usage and Apgar score were significant risk factors for overall and severe ROP incidence. Low Apgar score may be indicative of respiratory compromise immediately after birth and NICU admission period represents the duration of respiratory distress and hypoxemia which induce retinal hypoxia and promote ROP development[36-37]. We found significant relationships between ROP incidence and blood transfusion, sepsis, IVH and PDA. These findings are in agreement with previous studies which have shown an association between ROP and complex medical conditions[38-39].
In multivariate analysis, only three factors, i.e. BW, GA and blood transfusion, were shown as significant independent risk factors for ROP. Low BW and GA have been recognized as independent risk factors for the development of ROP in many studies[15,23,28,30]. Allegaert et al[40], Parekh et al[41] and Stutchfield et al[13], identified blood transfusion as a risk factor for ROP in multivariate regression analysis. Our results showed that blood transfusion is correlated with an 88% increase in the risk of ROP (RR: 1.888; Table 5). We did not find significant independent risk factors for severe ROP development in multivariate regression analysis, it might be due to few severe ROP cases (n=23) in our study.
For the first time in Iran, other risk factors such as MPV, platelet count, IGF-1 and hemoglobin levels (anemia) were investigated in our study. Unlike studies from other countries[6,9,42-43], we could not find any significant relationship between these risk factors and ROP incidence, perhaps due to the small sample size in our study.
The mean and
range of GA [28±2 (24-32)wk] and BW [1036±221 (700-1480) g] of infants with
severe ROP in our study was similar to the moderately developed countries such
as Argentina [GA: 29 (27-31)wk and BW: 1156 (950-1360) g] and Thailand [GA:
29.2±2.5 (24-35)wk and BW: 1046±257 (710
Our study was powered by its prospective method and consideration of many risk factors among ROP cases; however, the relatively small sample size could be considered as a shortcoming in our study. It should be noted that the present study was conducted in a referral hospital, so our results cannot be generalized for the entire premature population.
In
conclusion, the present study showed that the three factors of BW, GA and blood
transfusion had independent significant relationships with the incidence of ROP
in Iran, while having no effect on the severity of ROP. Therefore, in addition
to the first two factors, blood transfusion should be considered as an ROP risk
factor in premature infants. Considering the wide range of screening criteria
in Iran as well as the results of this and other studies, a revision of these
criteria is required in order to reduce costs and unnecessary examinations. We
recommend changing the ROP screening criteria to GA≤32wk or BW≤
ACKNOWLEDGEMENTS
The authors would like to thank Atefeh Heidary, for her great efforts.
Authors’ contributions: Khorshidifar M, writing the article, data collection, provision of patients and literature search. Nikkhah H, concept and design, analysis and interpretation, critical revision of the article, literature search, provision of patients. Ramezani A, concept and design, analysis and interpretation, critical revision of the article, provision of patients. Entezari M, concept and design, analysis and interpretation, critical revision of the article, provision of patients. Daftarian N, analysis and interpretation, critical revision of the article, provision of patients. Norouzi H, critical revision of the article, provision of patients and resources, literature search, administrative and logistic support. Shahriari M, writing the article, data collection, provision of patients and resources, administrative and logistic support. Radfar M, provision of the patients, data collection, critical revision of the article. Nourinia R, analysis and interpretation, critical revision of the article, provision of patients, statistical expertise. Karimi S, analysis and interpretation, critical revision of the article, data collection, provision of patients, statistical expertise. All authors read and approved the final manuscript.
Conflicts of Interest: Khorshidifar M, None; Nikkhah H, None; Ramezani A, None; Entezari M, None; Daftarian N, None; Norouzi H, None; Shahriari M, None; Radfar M, None; Nourinia R, None; Karimi S, None.
REFERENCES