
Citation: Han X, Dong XX, Shi MY, Feng L, Wang XL, Zhang JS, Yan
QC. SUMOylation and deacetylation affect NF-κB p65 activity induced by high
glucose in human lens epithelial cells. Int J Ophthalmol
2019;12(9):1371-1379 DOI:10.18240/ijo.2019.09.01
・Basic Research・
SUMOylation
and deacetylation affect NF-κB p65 activity induced by high glucose
in human lens epithelial cells
Xiao Han1, Xiao-Xuan Dong2, Ming-Yu
Shi1, Li Feng1, Xin-Ling Wang1, Jin-Song Zhang1,
Qi-Chang Yan1
1Department of
Ophthalmology, the Fourth Affiliated Hospital of China Medical University; Key
Laboratory of Lens Research of Liaoning Province; Eye Hospital of China Medical
University, Shenyang 110005, Liaoning Province, China
2Department
of Ophthalmology, the Fourth People’s Hospital of Shenyang, Shenyang 110031,
Liaoning Province, China
Correspondence
to: Qi-Chang
Yan. Department of Ophthalmology, the Fourth Affiliated Hospital of China
Medical University; Key Laboratory of Lens Research of Liaoning Province; Eye
Hospital of China Medical University, 11Xinhua Road, Heping District, Shenyang
110005, China. cmu4h_yqc@163.com
Received: 2018-12-25
Accepted: 2019-05-07
Abstract
AIM: To explore the effects of IκBα SUMOylation and NF-κB p65 deacetylation on NF-κB p65 activity induced by high glucose in cultured
human lens epithelial cells (HLECs).
METHODS: HLECs (SRA01/04) were cultured with 5.5, 25, and 50
mmol/L glucose media for 24h, and with 50 mmol/L glucose media for 0, 12, and
24h respectively. SUMO1 and SIRT1 expressions were detected by reverse
transcription-polymerase chain reaction (RT-PCR) and Western blot (WB). IκBα and NF-κB p65 expressions were detected
by WB. With NAC, DTT, MG132 or Resveratrol (RSV) treatment, SUMO1 and SIRT1
expressions were detected by WB. Protein expression localizations were examined
by immunofluorescence and co-immunofluorescence. The effects of SUMO1 or SIRT1
overexpression, as well as MG132 and RSV, on the nuclear expression and
activity of IκBα and NF-κB p65 were analyzed by
immunoblot and dual luciferase reporter gene assay.
RESULTS: SUMO1 and SIRT1 expressions were influenced by high
glucose in mRNA and protein levels, which could be blocked by NAC or DTT. SUMO1
was down-regulated by using MG132, and SIRT1 was up-regulated under RSV
treatment. IκBα nuclear expression was
attenuated and NF-κB p65 was opposite under high glucose, while IκBα and NF-κB p65 location was transferred
to the nucleus. SUMO1 or SIRT1 overexpression and MG132 or RSV treatment
affected the nuclear expression and activity of IκBα and NF-κB p65 under high glucose
condition.
CONCLUSION: IκBα SUMOylation and NF-κB p65 deacetylation affect NF-κB p65 activity in cultured
HLECs under high glucose, and presumably play a significant role in controlling
diabetic cataract.
KEYWORDS: SUMOylation; deacetylation; NF-κB
p65; IκBα; diabetic cataract; high glucose; lens epithelial cells
DOI:10.18240/ijo.2019.09.01
Citation: Han
X, Dong XX, Shi MY, Feng L, Wang XL, Zhang JS, Yan QC. SUMOylation and
deacetylation affect NF-κB p65 activity induced by high glucose in human lens
epithelial cells. Int J Ophthalmol 2019;12(9):1371-1379
INTRODUCTION
Diabetic
cataract (DC) is a common complication of type 1 diabetes mellitus[1]. High blood glucose can obviously accelerate cataract
progression in age-related cataract patients[2]
and promote cataract formation in type 2 diabetes mellitus patients[3]. High glucose treatment can cause oxidative stress to
damage the human lens epithelial cells (HLECs) and form cataractogenesis[4]. Meanwhile, it is widely acknowledged that oxidative
stress is one of major mechanisms of DC[5-6].
In previous studies, it is clarified that oxidative stress can motivate various
signal pathways, as well as posttranslational modification (PTM), such as
SUMOylation[7-8] and deacetylation[9-10]. Nuclear factor κB (NF-κB) as a
sensitive transcriptional factor plays an important role in regulating
oxidative stress[11] which contributes to DC[12-13]. However, whether SUMOylation
and deacetylation has relationship with NF-κB signal pathway involving in DC in
HLECs is still unknown.
SUMOylation
is one of PTM, which has been validated to protect target proteins from
degradation by ubiquitination[14-15].
There are four various isoforms of SUMO grouped into three classes, SUMO1,
SUMO2/3 and SUMO4 mediating SUMOylation in mammals[16].
SUMO1, the most well-known SUMO family member, is involved in kinds of cellular
processes and resulted in different diseases. It establishes an essential role
of regulating transcriptional factors[17], protecting
cell[18], inhibiting oxidative stress reaction[19], repairing DNA damage[20],
preventing apoptosis[21] and so on. Nevertheless,
there is few study of cataract to date have focused on SUMO1 and SUMOylation.
SIRT1, the
most crucial sirtuin family member, modifies deacetylation (another significant
PTM) and brings on various physiological and pathological processes. SIRT1, as
a longevity gene, is participated in reducing oxidative stress[22], preventing apoptosis[23],
resisting aging[24] and all that. It has
demonstrated the relationship between SIRT1 and age-related cataract in others
studies[25-26]. Increasing
evidence suggests that Resveratrol (RSV), a famous antioxidant and anti-aging
agent, can accelerate the expression and activity of SIRT1[27].
But the influence of SIRT1 and RSV in HLECs under high glucose remains poorly
understood.
NF-κB is a
significant stress responsive transcriptional factor located in the cytoplasm
in nonactivated state and its activity can be affected by various external
stimuli. NF-κB p65, as a prominent member of NF-κB family, is concerned with
various stimuli stress, especially oxidative stress[28].
When stimulated, NF-κB is activated and translocated into the nucleus. Its transcriptional
activity is controlled by inhibitor IκB proteins[29]
and IκBα is a main member of IκBs. NF-κB activity is determined by degradation
of IκB which is mediated by ubiquitin-proteasome pathway (UPP). Therefore, in
another study, it has verified the proteasome inhibitor MG132 reversed IκBα
degradation and decreased NF-κB expression and activity which was induced by
high glucose in rat mesangial cells[30]. MG132
treatment could also accumulate the conjugations of SUMO and target proteins[31]. Liu et al[32]
found that ubiquitin and SUMO competed for the same target lysine on K21/22
of IκBα in previous study. It was cleared that acetylation sites of NF-κB p65
were found on lysines K221, K310, and K122/123,
although there were different effects on different lysines[33].
This study
is the first to demonstrate whether high glucose could induce SUMO1 and SIRT1
expression owing to oxidative stress in cultured HLECs, and whether IκBα
SUMOylation and NF-κB p65 deacetylation could affect NF-κB p65 activity in
vitro. The results showed SUMO1 or SIRT1 overexpression could influence the
nuclear expression of IκBα and NF-κB p65, as well as the activity of NF-κB p65 in cultured HLECs. Meanwhile, it was the
first time to investigate the effects of MG132 and RSV on protecting lens
transparency from oxidative damage induced by high glucose through regulation
of NF-κB p65 activity in HLECs.
