
Citation: Lin H, Zhang J, Niu GZ, Huang XY, Zhang YS, Liu CY, Zheng CY, Bi YL. Phacoemulsification in eyes with corneal opacities after deep anterior lamellar keratoplasty. Int J Ophthalmol 2019;12(8):1344-1347. DOI:10.18240/ijo.2019.08.17
·Brief Report·
Phacoemulsification in eyes with corneal opacities after deep anterior lamellar keratoplasty
Hui Lin, Juan Zhang, Guo-Zhen Niu, Xin-Yu Huang, Yu-Shan Zhang, Chun-Yu Liu, Chang-Yue Zheng, Yan-Long Bi
Department of Ophthalmology, Tongji Hospital Affiliated with Tongji University School of Medicine, Shanghai 200065, China
Co-first authors: Hui Lin and Juan Zhang
Correspondence to: Yan-Long Bi. Department of Ophthalmology, Tongji Hospital Affiliated with Tongji University School of Medicine, No.389 Xincun Road, Putuo District, Shanghai 200065, China. biyanlong@tongji.edu.cn
Received:
Abstract
To
evaluate the maneuverability and efficacy of phacoemulsification and
intraocular lens (IOL) implantation in eyes with corneal opacities after deep
anterior lamellar keratoplasty (DALK), twelve eyes of 12 patients with mild to
moderate corneal opacities after DALK and coexisting cataracts were analyzed
retrospectively. Phacoemulsification and IOL implantation assisted with
anterior capsule staining, as well as non-invasive optical fiber illumination,
were performed on all eyes. No intraoperative or postoperative complications
were noted. Mean corrected distance visual acuity (logMAR) improved from
1.24±0.17 to 0.73±0.22. Post-phaco intraocular pressure was maintained between
13 to
KEYWORDS: phacoemulsification; corneal opacity; deep anterior lamellar keratoplasty; staining; illumination
DOI:10.18240/ijo.2019.08.17
Citation: Lin H, Zhang J, Niu GZ, Huang XY, Zhang YS, Liu CY, Zheng CY, Bi YL. Phacoemulsification in eyes with corneal opacities after deep anterior lamellar keratoplasty. Int J Ophthalmol 2019;12(8):1344-1347
introduction
Deep anterior lamellar keratoplasty (DALK) that only replaces the diseased part of the cornea while preserving the recipient’s Descemet membrane and endothelium minimizes the complications of replacing the full-thickness cornea [penetrating keratoplasty (PK)][1-2]. Moreover, improvements in DALK techniques have also enhanced its usefulness for treatment of corneal disease in patients with non-compromised endothelium[3-4].
Although less than that associated with PK[5-6], the risk of recurrence and graft rejection episodes after DALK also exists, potentially leaving a mild to moderate corneal opacity. In modern China, keratoplasty still faces many challenges, including the large numbers of patients, a lack of corneal donors, and limited medical funding[7]. These challenges greatly reduce the chance of a patient receiving another keratoplasty after suffering a mild to moderate corneal opacity after the first procedure. In patients with coexisting cataracts, capsulorhexis and phacoemulsification are difficult because of poor visibility of the crystalline lens and anterior capsule[8]. The protection of the graft and endothelium should also be considered during operation.
Previous studies have shown that phacoemulsification after DALK is safe and provides an improvement in the visual acuity of eyes with transparent corneas[4,9-10]. But few studies are available on phacoemulsification in eyes with mild to moderate corneal opacities after DALK. To overcome above difficulties, we performed a non-invasive optical fiber illumination-assisted phacoemulsification and IOL implantation procedure in eyes with mild to moderate corneal opacities after DALK and the efficacy of the procedure was evaluated.
subjects and Methods
Ethical Approval The study was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of Tongji Hospital Affiliated with Tongji University School of Medicine. Informed consent was waived due to the retrospective nature of the study.
