
Citation: Puspitasari A, Handayani N. Broccoli sprouts juice
prevents lens protein aggregation in streptozotocin-induced diabetic rat. Int
J Ophthalmol 2019;12(9):1380-1385
DOI:10.18240/ijo.2019.09.02
·Basic Research·
Broccoli sprouts juice prevents lens protein aggregation in streptozotocin-induced diabetic rat
Ayu Puspitasari, Nina Handayani
Department of Ophthalmology, Faculty of Medicine, Universitas Brawijaya, Dr. Saiful Anwar General Hospital, Malang 65145, Indonesia
Correspondence to: Nina Handayani. Department of Ophthalmology, Faculty of Medicine, Universitas Brawijaya, Dr. Saiful Anwar General Hospital, Malang 65145, East Java, Indonesia. ninahdyn@gmail.com
Received:
Abstract
AIM: To investigate the effect of broccoli sprouts juice in preventing lens protein aggregation in diabetic rat model.
METHODS: Totally 25 male Wistar rats were divided into
negative control group, diabetic group without juice treatment as positive
control and diabetic group given broccoli sprouts juice 0.25, 0.5 and
RESULTS: Positive control group had the highest cataract
score and lens aggregated αB-crystallin expression. Broccoli sprout juice dose of
CONCLUSION: Broccoli sprout juice has a significant effect in preventing lens protein aggregation in diabetic rat model. The higher dose gives better visual cataract scores, lower lens aggregated αB-crystallin expression and higher lens native αB-crystallin expression.
KEYWORDS: diabetic cataract; broccoli sprouts; oxidative stress; lens αB-crystallin; rat
DOI:10.18240/ijo.2019.09.02
Citation: Puspitasari A, Handayani N. Broccoli sprouts juice prevents lens protein aggregation in streptozotocin-induced diabetic rat. Int J Ophthalmol 2019;12(9):1380-1385
INTRODUCTION
Cataract is the leading cause of blindness in the world and diabetes mellitus is one of the risk factors. Studies have shown that chronic hyperglycemia can induce excessive production of reactive oxygen species (ROS), which plays a role in the pathogenesis of diabetes complications, including diabetic cataracts. Cataract extraction with intraocular lens implantation, is the main treatment for diabetic cataract. However, surgery can also provide higher postoperative complications in patients with diabetes. So, it is important to develop effective therapeutic strategies to prevent and inhibit the progression of diabetic cataracts, including multivitamins and antioxidants[1-3].
Broccoli is a source of bioactive compounds, such as carotenoids, phenolic compounds, vitamin C, and mainly glucosinolate. Broccoli sprouts contain greater antioxidant activity than adult broccoli, particularly because the 4-methysulphinylbutyl glucosinolate (glucoraphanin) content is 10-100 times greater. Sulforaphane (SFN) is a hydrolytic conversion product of glucoraphanin by myrosinase. SFN is a very potent long acting antioxidant and has been established as a cellular defense enhancer, to suppress oxidative stress. Oxidative stress due to chronic hyperglycemia can damage the lens protein so that the lens protein undergoes aggregation and results in loss of lens transparency. So, the aim of this study was to investigate the effect of broccoli sprout juice in preventing lens protein aggregation in diabetes rat model[4-6].
MATERIALS AND METHODS
Ethical Approval All experiments in this study were performed with approval from Health Research Ethics Committee of Faculty of Medicine, Universitas Brawijaya. And all animal procedures complied with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research.
Induction of Diabetes and
Treatment Six-week-old male Rattus novergicus
Wistar strain rats (200±
Cataract Grading Cataract score of each eye were examined at the end of the study before rats were sacrificed on dilated pupil using a slit lamp biomicroscope (Topcon DC3) with 25× magnification. The visual cataract score were assessed according to the following classification: normal clear lens (grade 0); peripheral vesicles (grade 1); peripheral vesicles and cortical opacities (grade 2); diffuse central opacities (grade 3); and mature cataract (grade 4)[7].
Lens Collection and Western Blot
Analysis Rats were sacrificed and the lenses
were dissected using posterior approach method. The lens was washed with
ice-cold saline to remove blood, then stored at
Statistical Analysis SPSS 22.0 software was used to analyze the data. All data were expressed as mean±SD and analyzed by one way ANOVA with post hoc test, correlation test, and simple linear regression. A P value less than 0.05 was considered statistically significant.