MATERIALS AND METHODS
Cell Culture
and Treatments Human lens epithelial cells
(SRA01/04) were gifts from Key Lens Laboratory of Lens Research of Liaoning
Province. The cells were cultured in Dulbecco’s modified Eagle’s media (DMEM;
Hyclone) with 5.5 mmol/L glucose, 10% fetal bovine serum (FBS; Invitrogen), 100
mg/mL streptomycin (Hyclone) and 100 IU/mL penicillin (Hyclone) in a 5% CO2
humidified atmosphere at 37℃. The
SRA01/04 cells were grown to 75%-80% confluence and divided randomly into
several groups: normal control glucose group (NC; media with 5.5 mmol/L
glucose), high glucose 1 group (HG1; media with 25 mmol/L glucose), high
glucose 2 group (HG2; media with 50 mmol/L glucose), and osmotic pressure
control group (OP; media with 50 mmol/L mannitol). N-acetyl cysteine (NAC;
Sigma) 5 mmol/L for 4h or the thiol-reducing agent dithiothreitol (DTT; Sigma)
10 mmol/L for 1h was as anti-oxidant addressed in high glucose media. MG132
(Sigma) 10 µmol/L as the proteasome inhibitor added in media for 4h. RSV
(Sigma) 10 µmol/L as SIRT1 activator was participated in media for 4h.
Reverse
Transcription-Polymerase Chain Reaction
Total RNA
from SRA01/04 cells was extracted using TRIzol (TaKaRa) and reverse transcribed
using M-MLV First Kit (Invitrogen) to get cDNA, which was amplified using Taq
DNA polymerase Recombinant Kit (Invitrogen). The results were determined using
chemiluminescent gel imaging system and normalized to β-actin gene expression.
The primer sequences were as followed: SUMO1 (forward: 5’-tgg aca gga tag cag tga ga-3’; reverse: 5’-tct tcc tcc att ccc agt tct-3’; product size: 174 bp), SIRT1 (forward: 5’-cca gcc atc tct ctg tca ca-3’; reverse: 5’-tcc tcg tac agc ttc aca gt-3’;
product size: 193 bp), β-actin (forward: 5’-cat ccg taa aga cct cta tgc caa c-3’; reverse: 5’-atg gag cca ccg atc cac a-3’;
product size: 171 bp).
Western Blot Total proteins from SRA01/04 cells
were extracted using RIPA lysis buffer with PMSF and protease inhibitor
cocktail set (Calbiolchem, Germany). Nuclear proteins from SRA01/04 cells were
extracted with nuclear protein extraction kit (Beyotime, China). The lysates
were separated by NuPAGE 10% Bis-Tris Gel (Invitrogen), and transferred to
polyvinylidene difluoride (PVDF) membrane (Millipore, USA). Primary antibodies
against SUMO1 (Abcam), SIRT1 (Abcam), NF-κB p65 (Bioss, China), IκBα (Bioss,
China), and β-actin (Proteintech, USA) were used, as well as
peroxidase-conjugated affinipure goat anti-rabbit IgG and peroxidase-conjugated
affinipure goat anti-mouse IgG secondary antibodies (Jackson immunoresearch,
USA). The proteins were detected by enhanced hemagglutinin (Thermo), quantified
by chemiluminescent gel imaging system, and normalized to β-actin protein
expression.
Immunofluorescence The SRA01/04 cells were cultured on
cover lips in 24-well plates and were treated with 5.5 mmol/L (NC) and 50
mmol/L (HG2) glucose in media for 24h. The cells were fixed with 4%
paraformaldehyde (PFA, Invitrogen) solubilized in 0.1% Triton×100-PBS for
20min, and were blocked with 5% BSA-PBS (Sigma) for 1h. The cells were
incubated with anti-IκBα and anti-NF-κB p65 antibodies in 2% BSA-PBS overnight
at 4℃.
Alex Fluor 596 goat anti-rabbit IgG (H+L) (Invitrogen) in 2% BSA-PBS was as
secondary antibody incubated for 1h in the dark room. DAPI (Beyotime, China)
used to stain the nucleus for 1min. Images were taken with fluorescence
microscope.
SUMO1 or
SIRT1 Overexpression and Immunoblot Analysis GFP-SUMO1 (gift from Prof. Chen[34]), GFP-SIRT1 (gift from Doctor Jiang) and empty
GFP-vector (Invitrogen) were transfected with lipofectamin 2000 (Invitrogen)
into cells for 6h. After cultured 40h, the cells were incubated with 5.5 mmol/L
(NC) and 50 mmol/L (HG2) glucose media respectively for 24h. The nuclear
protein of transfected SUMO1 or SIRT1 cells was extracted and detected the
nuclear protein expressions of IκBα and NF-κB p65 by immunoblot. The cells
transfected with empty GFP-vector were as a blank group.
MG132 or RSV
Treatment and Immunoblot Analysis SRA01/04 cells were cultured with
normal (NC) or high glucose (HG2) media for 24h. Then, cells were treated with
10 µmol/L MG132 or 10 µmol/L RSV for 4h respectively. The nuclear proteins of
treated cells were extracted and the nuclear expression of IκBα and NF-κB p65
was detected by immunoblot.
Dual
Luciferase Reporter Gene Assay SRA01/04 cells were cultured in 6
well plates and transiently transfected with pNF-κB-TA-luc, the control pGL6-TA
(Beyotime, China) reporters, GFP-SUMO1, GFP-SIRT1, GFP-vector, and together
with Renilla luciferase control plasmid (pRL-TK) as internal control plasmid.
After 24h co-transfection, cells were treated with or without high glucose, and
MG132 or RSV 10 µmol/L treatment for 4h. Absolute luminescence was measured
according to the Dual-Luciferase Reporter Assay protocol (Beyotime, China). The
relative NF-κB dual luciferase activities were measured and firefly values were
normalized by Renilla values.
Statistical
Analysis All data were presented as the mean±SD
for at least 3 independent experiments and statistical analysis was evaluated
using one-way ANOVA of SPSS program version 19.0. P<0.05 was
considered statistically significant.
RESULTS
SUMO1 and
SIRT1 Expression Influenced by High Glucose-Induced Oxidative Stress The relative SUMO1 expression of HG1
and HG2 groups were higher than that of NC group in both mRNA and protein
levels. In contrast, the expression of SIRT1 was decreased in HG1 and HG2
groups, which compared with NC group in both mRNA and protein levels (Figure 1A, 1C).
Compared with 0h group, SUMO1 expression in mRNA and protein levels were
enhanced in 12h and 24h group. There were also had different results for SIRT1
(Figure 1B, 1D). Importantly, it was confirmed that SUMO1 and SIRT1 expression
changed by high glucose owing to oxidative stress rather than osmotic pressure
stress (compared with NC, P>0.05, Figure 1A, 1C).
The increase in SUMO1 protein could be blocked by NAC or DTT (antioxidant)
treatment under high glucose condition. In the same way, the decrease in SIRT1
protein could be reversed by NAC or DTT addition in high glucose media (Figure
1E).
