
·Basic Research·
Blockade
of insulin receptor substrate-1 inhibits biological behavior of choroidal
endothelial cells
Yi-Yong Qian1,2, Hong-Ya Wu3,
Gao-Qin Liu1,3, Chi Ren1, Pei-Rong Lu1,3,
Xue-Guang Zhang3
1Department of Ophthalmology, the
First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu
Province, China
2Department of Ophthalmology,
Shanghai Tenth People’s Hospital, Tongji University School of Medicine,
Shanghai 200072, China
3Jiangsu Key Laboratory of Clinical
Immunology, the First Affiliated Hospital of Soochow University, Suzhou 215006,
Jiangsu Province, China
Co-first author: Yi-Yong Qian and Hong-Ya Wu
Corresponding to: Pei-Rong Lu and Gao-Qin Liu.
Department of Ophthalmology, the First Affiliated Hospital of Soochow
University, 188 Shizi Street, Suzhou 215006, Jiangsu Province, China.
lupeirong@suda.edu.cn; liugaoqin2006@sina.com
Received: 2018-07-27
Accepted: 2018-12-15
Abstract
AIM: To investigate the effects of blockade of insulin receptor substrate-1
(IRS-1) on the bio-function of tube formation of human choroidal endothelial
cells (HCECs).
METHODS: Quantitative reverse transcription-polymerase chain
reaction (RT-PCR) and Western blot were performed to determine the expression
level of IRS-1 and phospho-IRS-1 in
HCECs. Tube formation of HCECs was analyzed using three dimensional in vitro
Matrigel assay with or without IRS-1 blockage via IRS-1 inhibitor
(GS-101) and vascular endothelial growth factor receptor 2 (VEGFR2) inhibitor.
In addition, cell counting kit (CCK)-8 and Transwell migration assay were
exerted to analyze the effects of blockade of IRS-1 on the bio-function of
proliferation and migration of HCECs, respectively. The apoptosis of HCECs was
examined using flow cytometry (FCM).
RESULTS: RT-PCR and Western blot revealed that IRS-1
phospho-IRS-1 were expressed in HCECs and the expression level was enhanced by
stimulation of VEGF-A. The number of tube formation was decreased significantly
in GS-101 treated groups compared to phosphate buffered saline (PBS) treated
control groups. Furthermore, both cell proliferation and migration of HCECs
were decreased in the presence of GS-101. FCM analysis showed that the
apoptosis of HCECs was enhanced when the cells were treated with GS-101.
Western blot also showed that the expression level of cleaved-caspase 3 in GS-101 treated group was higher than
that in control group.
CONCLUSION: Blockade of IRS-1 can inhibit tube formation of
HCECs through reducing cell proliferation and migration and promoting cell
apoptosis.
KEYWORDS: insulin receptor substrate-1;
choroidal endothelial cells; neovascularization; proliferation
DOI:10.18240/ijo.2019.09.03
Citation: Qian
YY, Wu HY, Liu GQ, Ren C, Lu PR, Zhang XG. Blockade of insulin receptor
substrate-1 inhibits biological behavior of choroidal endothelial cells. Int
J Ophthalmol 2019;12(9):1386-1394
INTRODUCTION
Choroidal neovascularization (CNV),
known as a crucial late complication for pathological myopia and wet
age-related macular degeneration (AMD), is characterized by new capillary
vessels growing in choroid and breaking through Bruch’s membrane and further
growing into the subretinal space, frequently causing severe visual impairment[1-4]. It is well known that targeting on
vascular endothelial growth factor (VEGF)-A is currently the most efficient
treatment to exudative AMD in clinical settings, despite numerous regulatory
factors involved in angiogenic, inflammatory and immune modulatory cascade have
been confirmed in CNV occurrence and development[5-8]. However, anti-VEGF agents can also induce local and
systemic side effects[9]. Therefore, in order to
create novel therapeutic targets, it is necessary to obtain better knowledge of
the mechanisms of CNV development[10].
Insulin receptor substrate (IRS)-1,
currently known as the first indentified member of IRS protein (or cytoplasmic
adaptor proteins) family, is extensively expressed in various mammalian cells[11]. It functions as crucial ligand in triggering
insulin-induced response in human cells by binding to its cognate receptor[11-12]. Like other members in IRS protein family,
IRS-1 has no intrinsic enzymatic property but it can be activated after
phosphorylation[12]. It plays key role in
lifespan determination and cellular stress resistance[13-14], adipogenesis[15],
glucose homeostasis[16-17] and
cancer metabolism[18] under physiological and
pathological conditions. Accumulating evidence indicated that IRS-1 has an
important role in occurrence and development of neovascularization in some
ocular neovascularization diseases[19-21].
It was reported that the occurrence of experimental CNV was restrained in IRS-1
knockout mice when compared to that in wild type mice[21].
Furthermore, there was evidence also showing that application of the antisense
oligonucleotide targeting on human IRS-1 mRNA, GS-101 (5’-TATCCGGAGGGCTCGCCATGCTGCT-3’), acting as IRS-1 inhibitor, can suppress
corneal neovascularization significantly both in experimental corneal
neovascularization animal models and patients with keratitis[22-24]. These findings have proven and highlighted the
anti-angiogenic efficacy of IRS-1 inhibitor in treating ocular
neovascularization diseases.
Though IRS-1 has an important role
in some ocular neovascularization diseases, the mechanism underlying IRS-1
promoting neovascularization or IRS-1 inhibitor suppressing neovascularization
in this process has not yet been fully elucidated. Some reports revealed that
the pro-angiogenic effects of IRS-1 may be related to interaction with VEGF-A[21,25] and integrin signaling[26-27], but it still need further
exploration to verify and delineate the mechanism of this process. In present
work, we detected the influences of blockade of IRS-1 on capabilities of tube
formation, proliferation and migration of HCECs, and further examined the gene
and protein expression of cytokines associated with tube formation of HCECs,
then analyzed the exact mechanisms of blockade of IRS-1 affecting the
bio-function of HCECs in this process. Our findings provide a novel insight
into the mechanism of IRS-1 being involved in ocular neovascularization.