RESULTS
Fasting Blood Sugar level At the end of treatment, positive control group remained hyperglycemic (FBS>150 mg/dL) and negative controls still had normal FBS. No rats in our study died. Treatment groups 1, 2 and 3 experienced significant decrease in FBS levels compared to the positive control group (P<0.05), although there was no significant difference among treatment groups 1, 2, and 3. The broccoli sprout juice significantly reduces FBS in diabetic rat models (correlation coeff.: -0.697, P<0.05; R2: 0.461, P<0.05).
Cataract Grading Positive control group had the highest cataract score and remarkable difference compared to the other groups (P<0.05), and treatment group 3 had the mildest cataract score compared to treatment groups 1 and 2 however there was no significant difference among treatment groups 1, 2 and 3. The broccoli sprout juice considerably reduces cataract score in diabetic rat models (correlation coeff.: -0.891, P<0.05; R2: 0.793, P<0.05).
Expression of αB-crystallin in
Lens Positive control group had the
lowest expression of lens native αB-crystallin, and significant difference from
the treatment groups 1, 2, and 3 (P<0.05), opposite to lens
aggregrated αB-crystallin expression (Table 1, Figures 1-2). The positive
control group was remarkably different from the treatment group 3 (P<0.05),
but there was no significant difference from treatment groups 1 and
Table 1 Average results for each group
Groups |
Final FBS (mg/dL) |
Cataract score |
Lens native αB-crystallin (% band) |
Lens aggregated αB-crystallin (% band) |
Negative control |
115.2±
|
0±
|
8.78±
|
1.64±
|
Positive control |
409±128.88b |
5.8±0.84b |
0.46±0.17b |
12.54±3.33b |
Treatment 1 |
203.6±
|
3.2±
|
1.48±
|
11.08±3.56b |
Treatment 2 |
172.4±
|
2.4±
|
1.82±
|
8.82±2.40b |
Treatment 3 |
147±
|
1±
|
5.98±
|
3.68±
|
Data are expressed as mean±SD.
Negative control: Normal rats (n=5); Positive control: Diabetic rats (n=5);
Treatment 1: Diabetic rats+
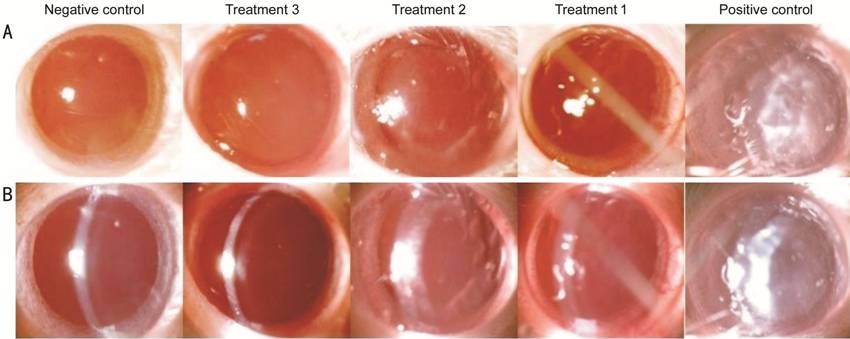
Figure 1 Picture of lens opacity of each group The lens was observed by slit lamp biomicroscope on diffuse illumination (A) and slit illumination (B). Data are expressed as representative images of each group.
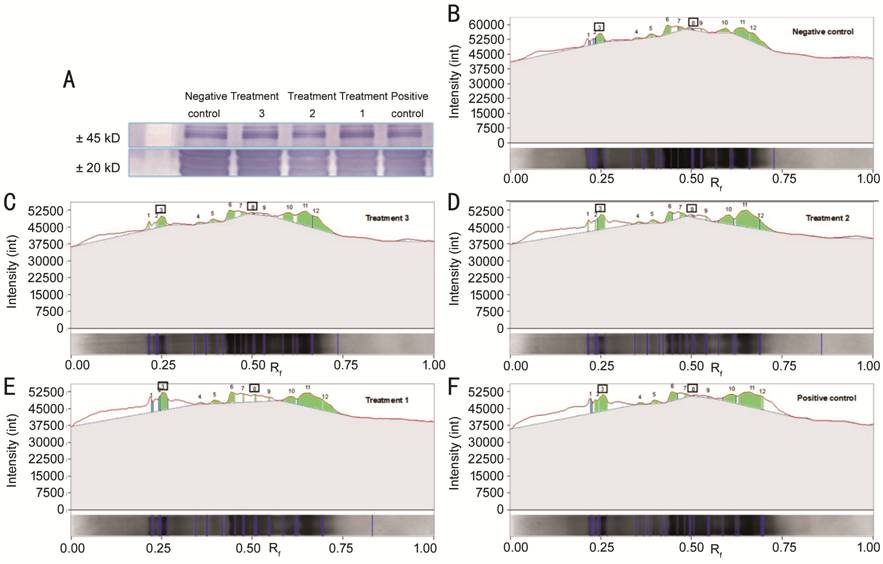
Figure 2 Immunoblot expression of
lens αB-crystallin of each group A: Immunoblot expression
image of lens αB-crystallin at ±20 kD (lower image) and ±45 kD (upper
image) molecular weight (Mw). B-F: Image of quantification of band intensity by
gel analyzing software. Number
DISCUSSION
Effects of Broccoli Sprout Juice on FBS Levels in Diabetic Rat Models STZ causes selective destruction of pancreatic β cells that produce insulin, resulting in insulin deficiency and hyperglycemia. In this study, we used a basic protocol for induction of diabetic animals with single dose STZ 65 mg/kg. In this study, it was found that in the broccoli sprout juice treatment group appeared to have a statistically significant decrease in the final FBS level compared to the positive control group and the larger dose of broccoli sprout juice given turned out to provide a greater decrease in final FBS. The fact that there was also a statistically significant relationship and effect between the dose of broccoli sprout juice and final FBS levels in diabetic rat models could be a secondary finding in this study[8-9]. Broccoli sprout is a source of bioactive compounds, including glucosinolate (SFN) and flavonoids. In addition to antioxidant effects, SFN has also been reported to be effective in conditions