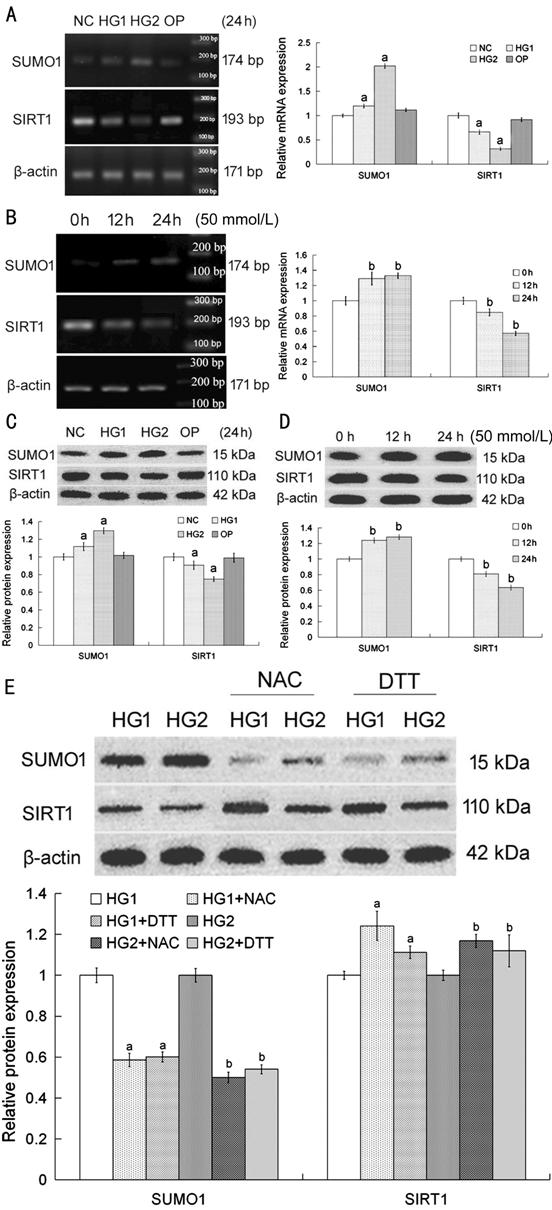
Figure 1
High glucose induced oxidative stress and influenced SUMO1 and SIRT1 expression
in HLECs The
expressions of SUMO1 and SIRT1 in
the mRNA (A, B) and protein (C, D) levels in SRA01/04 cells with different
concentrations of glucose media (A, C) and treated various times (B, D). There
was no obvious change in OP group (A, C). Compared with NC or 0h group, aP<0.05
or bP<0.05. SRA01/04 cells were cultured with high
glucose media for 24h, as well as 5 mmol/L NAC for 4h or 10 mmol/L DTT for 1h
respectively (E). Compared with HG1 or HG2 group, aP<0.05
or bP<0.05. The data were normalized to β-actin and
expressed as mean±SD of triplicates in an independent experiment, which was
repeated at least 3 times with the same results. NC: Media with 5.5 mmol/L
glucose; HG1: Media with 25 mmol/L glucose; HG2: Media with 50 mmol/L glucose;
OP: Media with 50 mmol/L mannitol; NAC: Media with 5 mmol/L NAC; DTT: Media
with 10 mmol/L DTT.
MG132 and
RSV Influenced SUMO1 and SIRT1 Expression
The SUMO1
expression was decreased in whether normal (NC) or high glucose (HG1) condition
when it was treated with MG132 (Figure 2A). As shown in Figure 2B, SIRT1 had an opposite
situation. After RSV was participated in normal or high glucose media, the
expression of SIRT1 was enhanced obviously.
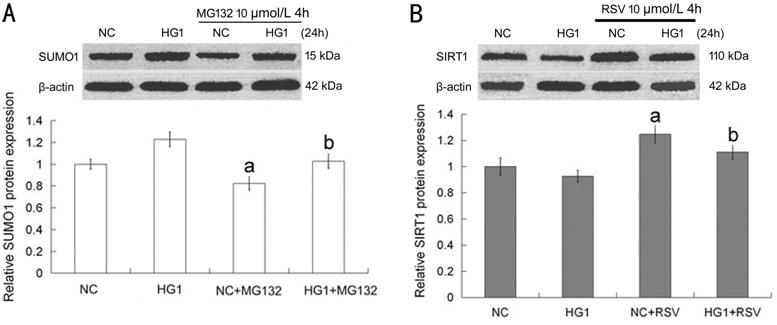
Figure 2
MG132 and RSV could influence SUMO1 and SIRT1 expression in HLECs A: With or without high glucose
added to the media for 24h, cells were treated with 10 µmol/L MG132 for 4h; B:
With or without high glucose added to the media for 24h, cells were treated
with 10 µmol/L RSV for 4h. Compared with NC or HG1 group, aP<0.05
or bP<0.05. The data were normalized to β-actin and
expressed as mean±SD of triplicates in an independent experiment, which was
repeated at least 3 times with the same results. NC: Media with 5.5 mmol/L
glucose; HG1: Media with 25 mmol/L glucose; MG132: Media with 10 µmol/L MG132;
RSV: Media with 10 µmol/L RSV.・
High Glucose
Affected the Nuclear Expression of IκBα and NF-κB p65 High glucose could attenuate IκBα
nuclear expression, while increase the nuclear expression of NF-κB p65 (Figure 3A). However, osmotic pressure had little
effect on the nuclear expressions of IκBα and NF-κB p65 (compared with NC, P>0.05;
Figure 3A). Immunofluorescence
(Figure 3B, 3C) showed
the locations of IκBα and NF-κB p65 were transferred to nucleus from cytoplasm
induced by high glucose.
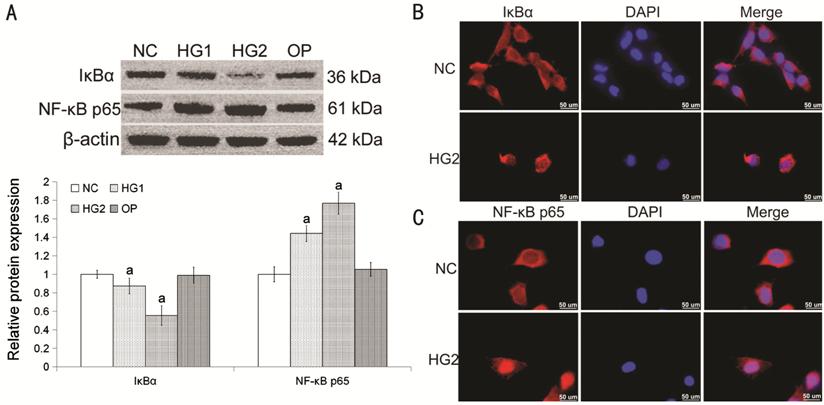
Figure 3
High glucose affected the nuclear expression of IκBα and NF-κB p65 in HLECs A: The nuclear expression of IκBα and
NF-κB p65 in HLECs
with different concentrations of glucose media for 24h. Compared with NC group,
aP<0.05. Osmotic pressure had little effect on the
expressions of IκBα and NF-κB p65. B, C: Immunofluorescence staining for IκBα
(red) and NF-κB p65 (red) in NC and HG2 group, nuclei with DAPI (blue), Bar=50
µm. The data were normalized to β-actin and expressed as mean±SD of triplicates
in an independent experiment, which was repeated at least 3 times with the same
results. NC: Media with 5.5 mmol/L glucose; HG1: Media with 25 mmol/L glucose;
HG2: Media with 50 mmol/L glucose; OP: Media with 50 mmol/L mannitol.