MATERIALS AND METHODS
Reagents And Antibodies The cell line of HCECs were obtained
from Yaji Biological Technologies (Shanghai, China). IRS-1 inhibitor of GS101
was obtained from Invitrogen Life Technologies (Carlsbad, CA, USA). Annexin
V-FITC Assay Kit (cat. No.556547) was obtained from BD Biosciences (Franklin
Lakes, NJ, USA). The cell counting kit (CCK)-8 was obtained from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). Primers were synthesized by Shanghai
Sangon Biological Engineering Technology and Service Co., Ltd. (Shanghai,
China). Trypsin-EDTA was obtained from Sigma-Aldrich; Merck Millipore
(Darmstadt, Germany). The total RNA extraction kit (RNeasy Mini kit) and
reverse transcription kit (Ominiscript RT kit) were obtained from Qiagen
Sciences, Inc. (Frederick, MA, USA). Matrigel was obtained from BD Biosciences
(Franklin Lakes, NJ, USA). Dulbecco’s modified Eagle’s medium (DMEM) was
obtained from HyClone; GE Healthcare Life Sciences (Logan, UT, USA). Fetal
bovine serum (FBS) was obtained from PAA Laboratories; GE Healthcare Life
Sciences. Transwell plates with 8.0 µm pore polycarbonate membrane insert were
obtained from Corning Life Science (New York, USA). Mouse anti-human
phospho-IRS-1 antibody (cat. No.3105-100) as well as rabbit anti human IRS-1
antibody (cat. No.3424-100) were purchased from BioVision (Milpitas, CA, USA).
Rabbit anti- human phospho-vascular endothelial growth factor receptor 2
(VEGFR2) antibody (cat. No.44-1052), rabbit anti-human VEGFR2 antibody (cat.
No.MA5-15157), rabbit anti-human cleaved-caspase 3 (cat. No.PA5-23921) and
rabbit anti-human caspase 3 antibody (cat. No.700182) were obtained from Thermo
Fisher Scientific (Waltham MA, USA). Mouse anti-human VEGFR2 antibody (cat.
No.GTX53462) was obtained from GeneTex (Irvine, CA, USA). Mouse anti- human
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (cat. No.AF0006) was
obtained from Beyotime Institute of Biotechnology (Shanghai, China).
Cell Culture Human choroidal endothelial cells
(HCECs) were cultured in 5 mL DMEM medium (Gibco, Shelton, CT, USA) containing
10% FBS (Gibco) and incubated in 37℃
incubator with humidified atmosphere of 5 percent CO2 and 95
percent air[28]. Fresh culture medium was then
added into the HCECs the next day. After incubation for 3-4d, confluent cells
were passaged at a 1:4 dilution, and culture medium was renewed every other
day. Functional assays in this study were performed using the cells in their
logarithmic growth phase. In some experiments, cells were treated with VEGF-A
and IRS-1 inhibitor (GS-101) at indicated concentrations or phosphate buffered
saline (PBS) as control for 12 or 24h. The proliferation assay and migration
assay were conducted in serum-free DMEM.
Semi-Quantitative Reverse
Transcription-Polymerase Chain Reaction Analysis Quantitative reverse
transcription-polymerase chain reaction (RT-PCR) analysis was performed as described
in detail previously[29]. Total RNA from the
HCECs was isolated using an RNeasy Mini kit (Qiagen, Inc. Frederick, MA, USA)
and cDNA was synthesized with the PrimeScriptTM RT Master mMix
(TaKaRa Biotechnology Co., Ltd., Dalian, China). The mRNAs encoding IRS-1,
VEGF-A, VEGFR2 as well as GAPDH were amplified using
appropriate convenient primers. The sequences of the PCR primer pairs are
listed in Table 1. Amplification of PCR was performed using a GeneAmp®
PCR System 9700 (Perkin-Elmer, Foster City, CA, USA). The PCR parameters
involved in initial denaturation at 94℃ for
2min, followed by 37 or 25 (for GAPDH) cycles of denaturation at 95℃
for 30s, annealing at 58℃ for 35s
and extension at 72℃ for 35s, and a final
extension at 72℃ for 10min. Each sample was
assayed in triplicate for both target and internal control (GAPDH)
genes. These PCR products were fractionated on a 1.0% agarose gel and
visualized using ethidium bromide. The intensities of the bands were determined
and their ratios to GAPDH determined using Image J software, version 2.1.4.7
(National Institutes of Health, Bethesda, MD, USA).
Table 1 Sequences of the primers
used for reverse transcription-polymerase chain reaction analysis
|
Gene
|
Sequence (5´-3´)
|
Annealing temperature (℃)
|
Cycles (n)
|
|
IRS-1
|
(F) GCAACCAGAGTGCCAAAGTG
(R) CCTCTGGCTGCTTCTGGAAA
|
58
|
37
|
|
VEGF-A
|
(F) TGGTCCCAGGCTGCACCCAT
(R) CGCATCGCATCAGGGGCACA
|
58
|
37
|
|
VEGFR2
|
(F) GGTACATGCCAACGACACAG
(R) CTCAAAGTCTCTCACGAACACG
|
58
|
37
|
|
GAPDH
|
(F) ACCACAGTCCATGCCATCAC
(R) TCCACCACCCTGTTGCTGTA
|
58
|
25
|
F: Forward primer; R: Reverse
primer.
Tube Formation Assay The in vitro capillary-like
tube formation assay for assessment of the effect of blockade of IRS-1 on the
HCECs was examined using matrigel matrix as described in a previous report with
some modifications[30]. Briefly, a 96-well plate
was incubated on ice and coated with 50 μL per well of fully thawed MatrigelTM.
The samples were centrifuged at 300×g for 10min at 4℃
to remove the air bubbles. The samples were subsequently incubated at 37°C for 30min in order to allow matrigel
solidification. HCECs were cultured in different medium with or without GS-101
and/or VEGFR2 inhibitor. The cells were seeded on the solidified matrigel
immediately at a density of 1.5×104 cells per well. The plates were
placed in a humidified atmosphere of 5% CO2 and 95% air at 37℃
for 12h to allow formation of capillary-like structures. Angiogenesis is the
formation of capillary tubes and was assessed following 12h of cultivation. The
tube-like capillary structures were examined under an Olympus TMS inverted
phase contrast microscope (Olympus Corporation, Tokyo, Japan). The micrographs
were captured using an Olympus digital camera.
Western Blot Analysis As described in detail previously[31], HCECs were harvested using 0.25% Trypsin-EDTA. The
supernatant was discarded and lysed in 150 mL lysis buffer, to which a protease
inhibitor cocktail was added (Boehringer Mannheim, Indianapolis, IN, USA). The
samples were then boiled for 5min and separated using 12.5% SDS-polyacrylamide
gel electrophoresis under denaturing conditions. It was then electroblotted
onto a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membranes were finally incubated at room temperature
(RT) for 1h with the following antibodies: Anti- phospho-IRS-1 (1:200),
anti-IRS-1 (1:200), anti-phospho-VEGFR2 (1:1000), anti-VEGFR2 (1:1000),
anti-cleaved- caspase 3 (1:1000), anti-caspase 3 (1:1000) and anti-GAPDH
(1:5000) antibodies. The immunoblot assays were then washed with PBST and
incubated at RT for 1h with a horseradish peroxidase-labeled secondary antibody
(1:10 000; cat. No.5196-2504 or 5178-2504; R&D Systems; Hercules, CA, USA).