related to inflammation through inhibition of NF-κB activation. The mechanism of SFN in glucose metabolism is shown by Song et al[10], in STZ-induced diabetic rat model, which showed a decrease in reactive oxygen species (ROS) production and inhibition of the NF-κB pathway in pancreas, which maintains insulin secretion. Another study by Axelsson et al[11] also showed that SFN reduced glucose production, through NRf2 translocation and decreased expression of important enzymes (PCK1) in gluconeogenesis, besides that SFN also protects against complications of diabetes in animal models due to its antioxidant effects[10-11].
Effects of Broccoli Sprout Juice on Cataract Score in Diabetic Rat Models There was a significant difference between the positive control group and the treatment group. In addition, there was also a significant and negative correlation between the doses of broccoli sprout juice and the cataract score in diabetic rat models. The higher doses of broccoli sprout juice give better visual cataract scores. According to our theory and hypothesis, broccoli sprout juice containing many antioxidant compounds reduces ROS and prevents lens protein aggregation that cause lens opacities. The antioxidant effect produced by broccoli sprout juice is derived from SFN and other phenolic compounds such as flavonoids enhancing the defense mechanism against oxidative stress through the Nrf2/ARE pathway. Both of these bioactive compounds increase Nrf2 translocation to the nucleus which causes Nrf2 bind to ARE and activate the transcription of several antioxidant genes, including glutathione. In addition, high antioxidants are also able to reduce blood sugar levels through NRf2 translocation and partly through a decrease in the expression of important enzymes in gluconeogenesis, The lower blood sugar levels also minimizes sorbitol accumulation resulting in lens opacities[6,12-13].
Effects of Broccoli Sprout Juice on Lens αB-crystallin Expression in Diabetic Rat Models The α-crystallin is the most dominant protein in the lens, has a monomer molecular weight of about 20 kD, so the bands at the 20 kD in the gel are most likely to be native αB-crystallin. Whereas the expression of lens αB-crystallin in bands with greater intensity at higher molecular weights around 45-50 kD may be an aggregation of the complex heteromic αB-crystallin[14-15].
α-crystallin plays an important role in preserving lens transparency, not only as a structural protein, but also as a lens chaperone, conserving proteins in their native state. Crystallin is a stable protein, tightly folded but undergoes major non-enzymatic modifications to its structure and function since prenatally which causes crystallin to be unfolded. Denatured and unfolded proteins are susceptible to oxidation. Oxidation of αA- and αB-crystallin may also occur, triggering structural changes and loss of chaperone activity. Oxidative attacks on unfolded or otherwise modified crystallin cause cross-linking, insolubility and formation of high molecular weight aggregates, leading to loss of lens transparency[16-24].
The high concentration of crystallin protein determines the high refractive index needed for transparency of the lens. In a study by Rumyantseva et al[16], showed that the early stages of cataract development were characterized by a significant reduction in the expression of the αA- and αB-crystallin genes in OXYS mice, which were increasingly apparent with cataract progression. This result is also consistent with several studies by Yang et al[25-26].