SUMO1 or
SIRT1 Overexpression Influenced IκBα Nuclear Expression and NF-κB p65
Activity SRA01/04 cells were highly efficient
transfected with GFP-SUMO1 or SIRT1 respectively and cultured with high glucose
media for 24h. Compared with transfected empty GFP-vector cells, IκBα nuclear
expression was increased and NF-κB p65 nuclear expression was decreased in
transfected SUMO1 or SIRT1 cells under high glucose condition (Figure 4A). SRA01/04 cells were highly
efficient transfected with GFP-SUMO1, GFP-SIRT1, GFP-vector, pNF-κB-TA-luc,
pGL6-TA, and pRL-TK respectively and cultured with high glucose media for 24h.
Compared with transfected empty GFP-vector cells, NF-κB p65 activity was
decreased in transfected SUMO1 or SIRT1 cells under high glucose condition
(Figure 4B). There was no obvious change in pGL6-TA control groups (P>0.05;
Figure 4B).
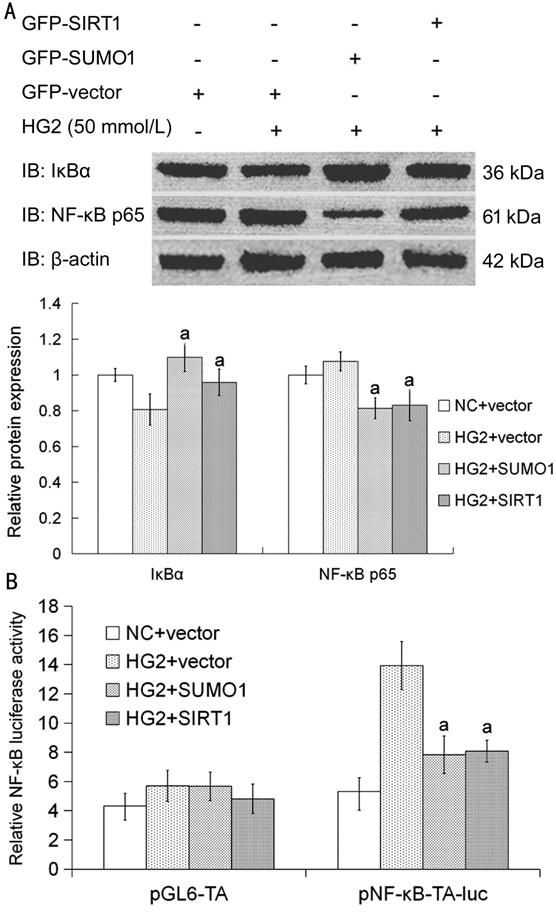
Figure 4
SUMO1 or SIRT1 overexpression influenced the nuclear expression of IκBα and
affected the expression and activity of NF-κB p65 in HLECs A: The cells transfected with SUMO1
or SIRT1 were cultured with high glucose media to detect the nuclear expression
of IκBα and NF-κB p65. Compared with HG2+vector group, aP<0.05.
The data were normalized to β-actin and expressed as mean±SD of triplicates in
an independent experiment, which was repeated at least 3 times with the same
results. B: The cells transfected with GFP-SUMO1, GFP-SIRT1, GFP-vector,
pNF-κB-TA-luc, pGL6-TA and pRL-TK respectively were cultured with high glucose
media to detect the relative NF-κB luciferase activity. Compared with
transfected HG2+GFP-vector group, aP<0.05. There was no
obvious change in pGL6-TA control groups. The value of fluorescence was
normalized by Renilla values and expressed as mean±SD of triplicates in an
independent experiment, which was repeated at least 9 times with the same
results. NC: Media with 5.5 mmol/L glucose; HG2: Media with 50 mmol/L glucose.
MG132 and
RSV Influenced IκBα Nuclear Expression and NF-κB p65 Activity After cultured with or without high glucose
for 24h, the nuclear expression of IκBα and NF-κB p65 was changed by MG132 and
RSV treatment. Both MG132 and RSV could enhance IκBα nuclear expression; in
contrast, reduce the nuclear expression of NF-κB p65. The effects of MG132 and
RSV were just like SUMO1 and SIRT1 overexpression on the nuclear expression of
IκBα and NF-κB p65 in HLECs
(Figure 5A). SRA01/04
cells were highly efficient transfected with GpNF-κB-TA-luc, pGL6-TA and pRL-TK
respectively. After cultured with or without high glucose for 24h, the activity
of NF-κB p65 was changed by MG132 and RSV treatment. Compared with HG2 group,
both MG132 and RSV could reduce the activity of NF-κB p65 under high glucose
condition. The effects of MG132 and RSV were just like SUMO1 and SIRT1
overexpression on the activity of NF-κB p65 in HLECs (Figure 5B). There was no obvious change
in pGL6-TA control groups (P>0.05; Figure 5B).
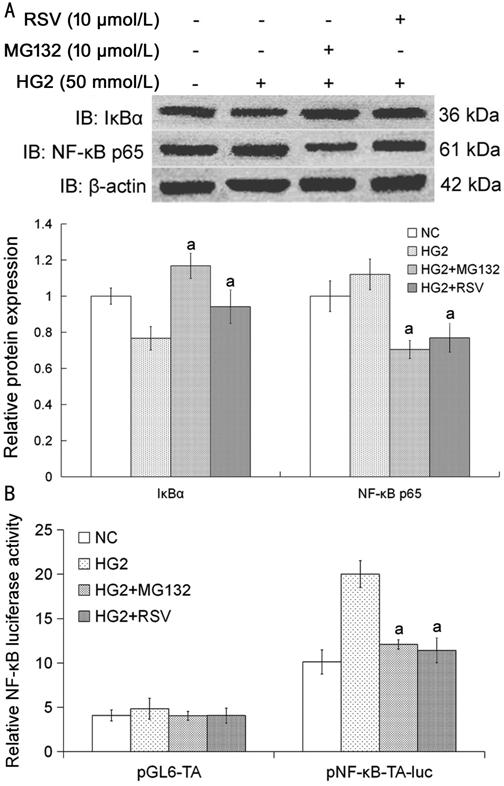
Figure 5
MG132 and RSV influenced the nuclear expression of IκBα and affected the
expression and activity of NF-κB p65 in
HLECs A: The
cells were cultured with or without high glucose media for 24h, and were
treated with MG132 and RSV 10 µmol/L for 4h respectively. Compared with HG2
group, aP<0.05. The data were normalized to β-actin and
expressed as mean±SD of triplicates in an independent experiment, which was
repeated at least 3 times with the same results. B: The cells transfected with
pNF-κB-TA-luc, pGL6-TA and pRL-TK were cultured with or without high glucose
media for 24h, and were treated with MG132 and RSV 10 µmol/L for 4h
respectively. The relative NF-κB luciferase activity compared with HG2 group, aP<0.05.
There was no obvious change in pGL6-TA control groups. The value of
fluorescence was normalized by Renilla values and expressed as mean±SD of
triplicates in an independent experiment, which was repeated at least 9 times
with the same results. NC: Media with 5.5 mmol/L glucose; HG2: Media with 50
mmol/L glucose; MG132: Media with 10 µmol/L MG132; RSV: Media with 10 µmol/L
RSV.