Enhanced chemiluminescence was used to visualize the blots (ECL Plus; Amersham;
GE Healthcare Life Sciences) according to the manufacturer’s protocol. The
intensities of the protein bands were determined and their ratios to GAPDH
determined using Image J software, version 2.1.4.7
(National Institutes of Health, Bethesda, MD, USA).
Proliferation Assay To evaluate the effect of GS-101 on
the proliferation of HCECs, we carried out the cell CCK-8 assay as described in
detail previously[32]. HCECs were seeded in a
96-well plate (2×103 cells per well). The cells were then treated
with or without IRS-1 inhibitor of GS-101. Following incubation for 24h, the
medium was replaced with fresh DMEM containing CCK-8 (10 μL per well). The
cells were subsequently incubated for an additional 2h. The absorbance was
measured at 450 nm using a microplate reader (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The inhibition rate (IR) of the proliferation of cells in the
groups was compared with the control group.
Migration Assay To evaluate
the effect of blockade of IRS-1 on the migration of HCECs cells, a modified
Boyden chamber assay was performed as described previously[33].
Briefly, 1×104 cells in 100 μL DMEM medium were seeded in the
upper chambers. After 24h incubation, the medium was replaced and fresh DMEM
medium was added. Totally 500 μL of DMEM medium were added in lower chambers and treated
with GS-101 for another 24h. The migrated HCECs were fixed prior to staining
with 0.5% crystal violet solution. Non-invading cells were swapped with a
cotton swab. The infiltrated cells were counted under a phase contrast
microscopy. Each assessment of each experimental group was repeated several times.
Flow Cytometrical Analysis Apoptosis was assayed by using the dual
staining with Annexin V: FITC (BD Biosciences, Franklin Lakes, NJ, USA) and
propidium iodide (PI)[34]. Briefly, cells were
harvested at 24h post-addition GS-101. Annexin V: FITC and PI were added to the
cellular suspension, according to the manufacturer’s instructions, and were
analyzed using a FACS Calibur flow cytometer (Becton–Dickinson, San Jose, CA,
USA). Early apoptotic cells were counted for relative apoptotic changes. All
experiments were performed at least three times. Fluorescence intensities were
determined with the help of FACS Calibur (Becton‑Dickinson, Franklin Lakes, NJ,
USA).
Statistical Analysis All data were expressed as mean±
standard error of the mean (SEM) and analysed statistically by Student’s t-test
(two-tailed) between two groups or by one-way analysis of variance (ANOVA) with
Tukey’s multiple comparison within multiple groups with statistic software SPSS
18.0 (USA). A value of P<0.05 was considered as statistically
significant. Each experiment was independently repeated at least 3 times.
RESULTS
Gene and Protein Levels of IRS-1 in HCECs RT-PCR analysis and Western Blot
assay revealed that the gene level of IRS-1 and protein level of IRS-1 as well
as phospho-IRS-1 in HCECs
was enhanced in VEGF-A-treated groups compared to PBS-treated control group
(Figure 1). These results suggested that IRS-1 may have a role in bioactivities
of HCECs and is a possible target molecule to inhibit the activities. The
observation of the expression of IRS-1 along with phospho-IRS-1 in above HCECs suggested the possible
involvement of IRS-1 in HCEC
bio-function (Figure 1).
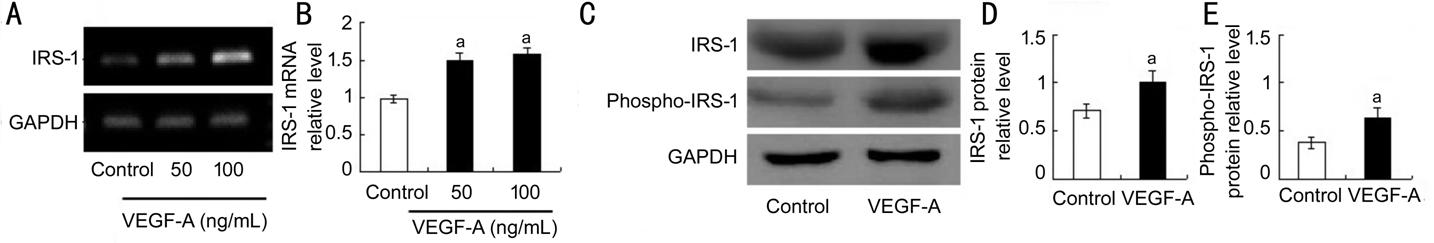
Figure 1 Expression (gene and
protein) of IRS-1 in
HCECs A: Semi-quantitative RT-PCR analysis
was applied to evaluate the mRNA expression of IRS-1; B: Quantitative data of
the ratio from three independent experiments; C: Representative Western blot
results from three independent experiments; D, E: Ratios of IRS-1 and
phospho-IRS-1 to GAPDH protein bands in the control and VEGF-A groups were
determined. All values are presented as the mean±standard error of the mean (n=3).
aP<0.05.
IRS-1 Signaling Blockade via
GS-101 Reduced Tube Formation of HCECs
To determine
whether IRS-1 had a role in activity of tube formation of HCECs, the HCEC cells
were seeded in plates of 96 wells which coated with Matrigel and were incubated
for 12h, the HCECs then formed capillary-like tubes. The results revealed that
the HCECs treated with GS-101 showed declined numbers of tube formation in
comparison with the numbers in control group (Figure 2). The results of tube
formation, which was quantified and statistically analyzed, indicated that
GS-101 suppressed tube formation. In addition, HCECs treated with GS-101 and
VEGFR2 inhibitor exhibited more decreased tube formation than the cells treated
with 40 μmol/L GS-101 alone, suggesting that VEGFR2 signaling is indispensable
for angiogenesis, and the treatment of GS-101 combined with VEGFR2 inhibitor
enhanced the anti-angiogenesis effect of blockade of IRS-1 signaling.
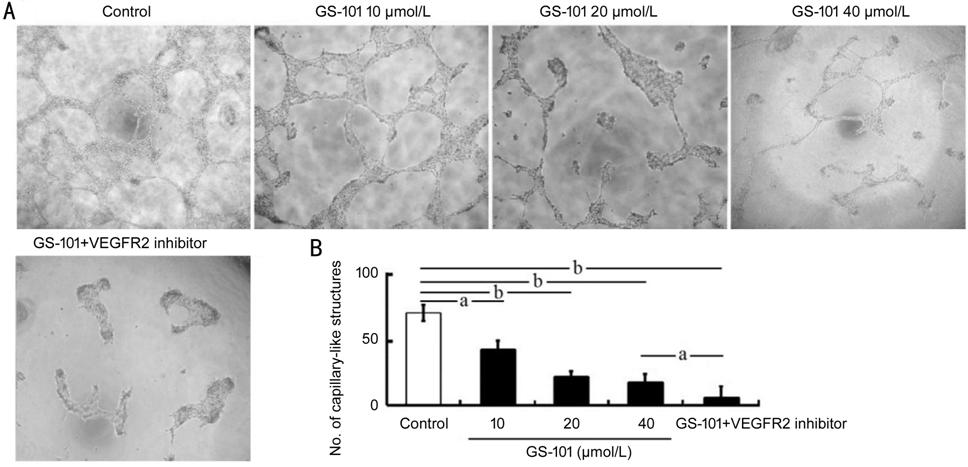
Figure 2 Effect of blockade of IRS-1
on the tube formation of HCECs A: Tube
formation assays showed that blockade of IRS-1 significantly suppressed the
tube formation of HCECs (magnification, ×200). B: The numbers of capillary-like
structures of tube formation were quantified from three independent in vitro
experiments. All data are showed as the mean±standard error of the mean (n=3).
aP<0.05; bP<0.01.