However, several studies have shown that the expression of the αB-crystallin gene is associated with oxidative stress. In a study by Kumar et al[19], which investigated the increase of αA- and αB-crystallin expression in STZ-induced diabetic rats in various tissues compared to controls where increased oxidative stress seems to be the main stimulus. Persistent hyperglycemia during uncontrolled diabetes is known to cause oxidative stress. An increase in αA- and αB-crystallin expression may be a cellular adaptation as a protective mechanism against oxidative stress. Whereas in the study by Kamei et al[20], it was shown that αA- and αB-crystallin phosphorylation increased after cataracts occurred in rat lenses[18-27].
In our study we found that the reduction of native αB-crystallin lens expression accompanied by an increase in αB-crystallin aggregation in the positive control group, compared to the negative control group and the difference was statistically significant. Actually αA- and αB-crystallin are included in the small heat shock protein (sHsp) family which is triggered or enhanced by heat stress, osmotic stress and oxidative stress. It is possible that there has been an increase in lens α-crystallin levels as a cellular response to stress caused by conditions of hyperglycemia. Increased expression of α-crystallin is an adaptation of lens cells that is very trivial and vital as a mechanism of protection against environmental and/or metabolic stress. However, it may be an increase in α-crystallin which functions as a chaperone unbalanced with the aggregation of lens proteins due to existing oxidative stress. Oxidative stress causes phosphorylation of lens crystallin including α-crystallin. Oxidized αA- and αB-crystallin trigger their own structural changes and loss of chaperone activity, so they are unable to prevent the aggregation of other proteins or their own proteins which in this study are indicated by a decrease in native αB-crystallin expression and an increase in aggregated αB-crystalline, which subsequently impacts lens opacities[18-28].
In this study we also found that the broccoli sprout juice prevented the αB-crystallin lens aggregation and the loss of native αB-crystallin lens. It seems that giving broccoli sprout juice with its antioxidant content may suppress oxidative stress which results in reduction of lens protein aggregation. So, if there is no balance between an increase in α-crystallin as a chaperone and lens protein aggregation occurring due to existing oxidative stress, damage to the lens continues to accumulate by time and lens opacities/cataracts cannot be avoided in the future.
In our study, we found that rats with the highest glucose levels had the most severe cataracts, the lowest expression of lens native αB-crystallin and the highest expression of lens aggregated αB-crystallin compared to groups with lower glucose levels. We assume that the effect of broccoli sprout on cataract formation is through both directly to the lens and through regulation of blood glucose, and it is not guaranteed which one was superior between the two. As in the theory where SFN is able to reduce blood glucose levels in part through Nrf2 translocation and partly through a decrease in the expression of important enzymes in gluconeogenetic. But the inhibition of cataract formation can also be caused due to the direct effect of antioxidant content in broccoli sprout (SFN, and other phenolic compounds) which reduces oxidative stress through activation of the Nrf2/ARE pathway and prevents aggregation of lens proteins that cause lens opacity. We also observed that the degree of cataract was not the same in both eyes in one rat, even though their blood glucose level was the same. This is also because oxidative stress that exists will affect many organs in the body, one of them is the lens. So that lens opacities that occur due to oxidative stress are also not necessarily the same in the same oxidative stress conditions[12-13].
This study cannot be separated from several limitations. This research is first study conducted to investigate the effect of broccoli sprout juice on aggregation of lens proteins, so that the dosage used and its variation apparently lacks a significant range in providing the effect of preventing the aggregation of lens proteins. We also did not measure antioxidant levels and content in a broccoli sprout juice. In addition, in this study, we only conducted an examination at the end of the study (not done regularly), so we were not able to assess the onset and progression of the diabetic cataract itself. Further research is needed by adding dose variations and also examining the antioxidant content so that effective doses can be determined and the antioxidant content playing a role in maintaining the expression of lens αB-crystallin in each gram dose of broccoli sprout juice used can be better understood. In addition, it is also necessary to do regular checks starting with earlier observation, for example observations in the first, second and subsequent weeks to observe the effect of broccoli sprout juice on inhibiting the onset and progression of cataracts.
In summary, our data indicated that broccoli sprout juice administration has a significant effect in preventing lens protein aggregation in diabetic rat models. The higher dose, give better visual cataract scores, lower aggregated lens αB-crystallin expression and higher native lens αB-crystallin expression.
ACKNOWLEDGEMENTS
We are grateful to Laboratorium BioSains dan Laboratorium Sentral Ilmu Hayati of Faculty of Medicine, Universitas Brawijaya for facilitating this research. Then we also would like to thank all those whose assistance proved to be a milestone in the accomplishment of our research. Our special thanks to the head of Department of Ophthalmology Faculty of Medicine Universitas Brawijaya, Dr. Saiful Anwar General Hospital for the permission to this research.
Conflicts of Interest: Puspitasari A, None; Handayani N, None.
REFERENCES