DISCUSSION
To date,
there was no previous experimental evidence for the function of SUMOylation and
deacetylation in HLECs or pathology of diabetic cataract. In the previous work,
we have discussed the expression and function of SUMO1-4 and SUMO E3 (Cbx4 and
PIASy) under high glucose environment in HLECs[35].
The increasing studies have reported the regulation of SUMOylation and
deacetylation by cellular stress, suggesting a key role of SUMOylation and
deacetylation in the cellular response. Therefore, it is significant to explore
study the relative proteins of SUMOylation and deacetylation under stress in
HLECs.
For the
first time, this finding was demonstrated that SUMO1 and SIRT1 expression was
influenced by high glucose in mRNA and protein levels in HLECs. They were also
changed time-dependently in mRNA and protein levels. Huang et al’s[30] study clarified that SUMO1-3 expression was also
enhanced by high glucose in rat mesangial cells. High glucose-induced oxidative
stress represses SIRT expression and increases histone acetylation leading to
neural tube defects[36]. We tried to explore the
reason for the changes of SUMO1 and SIRT1 under high glucose microenvironments,
because osmotic pressure stress had little effect on regulating SUMO1 and SIRT1
expression in HLECs. We found the change of SUMO1 and SIRT1 expression under
high glucose could be blocked and reversed by anti-oxidants, NAC or DTT. It was
guessed that high glucose could regulate SUMO1 and SIRT1 protein expression
owing to oxidative stress reaction, which allowed for a new
SUMOylation/deSUMOylation and acetylation/deacetylation balance in response to
oxidative stress. We hypothesized that high glucose-mediated oxidative stress
might decline the conjugation of SUMO1 and its target proteins, leading to
endogenous free SUMO1 proteins increase. In our results, MG132 (as antioxidant)
might improve SUMO1 conjugating with target proteins, which could lead to
decrease of endogenous free SUMO1 under MG132 treatment in HLECs. In another
study, MG132 could induce accumulation of SUMO2/3 conjugates, while reduce the
expression of free endogenous SUMO2/3 in
HEK 293T cells[31]. According to the change of
SIRT1 under high glucose, we hypothesized that oxidative stress might reduce
SIRT1 expression and activity mediated deacetylation under high glucose. It has
been recognized that H2O2-mediated oxidative stress
reduces the expression and activity of SIRT1
in human lung epithelial cells[37].
In our study, it was striking that RSV was still SIRT1 activator in HLECs.
In addition
to regulating SUMO1 and SIRT1, oxidative stress is also regulated by SUMO1 and
SIRT1. Researchers have revealed that SUMO1 conjugated to proteins involving in
the regulation of diverse cellular events, including transcriptional
regulation, stress resistance, cellular senescence, apoptosis, responses to
extracellular stimuli, and especially oxidative stress. SUMOylated lysines
cannot be ubiquitinated, which contribute to the stabilization of target
proteins[38]. Deacetylation of target proteins
mediated by SRIT1 involved in activity/inactivity of substance[33], which also contributes to regulating aging,
inflammation, metabolic processes, oxygen sensing, redox-dependent cellular
processes, among others[39]. SUMO1 mediated
SUMOylation to prevent degradation of IκBα from ubiquitin-proteasome pathway,
and to stabilize IκBα of IκB by SUMO, which results in the inhibition of NF-κB
transcriptional activity[40]. Moreover, the
activation of NF-κB p65 was reduced through deacetylation mediated by SIRT1[41]. SIRT1 interacts with NF-κB p65 leading to its
deacetylation and resulting in decreased NF-κB-dependent transcription[42]. As a transcriptional factor, NF-κB has function for
many target genes that control various cellular responses such as apoptosis,
stress and inflammation. More importantly, clinical and laboratory studies have
demonstrated NF-κB pathway participated in diverse human diseases, such as
myocardial disease[43], diabetic nephropathy[44], cataract[45] and
cancer[46]. A crucial observation made in this
study was NF-κB p65 nuclear expression induced by high glucose in HLECs, while
IκBα nuclear expression was opposite. It was confirmed that the changes of
NF-κB p65 and IκBα was due to oxidative stress rather than osmotic pressure
stress. The finding suggested NF-κB p65 and IκBα were mainly located in the
cytoplasm normally, but were transferred into nucleus by high glucose mediated
oxidative stress. It also evidenced that high glucose activated and
translocated NF-κB p65 and IκBα protein. Redox derived by high glucose led to
covalent post-translational modifications, SUMOylation and deacetylation.
SUMOylated
IκBα as inhibitor of NF-κB has been demonstrated in attenuating NF-κB
activation. It was verified SUMO1 overexpression promoted IκBα SUMOylation and
attenuated the activity of NF-κB p65 in
HLECs. We also recognized that SIRT1 overexpression induced NF-κB p65
deacetylation, which could also reduce the activity of NF-κB p65. On the other
side, a study reported that SUMO1 mediated SUMOylation of SIRT1[47-48], and stabilized the deacetylase
activity of SIRT1[49]. Therefore, SUMO1
overexpression might activate SIRT1 deacetylase at the same time, which also
promoted SIRT1 which mediated deacetylation of NF-κB p65 in HLECs. There was complicated correlate
among SUMO1, SIRT1, NF-κB p65 and IκBα shown in Figure 6. All the results
above-mentioned suggest that SUMO1 and SIRT1 might be new potential therapeutic
targets for the treatment of DC. Furthermore, we also found MG132 and RSV could
also influence the expression of SUMO1 and SIRT1 respectively in some degree,
which meant MG132 and RSV could regulate SUMOylation and deacetylation.
Therefore, we chose MG132 and RSV as antioxidant to treat HLECs under normal or
high glucose condition. The fact proved that MG132 and RSV, both could
influence the activity of NF-κB p65 and IκBα. In addition, MG132 is one kind of
proteasome inhibitor which inhibits modification of ubiquitination and
accumulates conjugations of SUMO and its target proteins. It is universal that
IκBα degradation is attenuated through UPP. Meanwhile, MG132 can improve the
conjugation of SUMO1 and IκBα. Therefore, stabilization of IκBα can promote its
conjugating with NF-κB p65, and inhibit the activation of NF-κB p65. We also
found RSV had effect on reducing the activation of NF-κB p65. As an
antioxidant, RSV might influence cellular stress response through kinds of
pathway. In our study, as SIRT1 activator, RSV might regulate SIRT1 mediated
deacetylation to affect NF-κB p65 activity in HLECs. RSV could also inhibit the
degradation of IκBα in our study. The mechanism was discovered that RSV
inhibited IκBα phosphorylation to maintain the expression level of IκBα[50]. The relationship of MG132, RSV, IκBα and NF-κB p65
was described as shown in Figure 6. In previous studies, there have been
reports to identified that MG132 or RSV was involved in controlling high glucose
in diabetes respectively[51-52].
It was full proof that MG132 and RSV as antioxidant might play a significant
role in protecting lens from DC.