Blockade of IRS-1 Suppressed
Proliferation of HCECs To evaluate the role of IRS-1 in the bio-activities of HCECs, the effect
of blockade of IRS-1 on HCEC proliferation was assessed in vitro. The
HCECs were incubated in the presence of GS-101 for 24h, and cell viability was
subsequently examined using CCK-8 kit. The proliferation rates of HCECs which
treated with GS-101 were lower than those in the PBS treated control group
(Figure 3). The analysis data of quantified optical density (OD) and IR values
verified that GS-101 was capable of suppressing cell proliferation. It proposed
that the reduction in the proliferation degree of HCECs resulted from blockade
of IRS-1 signaling via GS-101 attributed to the IRS-1 potential of
promoting tube formation of HCEC in vitro.
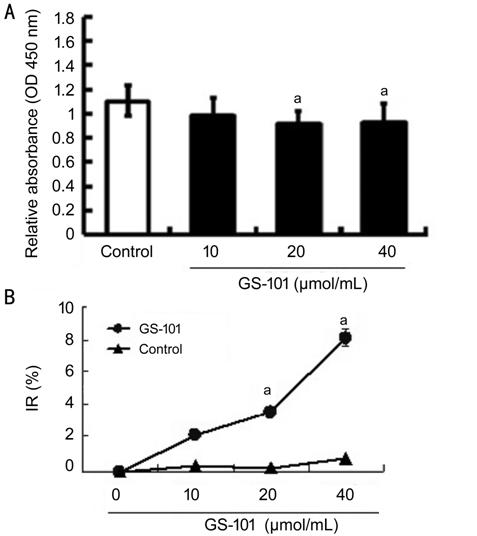
Figure 3 Effect of blockade of IRS-1
on the proliferation of HCECs Cell counting
kit-8 assays of examining HCEC proliferation showed that cell proliferation in
the GS-101 groups was reduced pronouncedly, in comparison with that in the
control group. The data are presented as mean±standard error mean. aP<0.05;
bP<0.01.
Blockade of IRS-1 Impaired Migration
of HCECs We also carried out a migration
assay to verify the effects of blockade of IRS-1 on HCECs. When co-cultured
with GS-101, the number of infiltrated HCECs which migrated through the upper
chambers of plates decreased compared to control group. The numbers of
positive-stained migrated cells in IRS-1 groups were 19±2 (10 μmol/L GS-101
group), 16±1 (20 μmol/L GS-101 group) and 15±2 (40 μmol/L GS-101 group) and
25±2 (per mm2) in control group (Figure 4). This suggests that Blockade
of IRS-1 inhibits migration of HCECs.
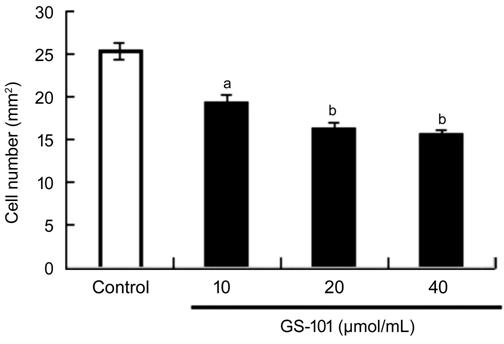
Figure 4 Effect of blockade of IRS-1 on the migration of
HCECs Quantitative data of the migrated cells from three
independent experiments. All values are presented as the mean±standard error of
the mean (n=3). aP<0.05 and bP<0.01
compared with the control.
Declined Expression Levels of VEGF-A
and its Receptor VEGFR2 in GS-101-treated HCECs In this work, the expression (gene and/or
protein) levels of angiogenic factors of VEGF-A along with its receptor of
VEGFR2 in HCECs
were detected. The expression (gene and/or protein) levels of VEGF-A, VEGFR2
and phospho-VEGFR2 were lower in the GS-101 treated cells, in comparison with
those in the PBS treated control groups (Figure 5). These in vitro
results indicated that blockade of IRS-1 may suppress HCEC migration, proliferation
and tube formation via decreasing angiogenesis by down-regulating the
gene and/or protein expression of VEGF-A and its receptor of VEGFR2 and/or
phospho-VEGFR2.
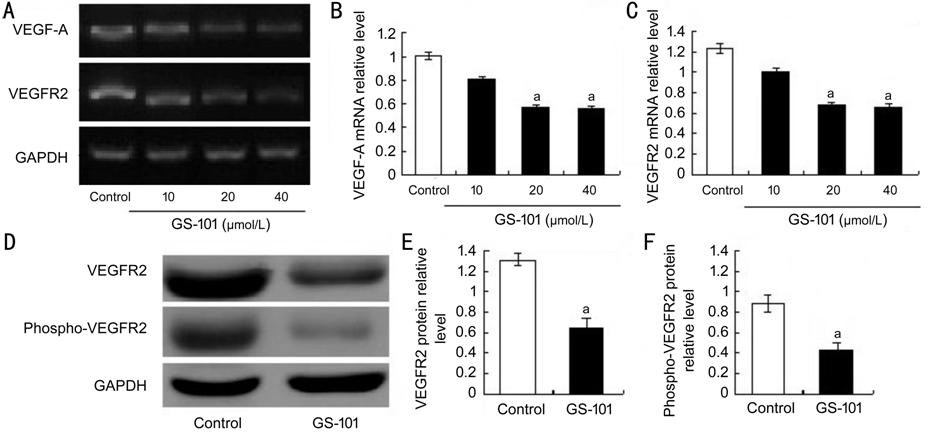
Figure 5 Effect of blockade of IRS-1 on mRNA and protein
expression of VEGF-A and VEGFR2 in
HCECS A: Semi-quantitative RT-PCR analysis was
used to evaluate the mRNA expression of VEGF-A and VEGFR2; B, C: Quantitative
data of the ratio from three independent experiments; D: Representative Western
blot results from three independent experiments; E, F: Ratios of VEGFR2 and
phospho-VEGFR2 to GAPDH protein bands in the control and GS-101 groups were
determined. All values are presented as the mean ± standard error of the mean (n=3).
aP<0.05 compared with the control.