Subjects This retrospective analysis
comprised 12 eyes with mild to moderate corneal opacities after DALK that
underwent optical fiber illumination-assisted phacoemulsification and IOL
implantation by the same senior surgeon (Bi YL) between March 2013 and April
Pre-phaco Examination Corrected distance visual acuity (CDVA) and slit-lamp microscopic examination were performed in all eyes. Intraocular pressure (IOP) was measured by a Tono-Pen (SW-500; SUOER, China). Endothelial cell density (ECD) was detected by confocal microscopy (Confoscan 3.0; NIDEK, Japan). The axial length was measured by partial coherence interferometry (PCI; IOLMaster 500; Carl Zeiss, Germany). Keratometry readings were obtained using corneal topography (ATLAS 9000; Carl Zeiss, Germany) and then manually inserted into the PCI device to determine the spherical IOL power using the SRK-T formula.
Surgical Technique All operations were performed by the
same senior surgeon (Bi YL) under retrobulbar anesthesia. Care was taken to
avoid contact with the graft-host junction when making two corneoscleral limbus
incisions. Through a
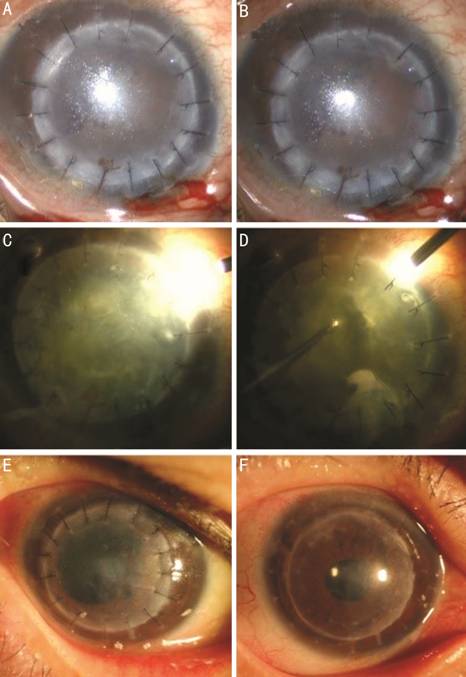
Figure 1 Microscope views taken during surgery, and the slit-lamp photo of
the right eye of patient
Post-phaco Protocol All eyes received levofloxacin 0.5%, TobraDex (tobramycin 0.3% and dexamethasone 0.1%) and pranoprofen 0.1% eye drops postoperatively, which were gradually tapered and then stopped after one month. Patients received follow-up regularly after discharge. CDVA, IOP, ECD, and slit-lamp microscopic examination accompanied each visit.
Statistical Analysis Statistical analyses were performed using SPSS 11.0. Data were shown as mean±SD. Comparisons between pre-phaco and post-phaco ECD were performed using a pared-samples t-test. P<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Twelve eyes of 12 patients were included in the study and the patient
characteristics are shown in Table 1. The mean age was 61.33±5.76y. The mean
interval between DALK and phacoemulsification was 13.50±3.48mo. For a final
accurate prediction of IOL power, phacoemulsification was performed in all eyes
after full suture removal[11] (at least 12mo
after DALK; Figures 2-3), except for one eye (Figure 1E) with preexisting
posterior synechia presenting phacomorphic glaucoma beyond the control of
topical antiglaucoma medications during the follow-up period after DALK. The
IOP in this eye maintained between 32 to
Table 1 Patient characteristics
Patient No./sex/age (y) |
Reason for DALK |
ECD (cells/mm2) |
CDVA |
||
Pre-phaco |
Post-phacoa |
Pre-phaco |
Post-phacoa |
||
1/M/54 |
Infective leucoma |
2570 |
2114 |
20/500 |
20/100 |
2/F/70 |
Infective leucoma |
2022 |
1687 |
20/667 |
20/200 |
3/M/61 |
Traumatic leucoma |
2285 |
1907 |
20/500 |
20/200 |
4/M/58 |
Fungal keretitis |
2334 |
2005 |
20/333 |
20/80 |
5/M/68 |
Mooren’s ulcer |
2087 |
1733 |
20/200 |
20/50 |
6/F/64 |
Infective leucoma |
2045 |
1721 |
20/250 |
20/133 |
7/M/52 |
Fungal keretitis |
2642 |
2245 |
20/500 |
20/133 |
8/M/63 |
Infective leucoma |
2125 |
1836 |
20/333 |
20/166 |
9/M/55 |
Fungal keretitis |
2475 |
2084 |
20/250 |
20/66 |
10/M/58 |
Traumatic leucoma |
2316 |
1968 |
20/250 |
20/80 |
11/M/69 |
Infective leucoma |
2032 |
1699 |
20/200 |
20/50 |
12/M/64 |
Infective leucoma |
2168 |
1876 |
20/500 |
20/200 |
DALK: Deep anterior lamellar keratoplasty; ECD: Endothelial cell density; CDVA: Corrected distance visual acuity. a12mo after phacoemulsification.