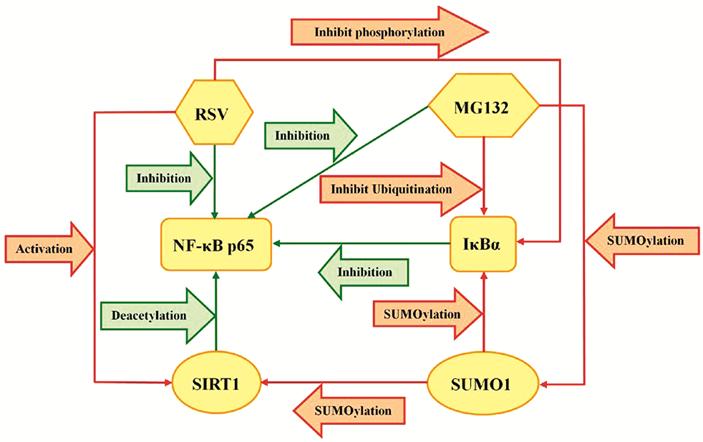
Figure 6 The
relationship of SUMO1, SIRT1, NF-κB p65 and IκBα in HLECs.
In
conclusion, the study was first to found that SUMO1 and SIRT1 expression was
influenced by high glucose due to oxidative stress in cultured HLECs. SUMO1 or
SIRT1 overexpression could enhance the modifications of IκBα SUMOylation and
NF-κB p65 deacetylation, and then influence the activation of NF-κB p65. In the
same time, MG132 and RSV as antioxidant also regulated NF-κB p65 activity
through influencing SUMOylation and deacetylation respectively. They had the
potential to protect HLECs from oxidative damage and maintain lens
transparency. This study supported that IκBα SUMOylation and NF-κB p65
deacetylation may be involved in the pathogenesis of DC through affecting NF-κB
p65 activity under high glucose conditions. SUMO and SIRT signaling molecules
may be potential therapeutic targets for the treatment of DC.
ACKNOWLEDGEMENTS
We are
grateful to Prof. Chen of Laboratory of Cell Differentiation and Apoptosis of
Chinese Ministry of Education (Shanghai Jiao-Tong University School of
Medicine) and Dr. Zhong-Xiu Jiang of Department of Oncology (Shengjing
Affiliated Hospital of China Medical University) supporting plasmids for the
gifts.
Foundations:
Supported by
the National Natural Science Foundation of China (No.81170836; No.81570838).
Conflicts of
Interest: Han X, None;
Dong XX, None; Shi MY, None; Feng L, None; Wang XL, None;
Zhang JS, None; Yan QC, None.
REFERENCES
|
1 Geloneck MM, Forbes BJ, Shaffer J, Ying
GS, Binenbaum G. Ocular complications in children with diabetes mellitus.
Ophthalmology 2015;122(12):2457-2464.
https://doi.org/10.1016/j.ophtha.2015.07.010
PMid:26341461 PMCid:PMC4769865
|
|
|
|
2 Ghaem Maralani H, Tai BC, Wong TY, Tai
ES, Li J, Wang JJ, Mitchell P. Metabolic syndrome and risk of age-related
cataract over time: an analysis of interval-censored data using a
random-effects model. Invest Ophthalmol Vis Sci 2013;54(1):641-646.
https://doi.org/10.1167/iovs.12-10980
PMid:23258144
|
|
|
|
|
3 Tan NC, Barbier S, Lim WY, Chia KS.
5-Year longitudinal study of determinants of glycemic control for
multi-ethnic Asian patients with type 2 diabetes mellitus managed in primary
care. Diabetes Res Clin Pract 2015;110(2):218-223.
https://doi.org/10.1016/j.diabres.2015.07.010
PMid:26385596
|
|
|
|
|
4 Mirsky N, Cohen R, Eliaz A, Dovrat A.
Featured Article: Inhibition of diabetic cataract by glucose tolerance factor
extracted from yeast. Exp Biol Med (Maywood) 2016;241(8):817-829.
https://doi.org/10.1177/1535370215627031
PMid:26825353 PMCid:PMC4950394
|
|
|
|
|
5 Kruk J, Kubasik-Kladna K, Aboul-Enein HY.
The role oxidative stress in the pathogenesis of eye diseases: current status
and a dual role of physical activity. Mini Rev Med Chem 2015;16(3):241-257.
https://doi.org/10.2174/1389557516666151120114605
|
|
|
|
|
6 Kandarakis SA, Piperi C, Topouzis F,
Papavassiliou AG. Emerging role of advanced glycation-end products (AGEs) in
the pathobiology of eye diseases. Prog Retin Eye Res 2014;42:85-102.
https://doi.org/10.1016/j.preteyeres.2014.05.002
PMid:24905859
|
|
|
|
|
7 Lee YJ, Bernstock JD, Nagaraja N, Ko B,
Hallenbeck JM. Global SUMOylation facilitates the multimodal neuroprotection
afforded by quercetin against the deleterious effects of oxygen/glucose
deprivation and the restoration of oxygen/glucose. J Neurochem
2016;138(1):101-116.
https://doi.org/10.1111/jnc.13643
PMid:27087120 PMCid:PMC4916017
|
|
|
|
|
8 Wang T, Xu W, Qin M, Yang Y, Bao P, Shen
F, Zhang Z, Xu J. Pathogenic mutations in the valosin-containing
protein/p97(VCP) N-domain inhibit the SUMOylation of VCP and lead to impaired
stress response. J Biol Chem 2016;291(27):14373-14384.
https://doi.org/10.1074/jbc.M116.729343
PMid:27226613 PMCid:PMC4933190
|
|
|
|
|
9 Ding YW, Zhao GJ, Li XL, Hong GL, Li MF,
Qiu QM, Wu B, Lu ZQ. SIRT1 exerts protective effects against paraquat-induced
injury in mouse type II alveolar epithelial cells by deacetylating NRF2 in
vitro. Int J Mol Med 2016;37(4):1049-1058.
https://doi.org/10.3892/ijmm.2016.2503
PMid:26935021
|
|
|
|
|
10 Li S, Zhao G, Chen L, Ding
Y, Lian J, Hong G, Lu Z. Resveratrol protects mice from paraquat-induced lung
injury: The important role of SIRT1 and NRF2 antioxidant pathways. Mol Med
Rep 2016;13(2):1833-1838.
https://doi.org/10.3892/mmr.2015.4710
PMid:26708779
|
|
|
|
|
11 Akyol S, Ugurcu V, Balci M,
Gurel A, Erden G, Cakmak O, Akyol O. Caffeic acid phenethyl ester: its
protective role against certain major eye diseases. J Ocul Pharmacol Ther
2014;30(9):700-708.
https://doi.org/10.1089/jop.2014.0046
PMid:25100535
|
|
|
|
|
12 Nambu H, Kubo E, Takamura Y,
Tsuzuki S, Tamura M, Akagi Y. Attenuation of aldose reductase gene suppresses
high-glucose-induced apoptosis and oxidative stress in rat lens epithelial
cells. Diabetes Res Clin Pract 2008;82(1):18-24.
https://doi.org/10.1016/j.diabres.2008.03.023
PMid:18835019
|
|
|
|
|
13 Kim J, Kim CS, Sohn E, Kim
H, Jeong IH, Kim JS. Lens epithelial cell apoptosis initiates diabetic
cataractogenesis in the Zucker diabetic fatty rat. Graefes Arch Clin Exp
Ophthalmol 2010;248(6):811-818.
https://doi.org/10.1007/s00417-010-1313-1
PMid:20162295
|
|
|
|
|
14 Ahner A, Gong X, Frizzell
RA. Divergent signaling via SUMO modification: potential for CFTR modulation.