Blockade of IRS-1 Increased
Apoptosis of HCECs As indicated by flow cytometry (FCM;
Figure 6), the GS-101 treated group exhibited higher expression level of
FITC-Annexin V in HCECs when compared to the control group, suggesting that
blockade of IRS-1 increased apoptosis of HCECs. Additional, the expression
level of apoptosis associated protein of cleaved-caspase-3 was also elevated in
GS-101 treated group in comparison to control group (Figure 6C). It showed statistically significance
between these two groups (Figure 6E). To the best of our knowledge, the level
of cleaved-caspase-3 protein expression directly reflects cell apoptosis
degree. Thus, our results suggest that GS-101 have negative effect on cell
viability by promoting cell apoptosis.
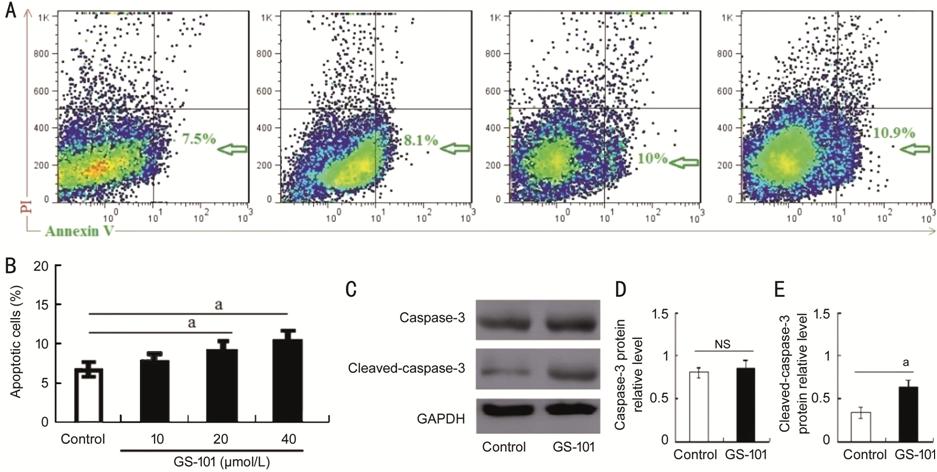
Figure 6 The proportion of Annexin V -positive HCECs and
cleaved-caspase 3 expression after treatment with GS-101 A: Representative results from three to four tests of Annexin V positive
cells from either control, GS-101 treated HCECs; B: Quantitative data of the
ratio from three independent experiments. C: Representative Western blot
results from three independent experiments; D, E: Ratios of caspase 3 and
cleaved-caspase 3 to GAPDH protein bands in the control and GS-101 groups were
determined. All values are presented as the mean±standard error of the mean (n=3).
aP<0.05 compared with the control.
DISCUSSION
The results in present study
verified that IRS-1 has an important role in tube formation, proliferation,
migration and apoptosis of HCECs, whereas this efficacy of IRS-1 on HCECs was
inhibited by IRS-1 inhibitor of GS-101. The results also demonstrated that blockade
of IRS-1 affect the bio-function of HCECs through down-regulating VEGF-A/VEGFR2
signaling. Our data implied that IRS-1 would probably be a novel target for
treating ocular neovascularization, such as CNV, in clinical settings.
As a signal receptor locating in
cytoplasm of various cells, IRS-1 can induce intracellular signaling cascade
and subsequently triggering cell bio-activities through its involvement in
various cellular signal transduction[35]. Bugner et
al[36] reported that IRS-1 was closely
involved in growth of Xenopus laevis eye. Some studies also
supported that IRS-1 played key role in process of ocular angiogenesis[37]. Berdugo et al[22]
found that the reduced expression of IRS-1
in subconjunctival tissue by injecting with IRS-1 antisense
(IRS-1 antagonist) leads to restrained corneal angiogenesis. Cloutier et al[19] revealed that IRS-1 inhibitor have anti-angiogenic
potential in suppressing retinal neovascularization (RNV) and CNV when topical
administrating with GS-101 in
non-human primate and rodent models, suggesting the promoting effect of IRS-1
on RNV or CNV. Additionally, Hos et al[38]
demonstrated that the blocking effect of GS-101 on IRS-1 signaling in
corneas can also inhibit corneal lymph-angiogenesis in animal experiments.
These results together with our findings provided novel insight on IRS-1
pro-angiogenesis or pro-lymph-angiogenesis efficacy in ocular diseases and
suggested the latent application for IRS-1 inhibitor to treat ocular
neovascularization, such as corneal neovascularization and CNV clinically[37,39].
Several evidences documented that
IRS-1 signaling was capable of influence cell migration[40-41] and proliferation when the cells were treated with
IRS-1 recombinant protein or IRS-1 inhibitor[42-44]. Besides, other reports revealed that IRS-1 was
involved in process of cell apoptosis in breast cancer cells[45]
and retinal endothelial cells[46]. In present
study, we also carried out relevant in vitro experiments to explore the
precise mechanism of IRS-1 regulating the tube formation behavior of HCECs. The
results verified that blockade of IRS-1 can suppress proliferation and
migration, and increase apoptosis of HCECs. These results are consistent with
above findings from other reports, suggesting that the anti-angiogenesis effect
of blockade of IRS-1 on HCECs tube formation may be through inhibiting cell
migration and cell proliferation and promoting cell apoptosis by IRS-1
inhibitor of GS-101.
Since vascular endothelial cells are
fundamental for angiogenic process, any components that affect the bio-function
of choroidal capillary endothelial cells may alter the development degree of
CNV[47-50]. To the best of our
knowledge, VEGF-A is the most important factor that promoting vascularization
both under physiological and pathological condition. It exerts pro-angiogenesis
effects partially via facilitating vascular endothelial cell
proliferation and migration. For the reason that verifying whether IRS-1 exerts
pro-angiogenesis effect through VEGF-A/VEGFR2 signaling pathway, we examined
VEGF-A and VEGFR2 expression in HCECs after treating the cells with serial
concentrations of IRS-1 inhibitor of GS-101. Our results demonstrated that the
gene and protein of VEGF-A is expressed in HCECs and decreased by IRS-1
inhibitor treatment in a dose-dependent way. In addition, the protein level of
VEGFR2/phospho-VEGFR2 in HCECs, a
receptor for VEGF-A, was also declined when the IRS-1 signaling was blocked by
the inhibitor of GS-101. It suggested that blockade of IRS-1 exerting
anti-angiogenesis effect may be, at least partly, through VEGF-A/VEGFR2
signaling pathway in the way of down-regulating the levels of VEGF-A and
VEGF-A-cognate receptor of VEGFR2/phospho-VEGFR2.