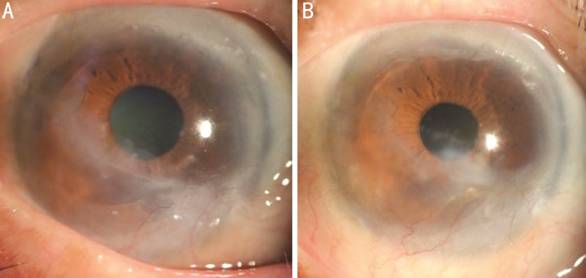
Figure 2 Pre-phaco and post-phaco slit-lamp photos of patient
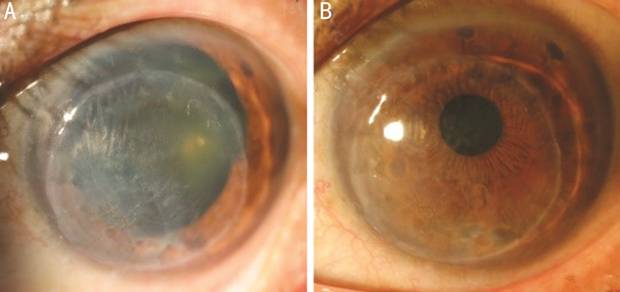
Figure 3 Pre-phaco and post-phaco slit-lamp photos of patient
Operations were uneventful in all cases. No complications were noted during
or after phacoemulsification and IOL implantation. IOP maintained between 13 to
Performing phacoemulsification in eyes with preexisting corneal opacities is challenging because of poor visibility of the lens and anterior chamber. Anterior chamber endoillumination and transconjunctival chandelier retroillumination have reportedly been used in cataract surgery to enhance visibility[8,16]. But these procedures also have limits. Anterior chamber endoillumination occupies space in the anterior chamber and the position is inconvenient to change. Transconjunctival chandelier retroillumination used in vitreoretinal surgery may result in retinal phototoxicity[17-19]. In this study, a non-invasive optical fiber was used as the only light source to decrease the light scatter caused by corneal opacity and further enhance visibility. The non-invasive optical fiber does not need additional incisions and has smaller influence on the cornea, which is especially important in eyes after DALK. Further, it has better mobility and does not occupy the space of the anterior chamber, making anterior chamber manipulation progress more smoothly. The slant projection angle also reduces microscope-induced retinal phototoxicity[20-21].
After solving the problem of illumination, the other thing to be noticed is the protection of the graft and endothelium. Corneoscleral limbus incisions should avoid contact with the graft-host junction. Staining of the anterior capsule under an air bubble reduced contact between the dye and the corneal endothelium. Minimize movement of the tip to reduce its influence on the graft-host junction. A low vacuum and flow rate were required to decrease turbulence in the anterior chamber and to minimize the damage to the graft-host junction and endothelium. For safety, this technique should be performed in eyes with a nucleus up to grade Ⅲ (according to the Emery-Little classification), to decrease endothelial cell loss caused by high ultrasonic energy[14,22].
There were also some limitations in this study, such as the small sample size and the need for an assistant during the surgery. More appropriate cases will be comprised in the future. Moreover, the holder for the non-invasive optical fiber is in the design.
In conclusion, for patients with coexisting cataracts and mild to moderate corneal opacities after keratoplasty but who are unable to undergo another keratoplasty due to a lack of corneal donors or financial concerns, this study provided a safe and valid technique for enhancing their postoperative visual acuity to some extent and at lower cost.
ACKNOWLEDGEMENTS
Foundations: Supported by a Municipal Human Resources Development Program for Outstanding Leaders in Medical Disciplines in Shanghai (No.2017BR060); Shanghai Scientific and Technical Innovation Plan 2016 (No.16140900900).
Conflicts of Interest: Lin H, None; Zhang J, None; Niu GZ, None; Huang XY, None; Zhang YS, None; Liu CY, None; Zheng CY, None; Bi YL, None.
references