Am J Physiol Cell Physiol 2016;310(3):C175-C180.
https://doi.org/10.1152/ajpcell.00124.2015
PMid:26582473 PMCid:PMC4838058
|
|
|
|
|
15 Vishwamitra D, Curry CV, Shi
P, Alkan S, Amin HM. SUMOylation confers posttranslational stability on
NPM-ALK oncogenic protein. Neoplasia 2015;17(9):742-754.
https://doi.org/10.1016/j.neo.2015.09.005
PMid:26476082 PMCid:PMC4611074
|
|
|
|
|
16 Enserink JM. Sumo and the
cellular stress response. Cell Div 2015;10:4.
https://doi.org/10.1186/s13008-015-0010-1
PMid:26101541 PMCid:PMC4476178
|
|
|
|
|
17 Nishida T, Yamada Y.
SUMOylation of the KRAB zinc-finger transcription factor PARIS/ZNF746
regulates its transcriptional activity. Biochem Biophys Res Commun
2016;473(4):1261-1267.
https://doi.org/10.1016/j.bbrc.2016.04.051
PMid:27086851
|
|
|
|
|
18 Hudson JJ, Chiang SC, Wells
OS, Rookyard C, El-Khamisy SF. SUMO modification of the neuroprotective
protein TDP1 facilitates chromosomal single-strand break repair. Nat Commun
2012;3:733.
https://doi.org/10.1038/ncomms1739
PMid:22415824 PMCid:PMC3316882
|
|
|
|
|
19 Lee A, Jeong D, Mitsuyama S,
Oh JG, Liang L, Ikeda Y, Sadoshima J, Hajjar RJ, Kho C. The role of SUMO-1 in
cardiac oxidative stress and hypertrophy. Antioxid Redox Signal
2014;21(14):1986-2001.
https://doi.org/10.1089/ars.2014.5983
PMid:24893265 PMCid:PMC4208582
|
|
|
|
|
20 Marchiani S, Tamburrino L,
Ricci B, Nosi D, Cambi M, Piomboni P, Belmonte G, Forti G, Muratori M, Baldi
E. SUMO1 in human sperm: new targets, role in motility and morphology and
relationship with DNA damage. Reproduction 2014;148(5):453-467.
https://doi.org/10.1530/REP-14-0173
PMid:25118297
|
|
|
|
|
21 Meinecke I, Pap G, Mendoza
H, Drange S, Ender S, Strietholt S, Gay RE, Seyfert C, Ink B, Gay S, Pap T,
Peters MA. Small ubiquitin-like modifier 1 [corrected] mediates the
resistance of prosthesis-loosening fibroblast-like synoviocytes against
Fas-induced apoptosis. Arthritis Rheum 2009;60(7):2065-2070.
https://doi.org/10.1002/art.24633
PMid:19565496
|
|
|
|
|
22 Emamgholipour S,
Hossein-Nezhad A, Sahraian MA, Askarisadr F, Ansari M. Evidence for possible
role of melatonin in reducing oxidative stress in multiple sclerosis through
its effect on SIRT1 and antioxidant enzymes. Life Sci 2016;145:34-41.
https://doi.org/10.1016/j.lfs.2015.12.014
PMid:26679105
|
|
|
|
|
23 Zhang C, Qu S, Wei X, Feng
Y, Zhu H, Deng J, Wang K, Liu K, Liu M, Zhang H, Xiao X. HSP25
down-regulation enhanced p53 acetylation by dissociation of SIRT1 from p53 in
doxorubicin-induced H9c2 cell apoptosis. Cell Stress Chaperones
2016;21(2):251-260.
https://doi.org/10.1007/s12192-015-0655-3
PMid:26515559 PMCid:PMC4786524
|
|
|
|
|
24 Wang RH, Zhao T, Cui K, Hu
G, Chen Q, Chen W, Wang XW, Soto-Gutierrez A, Zhao K, Deng CX. Negative
reciprocal regulation between Sirt1 and Per2 modulates the circadian clock
and aging. Sci Rep 2016;6:28633.
https://doi.org/10.1038/srep28633
PMid:27346580 PMCid:PMC4922021
|
|
|
|
|
25 Mimura T, Kaji Y, Noma H,
Funatsu H, Okamoto S. The role of SIRT1 in ocular aging. Exp Eye Res
2013;116:17-26.
https://doi.org/10.1016/j.exer.2013.07.017
PMid:23892278
|
|
|
|
|
26 Kang L, Zhao W, Zhang G, Wu
J, Guan H. Acetylated 8-oxoguanine DNA glycosylase 1 and its relationship
with p300 and SIRT1 in lens epithelium cells from age-related cataract. Exp
Eye Res 2015;135:102-108.
https://doi.org/10.1016/j.exer.2015.02.005
PMid:25660075
|
|
|
|
|
27 Zhang E, Guo Q, Gao H, Xu R,
Teng S, Wu Y. Metformin and resveratrol inhibited high glucose-induced
metabolic memory of endothelial senescence through SIRT1/p300/p53/p21
pathway. PLoS One 2015;10(12):e0143814.
https://doi.org/10.1371/journal.pone.0143814
PMid:26629991 PMCid:PMC4668014
|
|
|
|
|
28 Pei H, Yang Y, Cui L, Yang
J, Li X, Yang Y, Duan H. Bisdemethoxycurcumin inhibits ovarian cancer via
reducing oxidative stress mediated MMPs expressions. Sci Rep 2016;6:28773.
https://doi.org/10.1038/srep28773
PMid:27349797 PMCid:PMC4923879
|
|
|
|
|
29 Hochrainer K, Pejanovic N,
Olaseun VA, Zhang S, Iadecola C, Anrather J. The ubiquitin ligase HERC3
attenuates NF-κB-dependent transcription independently of its enzymatic
activity by delivering the RelA subunit for degradation. Nucleic Acids Res
2015;43(20):9889-9904.
https://doi.org/10.1093/nar/gkv1064
PMid:26476452 PMCid:PMC4787756
|
|
|
|
|
30 Huang W, Xu L, Zhou X, Gao
C, Yang M, Chen G, Zhu J, Jiang L, Gan H, Gou F, Feng H, Peng J, Xu Y. High
glucose induces activation of NF-κB inflammatory signaling through IκBα
sumoylation in rat mesangial cells. Biochem Biophys Res Commun
2013;438(3):568-574.
https://doi.org/10.1016/j.bbrc.2013.07.065
PMid:23911785
|
|
|
|
|
31 Castorálová M, Březinová D,
Svéda M, Lipov J, Ruml T, Knejzlík Z. SUMO-2/3 conjugates accumulating under
heat shock or MG132 treatment result largely from new protein synthesis.