It is well known that migration and
proliferation of vascular endothelial cells were initial and vital steps for
angiogenesis[51-53]. The
angiogenic cascade involves in many complicated and integrated sequential
steps, in which vascular endothelial cells proliferation and migration are
initial steps in the process of angiogenesis, followed by vascular endothelial
cells behaviors of establishing and developing a capillary-like structure[54-55]. Accordingly, the results in
this work showed that blockade of IRS-1 exerted negative role in the
bio-functions of proliferation and migration of HCECs, and enhanced apoptosis
of HCECs, suggesting blockade of IRS-1 signaling exerted anti-angiogenic
effects on HCECs via in-activating the initial steps of angiogenic
cascade.
To summarize, the results of present
study confirmed a novel biological role for IRS-1
in HCECs. Blockade of IRS-1 suppressed capillary tube formation
through inhibiting cell migration and proliferation of the initial step of
angiogenic cascade. The effects may be, at least partly, through down-regulating
the expression level of VEGF-A and VEGFR2/phospho-VEGFR2 (VEGF-A receptor),
along with promoting cell apoptosis of HCECs. These findings indicate the
potential of anti-angiogenesis by GS-101 in HCECs, which may assist to clinical treatment
on ocular neovascularization in future.
ACKNOWLEDGEMENTS
The authors
would like to thank Xue-Guang Zhang for excellent technical assistance in this
study.
Foundations: Supported by the National Natural
Science Foundation in China (No. 81671641; No.81970830; No.31600736); Suzhou
Municipal Natural Science Foundation (No.SYS201745); Soochow University
Doctoral Academic Talents Program (No.5832001313); Jiangsu Provincial Medical
Youth Talent (No.QNRC2016718); Jiangsu Provincial Medical Innovation Team
(No.CXTDA2017039); Jiangsu Provincial Natural Science Foundation
(No.BK20151208); the Soochow Scholar Project of Soochow University
(No.R5122001).
Conflicts of Interest: Qian YY, None; Wu HY, None; Liu
GQ, None; Ren C, None; Lu PR, None; Zhang XG, None.
REFERENCES
|
1 Green WR.
Histopathology of age-related macular degeneration. Mol Vis 1999;5:27.
|
|
|
|
2 Fine SL. Age-related
macular degeneration 1969-2004: a 35-year personal perspective. Am J
Ophthalmol 2005;139(3):405-420.
https://doi.org/10.1016/j.ajo.2004.11.050
PMid:15767048
|
|
|
|
|
3 Argon laser
photocoagulation for neovascular maculopathy. Five-year results from
randomized clinical trials. Macular Photocoagulation Study Group. Arch
Ophthalmol 1991;109(8):1109-1114.
https://doi.org/10.1001/archopht.1991.01080080069030
PMid:1714270
|
|
|
|
|
4 Anderson DH, Mullins
RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of
drusen in the aging eye. Am J Ophthalmol 2002;134(3):411-431.
https://doi.org/10.1016/S0002-9394(02)01624-0
|
|
|
|
|
5 Kvanta A, Algvere
PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related
macular degeneration express vascular endothelial growth factor. Invest
Ophthalmol Vis Sci 1996;37(9): 1929-1934.
https://doi.org/10.1016/S0002-9394(14)70522-7
|
|
|
|
|
6 Ishibashi T, Hata Y,
Yoshikawa H, Nakagawa K, Sueishi K, Inomata H. Expression of vascular
endothelial growth factor in experimental choroidal neovascularization.
Graefes Arch Clin Exp Ophthalmol 1997; 235(3):159-167.
https://doi.org/10.1007/BF00941723
PMid:9085111
|
|
|
|
|
7 Kwak N, Okamoto N,
Wood JM, Campochiaro PA. VEGF is major stimulator in model of choroidal
neovascularization. Invest Ophthalmol Vis Sci 2000;41(10):3158-3164.
|
|
|
|
|
8 Heier JS, Brown DM,
Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related
macular degeneration. Ophthalmology 2012;119(12):2537-2548.
https://doi.org/10.1016/j.ophtha.2012.09.006
PMid:23084240
|
|
|
|
|
9 Yu WZ, Bai YJ, Han
N, Wang F, Zhao M, Huang L, Li XX. Inhibition of pathological retinal
neovascularization by semaphorin 3A. Mol Vis 2013;19:1397-1405.
|
|
|
|
|
10 Ameri
H, Liu H, Liu R, Ha Y, Paulucci-Holthauzen AA, Hu SQ, Motamedi M, Godley BF,
Tilton RG, Zhang WB. TWEAK/Fn14 pathway is a novel mediator of retinal
neovascularization. Invest Ophthalmol Vis Sci 2014;55(2):801-813.
https://doi.org/10.1167/iovs.13-12812
PMid:24408972 PMCid:PMC3920863
|
|
|
|
|
11 White
MF, Maron R, Kahn CR. Insulin rapidly stimulates tyrosine phosphorylation of
a Mr-185, 000 protein in intact cells. Nature 1985;318(6042):183-186.
https://doi.org/10.1038/318183a0
PMid:2414672
|
|
|
|
|
12 Sun XJ,
Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein
BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a
unique signal transduction protein. Nature 1991;352(6330):73-77.
https://doi.org/10.1038/352073a0
PMid:1648180
|
|
|
|
|
13 Selman
C, Lingard S, Choudhury AI, et al. Evidence for lifespan extension and
delayed age-related biomarkers in insulin receptor substrate 1 null mice.
FASEB J 2008;22(3):807-818.
https://doi.org/10.1096/fj.07-9261com
PMid:17928362
|
|
|
|
|
14 Selman
C, Partridge L, Withers DJ. Replication of extended lifespan phenotype in
mice with deletion of insulin receptor substrate 1. PLoS One
2011;6(1):e16144.
https://doi.org/10.1371/journal.pone.0016144
PMid:21283571 PMCid:PMC3026792
|
|
|
|
|
15
Laustsen PG, Michael MD, Crute BE, Cohen SE, Ueki K, Kulkarni RN, Keller SR,
Lienhard GE, Kahn CR. Lipoatrophic diabetes in Irs1(-/-)/Irs3(-/-) double
knockout mice. Genes Dev 2002;16(24):3213-3222.
https://doi.org/10.1101/gad.1034802
PMid:12502742 PMCid:PMC187498
|
|
|
|
|
16 Dong
XC, Park S, Lin XY, Copps K, Yi XJ, White MF. Irs1 and Irs2 signaling is
essential for hepatic glucose homeostasis and systemic growth. J Clin Invest 2006;116(1):101-114.
https://doi.org/10.1172/JCI25735
PMid:16374520 PMCid:PMC1319221
|
|
|
|
|
17 Yang
WM, Jeong HJ, Park SY, Lee W. Induction of miR-29a by saturated fatty acids
impairs insulin signaling and glucose uptake through translational repression
of IRS-1 in myocytes. FEBS Lett 2014;588(13): 2170-2176.