Biochim Biophys Acta 2012;1823(4):911-919.
https://doi.org/10.1016/j.bbamcr.2012.01.010
PMid:22306003
|
|
|
|
|
32 Liu Q, Li J, Khoury J,
Colgan SP, Ibla JC. Adenosine signaling mediates SUMO-1 modification of
IkappaBalpha during hypoxia and reoxygenation. J Biol Chem
2009;284(20):13686-13695.
https://doi.org/10.1074/jbc.M809275200
PMid:19297320 PMCid:PMC2679470
|
|
|
|
|
33 Hwang JW, Yao H, Caito S,
Sundar IK, Rahman I. Redox regulation of SIRT1 in inflammation and cellular
senescence. Free Radic Biol Med 2013;61:95-110.
https://doi.org/10.1016/j.freeradbiomed.2013.03.015
PMid:23542362 PMCid:PMC3762912
|
|
|
|
|
34 Li J, Xu Y, Long XD, Wang W,
Jiao HK, Mei Z, Yin QQ, Ma LN, Zhou AW, Wang LS, Yao M, Xia Q, Chen GQ. Cbx4
governs HIF-1α to potentiate angiogenesis of hepatocellular carcinoma by its
SUMO E3 ligase activity. Cancer Cell 2014;25(1):118-131.
https://doi.org/10.1016/j.ccr.2013.12.008
PMid:24434214
|
|
|
|
|
35 Han X, Wang XL, Li Q, Dong
XX, Zhang JS, Yan QC. HIF-1α SUMOylation affects the stability and
transcriptional activity of HIF-1α in human lens epithelial cells. Graefes
Arch Clin Exp Ophthalmol 2015;253(8):1279-1290.
https://doi.org/10.1007/s00417-015-2999-x
PMid:25877955
|
|
|
|
|
36 Yu J, Wu Y, Yang P. High
glucose-induced oxidative stress represses sirtuin deacetylase expression and
increases histone acetylation leading to neural tube defects. J Neurochem
2016;137(3):371-383.
https://doi.org/10.1111/jnc.13587
PMid:26896748 PMCid:PMC4837015
|
|
|
|
|
37 Caito S, Rajendrasozhan S,
Cook S, Chung S, Yao H, Friedman AE, Brookes PS, Rahman I. SIRT1 is a
redox-sensitive deacetylase that is post-translationally modified by oxidants
and carbonyl stress. FASEB J 2010;24(9):3145-3159.
https://doi.org/10.1096/fj.09-151308
PMid:20385619 PMCid:PMC2923349
|
|
|
|
|
38 Carbia-Nagashima A, Gerez J,
Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E.
RSUME, a small RWD-containing protein, enhances SUMO conjugation and
stabilizes HIF-1alpha during hypoxia. Cell 2007;131(2):309-323.
https://doi.org/10.1016/j.cell.2007.07.044
PMid:17956732
|
|
|
|
|
39 Radak Z, Zhao Z, Koltai E,
Ohno H, Atalay M. Oxygen consumption and usage during physical exercise: the
balance between oxidative stress and ROS-dependent adaptive signaling.
Antioxid Redox Signal 2013;18(10):1208-1246.
https://doi.org/10.1089/ars.2011.4498
PMid:22978553 PMCid:PMC3579386
|
|
|
|
|
40 Kracklauer MP, Schmidt C. At
the crossroads of SUMO and NF-kappaB. Mol Cancer 2003;2:39.
https://doi.org/10.1186/1476-4598-2-39
PMid:14613580 PMCid:PMC280695
|
|
|
|
|
41 Shinozaki S, Chang K, Sakai
M, Shimizu N, Yamada M, Tanaka T, Nakazawa H, Ichinose F, Yamada Y, Ishigami
A, Ito H, Ouchi Y, Starr ME, Saito H, Shimokado K, Stamler JS, Kaneki M.
Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase
SIRT1 to increase acetylation and activation of p53 and p65. Sci Signal 2014;
7(351):ra106.
https://doi.org/10.1126/scisignal.2005375
PMid:25389371 PMCid:PMC4340581
|
|
|
|
|
42 Yeung F, Hoberg JE, Ramsey
CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent
transcription and cell survival by the SIRT1 deacetylase. EMBO J
2004;23(12):2369-2380.
https://doi.org/10.1038/sj.emboj.7600244
PMid:15152190 PMCid:PMC423286
|
|
|
|
|
43 Li X, Luo R, Chen R, Song L,
Zhang S, Hua W, Chen H. Cleavage of IκBα by calpain induces myocardial NF-κB
activation, TNF-α expression, and cardiac dysfunction in septic mice. Am J
Physiol Heart Circ Physiol 2014;306(6):H833-H843.
https://doi.org/10.1152/ajpheart.00893.2012
PMid:24441549
|
|
|
|
|
44 Sun Y, Peng R, Peng H, Liu
H, Wen L, Wu T, Yi H, Li A, Zhang Z. miR-451 suppresses the
NF-kappaB-mediated proinflammatory molecules expression through inhibiting
LMP7 in diabetic nephropathy. Mol Cell Endocrinol 2016;433:75-86.
https://doi.org/10.1016/j.mce.2016.06.004
PMid:27264074
|
|
|
|
|
45 Tang X, Yao K, Zhang L, Yang
Y, Yao H. Honokiol inhibits H(2)O(2)-induced apoptosis in human lens
epithelial cells via inhibition of the mitogen-activated protein kinase and
Akt pathways. Eur J Pharmacol 2011;650(1):72-78.
https://doi.org/10.1016/j.ejphar.2010.09.076
PMid:20965163
|
|
|
|
|
46 Keshk WA, Zineldeen DH,
Wasfy RE, El-Khadrawy OH. Fatty acid synthase/oxidized low-density
lipoprotein as metabolic oncogenes linking obesity to colon cancer via
NF-kappa B in Egyptians. Med Oncol 2014;31(10):192.
https://doi.org/10.1007/s12032-014-0192-4
PMid:25173531
|
|
|
|
|
47 Bossis G, Melchior F. SUMO:
regulating the regulator. Cell Div 2006;1:13.
https://doi.org/10.1186/1747-1028-1-13
PMid:16805918 PMCid:PMC1524954
|
|
|
|
|
48 Tong C, Morrison A, Mattison
S, Qian S, Bryniarski M, Rankin B, Wang J, Thomas DP, Li J. Impaired SIRT1
nucleocytoplasmic shuttling in the senescent heart during ischemic stress.
FASEB J 2013;27(11): 4332-4342.
https://doi.org/10.1096/fj.12-216473
PMid:23024374 PMCid:PMC3804750
|
|
|
|
|
49 Yang Y, Fu W, Chen J,
Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates
its deacetylase activity and cellular response to genotoxic stress. Nat Cell
Biol 2007;9(11):1253-1262.
https://doi.org/10.1038/ncb1645
PMid:17934453 PMCid:PMC3201724
|
|
|
|
|
50 Zhang X, Jiang A, Qi B, Ma
Z, Xiong Y, Dou J, Wang J. Resveratrol protects against Helicobacter pylori-
associated gastritis by combating oxidative stress. Int J Mol Sci
2015;16(11):27757-27769.
https://doi.org/10.3390/ijms161126061
PMid:26610474 PMCid:PMC4661919
|
|
|
|
|
51 Liu H, Yu S, Xu W, Xu J.
Enhancement of 26S proteasome functionality connects oxidative stress and
vascular endothelial inflammatory response in diabetes mellitus. Arterioscler
Thromb Vasc Biol 2012;32(9):2131-2140.
https://doi.org/10.1161/ATVBAHA.112.253385
PMid:22772755 PMCid:PMC3432586
|
|
|
|
|
52 Pereira TM, Pimenta FS,
Porto ML, Baldo MP, Campagnaro BP, Gava AL, Meyrelles SS, Vasquez EC.
Coadjuvants in the diabetic complications: nutraceuticals and drugs with
pleiotropic effects. Int J Mol Sci 2016;17(8):pii:E1273.
https://doi.org/10.3390/ijms17081273
PMid:27527163 PMCid:PMC5000671
|
|