https://doi.org/10.1016/j.febslet.2014.05.011
PMid:24844433
|
|
|
|
|
18 Shaw
LM. The insulin receptor substrate (IRS) proteins: at the intersection of
metabolism and cancer. Cell Cycle 2011;10(11): 1750-1756.
https://doi.org/10.4161/cc.10.11.15824
PMid:21597332 PMCid:PMC3142458
|
|
|
|
|
19
Cloutier F, Lawrence M, Goody R, Lamoureux S, Al-Mahmood S, Colin S, Ferry A,
Conduzorgues JP, Hadri A, Cursiefen C, Udaondo P, Viaud E, Thorin E, Chemtob
S. Antiangiogenic activity of aganirsen in nonhuman primate and rodent models
of retinal neovascular disease after topical administration. Invest
Ophthalmol Vis Sci 2012;53(3):1195-1203.
https://doi.org/10.1167/iovs.11-9064
PMid:22323484
|
|
|
|
|
20 Fang
SF, Ma X, Guo SP, Lu JM. MicroRNA-126 inhibits cell viability and invasion in
a diabetic retinopathy model via targeting IRS-1. Oncol Lett 2017;14(4):4311-4318.
https://doi.org/10.3892/ol.2017.6695
PMid:28943945 PMCid:PMC5604121
|
|
|
|
|
21 Jiang
ZY, He ZH, King BL, Kuroki T, Opland DM, Suzuma K, Suzuma I, Ueki K, Kulkarni
RN, Kahn CR, King GL. Characterization of multiple signaling pathways of
insulin in the regulation of vascular endothelial growth factor expression in
vascular cells and angiogenesis. J Biol Chem 2003;278(34):31964-31971.
https://doi.org/10.1074/jbc.M303314200
PMid:12775712
|
|
|
|
|
22 Berdugo
M, Andrieu-Soler C, Doat M, Courtois Y, BenEzra D, Behar-Cohen F.
Downregulation of IRS-1 expression causes inhibition of corneal angiogenesis.
Invest Ophthalmol Vis Sci 2005;46(11):4072-4078.
https://doi.org/10.1167/iovs.05-0105
PMid:16249482
|
|
|
|
|
23
Al-Mahmood S, Colin S, Farhat N, Thorin E, Steverlynck C, Chemtob S. Potent
in vivo antiangiogenic effects of GS-101 (5'-TATCCGGAGGGCTCGCCATGCTGCT-3'),
an antisense oligonucleotide preventing the expression of insulin receptor
substrate-1. J Pharmacol Exp Ther 2009;329(2):496-504.
https://doi.org/10.1124/jpet.108.147496
PMid:19208899
|
|
|
|
|
24
Cursiefen C, Viaud E, Bock F, et al. Aganirsen antisense oligonucleotide eye
drops inhibit keratitis-induced corneal neovascularization and reduce need
for transplantation. Ophthalmology 2014;121(9):1683-1692.
https://doi.org/10.1016/j.ophtha.2014.03.038
PMid:24811963
|
|
|
|
|
25 Miele
C, Rochford JJ, Filippa N, Giorgetti-Peraldi S, Van Obberghen E. Insulin and
insulin-like growth factor-I induce vascular endothelial growth factor mRNA
expression via different signaling pathways. J Biol Chem
2000;275(28):21695-21702.
https://doi.org/10.1074/jbc.M000805200
PMid:10777488
|
|
|
|
|
26 Vuori
K, Ruoslahti E. Association of insulin receptor substrate-1 with integrins.
Science 1994;266(5190):1576-1578.
https://doi.org/10.1126/science.7527156
PMid:7527156
|
|
|
|
|
27
Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and
lymphangiogenesis. Nat Rev Cancer 2008;8(8):604-617.
https://doi.org/10.1038/nrc2353
PMid:18497750 PMCid:PMC2577722
|
|
|
|
|
28 Liu GQ,
Wu HY, Chen L, Xu J, Wang MJ, Li D, Lu PR. Effects of interleukin-17 on human
retinal vascular endothelial cell capillary tube formation in vitro. Mol Med
Rep 2017;16(1):865-872.
https://doi.org/10.3892/mmr.2017.6623
PMid:28560397
|
|
|
|
|
29 Lu PR,
Li LB, Liu GQ, van Rooijen N, Mukaida N, Zhang XG. Opposite roles of CCR2 and
CX3CR1 macrophages in alkali-induced corneal neovascularization. Cornea
2009;28(5):562-569.
https://doi.org/10.1097/ICO.0b013e3181930bcd
PMid:19421039
|
|
|
|
|
30 Liu GQ,
Zhang WP, Xiao YH, Lu PR. Critical role of IP-10 on reducing experimental
corneal neovascularization. Curr Eye Res 2015;40(9):891-901.
https://doi.org/10.3109/02713683.2014.968934
PMid:25309995
|
|
|
|
|
31 Chen
ZG, Liu GQ, Xiao YH, Lu PR. Adrenomedullin22-52 suppresses
high-glucose-induced migration, proliferation, and tube formation of human
retinal endothelial cells. Mol Vis 2014;20:259-269.
|
|
|
|
|
32 Chao
TI, Xiang SH, Chen CS, Chin WC, Nelson AJ, Wang CC, Lu J. Carbon nanotubes
promote neuron differentiation from human embryonic stem cells. Biochem
Biophys Res Commun 2009;384(4):426-430.
https://doi.org/10.1016/j.bbrc.2009.04.157
PMid:19426708
|
|
|
|
|
33 Senger
DR, Perruzzi CA, Streit M, Koteliansky VE, de Fougerolles AR, Detmar M. The
alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for
vascular endothelial growth factor signaling, endothelial cell migration, and
tumor angiogenesis. Am J Pathol 2002;160(1):195-204.
https://doi.org/10.1016/S0002-9440(10)64363-5
|
|
|
|
|
34 Liu GQ,
Wu HY, Xu J, Wang MJ, Lu PR, Zhang XG. Anti-apoptosis effects of vascular
endothelial cadherin in experimental corneal neovascularization. Int J
Ophthalmol 2015;8(6):1083-1088.
|
|
|
|
|
35 White
MF. The IRS-signaling system: a network of docking proteins that mediate
insulin and cytokine action. Recent Prog Horm Res 1998;53:119-138.
https://doi.org/10.1007/978-3-642-80481-6_8
PMid:9401207
|
|
|
|
|
36 Bugner
V, Aurhammer T, Kühl M. Xenopus laevis insulin receptor substrate IRS-1 is
important for eye development. Dev Dyn 2011;240(7): 1705-1715.
https://doi.org/10.1002/dvdy.22659
PMid:21574211
|
|
|
|
|
37 Kain H,
Goldblum D, Geudelin B, Thorin E, Beglinger C. Tolerability and safety of
GS-101 eye drops, an antisense oligonucleotide to insulin receptor
substrate-1: a 'first in man' phase I investigation. Br J Clin Pharmacol
2009;68(2):169-173.
https://doi.org/10.1111/j.1365-2125.2009.03450.x
PMid:19694734 PMCid:PMC2767278
|
|
|
|
|
38 Hos D,
Regenfuss B, Bock F, Onderka J, Cursiefen C. Blockade of insulin receptor
substrate-1 inhibits corneal lymphangiogenesis. Invest Ophthalmol Vis Sci
2011;52(8):5778-5785.
https://doi.org/10.1167/iovs.10-6816
PMid:21666240
|
|
|
|
|
39
Cursiefen C, Bock F, Horn FK, Kruse FE, Seitz B, Borderie V, Früh B, Thiel
MA, Wilhelm F, Geudelin B, Descohand I, Steuhl KP, Hahn A, Meller D. GS-101
antisense oligonucleotide eye drops inhibit corneal neovascularization:
interim results of a randomized phase II trial. Ophthalmology
2009;116(9):1630-1637.
https://doi.org/10.1016/j.ophtha.2009.04.016
PMid:19643487
|
|
|
|
|
40 Longato
L, de la Monte S, Kuzushita N, Horimoto M, Rogers AB, Slagle BL, Wands JR.
Overexpression of insulin receptor substrate-1 and hepatitis Bx genes causes premalignant
alterations in the liver. Hepatology 2009;49(6):1935-1943.
https://doi.org/10.1002/hep.22856
PMid:19475691 PMCid:PMC2754284
|
|
|
|
|
41 Cao MR,
Li YL, Lu HL, Meng QW, Wang L, Cai L, Dong XQ. MiR-23a-mediated
migration/invasion is rescued by its target, IRS-1, in non-small cell lung
cancer cells. J Cancer Res Clin Oncol 2014;140(10):1661-1670.
https://doi.org/10.1007/s00432-014-1725-0
PMid:24898878
|
|
|
|
|
42 Costa
MM, Violato NM, Taboga SR, Góes RM, Bosqueiro JR. Reduction of insulin
signalling pathway IRS-1/IRS-2/AKT/mTOR and decrease of epithelial cell
proliferation in the prostate of glucocorticoid-treated rats. Int J Exp
Pathol 2012;93(3):188-195.
https://doi.org/10.1111/j.1365-2613.2012.00817.x
PMid:22583132 PMCid:PMC3385916
|
|
|
|
|
43 Zhou Y,
Feng X, Liu YL, Ye SC, Wang H, Tan WK, Tian T, Qiu YM, Luo HS.
Down-regulation of miR-126 is associated with colorectal cancer cells
proliferation, migration and invasion by targeting IRS-1 via the AKT and
ERK1/2 signaling pathways. PLoS One 2013;8(11):e81203.
https://doi.org/10.1371/journal.pone.0081203
PMid:24312276 PMCid:PMC3843680
|
|
|
|
|
44 Wu YJ,
Yu R, Cheng XH, Wu H, Wu CR, Wei GD, Zhang Q. Effect of Jiangang Yishen
Recipe on high insulin induced cell proliferation of human glomerular
mesangial cells and the expression of insulin receptor substrate 1 and
phosphatidylinositol-3-kinase. Chin J Integr Tradit West Med
2014;34(5):597-601.
|
|
|
|
|
45 Tiwary
R, Yu W, Sanders BG, Kline K. Α-TEA cooperates with MEK or mTOR inhibitors to
induce apoptosis via targeting IRS/PI3K pathways. Br J Cancer
2011;104(1):101-109.
https://doi.org/10.1038/sj.bjc.6606019
PMid:21119656 PMCid:PMC3039802
|
|
|
|
|
46 Jiang
YD, Zhang QH, Soderland C, Steinle JJ. TNFα and SOCS3 regulate IRS-1 to
increase retinal endothelial cell apoptosis. Cell Signal
2012;24(5):1086-1092.
https://doi.org/10.1016/j.cellsig.2012.01.003
PMid:22266116 PMCid:PMC4073498
|
|
|
|
|
47 Becher
MU, Nickenig G, Werner N. Regeneration of the vascular compartment. Herz
2010;35(5):342-351.
https://doi.org/10.1007/s00059-010-3360-0
PMid:20842474
|
|
|
|
|
48 Izumi
N, Helker C, Ehling M, Behrens A, Herzog W, Adams RH. Fbxw7 controls
angiogenesis by regulating endothelial Notch activity. PLoS One
2012;7(7):e41116.
https://doi.org/10.1371/journal.pone.0041116
PMid:22848434 PMCid:PMC3407154
|
|
|
|
|
49
Sacilotto N, Chouliaras KM, Nikitenko LL, Lu YW, Fritzsche M, Wallace MD,
Nornes S, García-Moreno F, Payne S, Bridges E, Liu K, Biggs D, Ratnayaka I,
Herbert SP, Molnár Z, Harris AL, Davies B, Bond GL, Bou-Gharios G, Schwarz
JJ, De Val S. MEF2 transcription factors are key regulators of sprouting
angiogenesis. Genes Dev 2016;30(20): 2297-2309.
https://doi.org/10.1101/gad.290619.116
PMid:27898394 PMCid:PMC5110996
|
|
|
|
|
50
Salminen A, Kauppinen A, Hyttinen JM, Toropainen E, Kaarniranta K.
Endoplasmic reticulum stress in age-related macular degeneration: trigger for
neovascularization. Mol Med 2010;16(11-12):535-542.
https://doi.org/10.2119/molmed.2010.00070
PMid:20683548 PMCid:PMC2972399
|
|
|
|
|
51
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of
angiogenesis. Nature 2011;473(7347):298-307.
https://doi.org/10.1038/nature10144
PMid:21593862 PMCid:PMC4049445
|
|
|
|
|
52 Marcelo
KL, Goldie LC, Hirschi KK. Regulation of endothelial cell differentiation and
specification. Circ Res 2013;112(9):1272-1287.
https://doi.org/10.1161/CIRCRESAHA.113.300506
PMid:23620236 PMCid:PMC3768127
|
|
|
|
|
53 Adams
RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis.
Nat Rev Mol Cell Biol 2007;8(6):464-478.
https://doi.org/10.1038/nrm2183
PMid:17522591
|
|
|
|
|
54
Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic
intervention in the treatment of cancer, cardiovascular diseases, and chronic
inflammation. Pharmacol Rev 2000;52(2):237-268.
|
|
|
|
|
55 Liekens
S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications.
Biochem Pharmacol 2001;61(3):253-270.
https://doi.org/10.1016/S0006-2952(00)00529-3
|
|
|
|






