
Citation: Kang LH, Zhang S, Jiang S, Hu N. Activation of autophagy
in the retina after optic nerve crush injury in rats. Int J Ophthalmol
2019;12(9):1395-1401.
DOI:10.18240/ijo.2019.09.04
·Basic Research·
Activation
of autophagy in the retina after optic nerve crush injury in rats
Li-Hua Kang1, Su
Zhang2, Sheng Jiang1, Nan Hu1
1Eye
Institute, Affiliated Hospital of Nantong University, Nantong 226001, Jiangsu
Province, China
2Nanjing Medical
University Affiliated Eye Hospital, Nanjing 210000, Jiangsu Province, China
Co-first
authors: Li-Hua Kang
and Su Zhang
Correspondence
to: Nan Hu. Eye
Institute, Affiliated Hospital of Nantong University, 20 Xisi Road, Nantong
226001, Jiangsu Province, China. hunaneye@hotmail.com
Received:
Abstract
AIM: To investigate the activation of autophagy in rat retina after optic
nerve crush (ONC) and evaluate its relationship with apoptosis of retinal
ganglion cells (RGCs).
METHODS: The ONC model was established. Western blots were
performed to investigate expression of p62, LC3 and Beclin-1. Transmission
electron microscopy was performed to discover the autophagosomes in the retina after
ONC. Immunohistochemistry was used to confirm the distribution of LC3. TUNEL
was performed to confirm the relationship between autophagy and RGC apoptosis.
RESULTS: p62/Beclin-1 ratio was declined shortly after ONC
until to day 7 after ONC and then restored to a normal level at day 21. There
was an opposite change in the LC3-II/LC3I ratio in the retina compared to the
p62/Beclin-1 ratio. Increased autophagosomes were found after ONC using
transmission electron microscopy, and most of the LC3-stained cells were
colocalized with RGCs and Müller cells. More LC3-immunoreactive cells and
apoptotic RGCs were found on day 7 following ONC.
CONCLUSION: Possible activation of autophagy in RGCs after ONC;
autophagy mainly occurred in RGCs and Müller cells, and the apoptosis of RGCs
after ONC may be partly associated with autophagic activation.
KEYWORDS: autophagy;
optic nerve crush; apoptosis; retinal ganglion cells; rat
DOI:10.18240/ijo.2019.09.04
Citation:
Kang LH, Zhang S, Jiang S, Hu N. Activation of autophagy in the retina after
optic nerve crush injury in rats. Int J Ophthalmol 2019;12(9):1395-1401
INTRODUCTION
The
neurodegenerative process after optic nerve crush (ONC) is similar to the
pathological process of glaucomatous optic neuropathy[1].
The pathological basis of ONC is progressive retinal ganglion cells (RGCs) loss
in retina and optic nerve fibers loss, leading to irreversible changes in
visual function[2]. RGCs are one of the three
major retinal neurons in the retina. Their axons form the optic nerve and send
visual information to higher brain[3]. RGCs
degeneration is often modeled using ONC, which can better simulate the
secondary apoptosis of RGCs[4-6].
The mechanism of optic nerve injury and the repair of RGCs after injury[7] has become one of the hot topics in ophthalmology. It
is also an urgent problem for ophthalmology.
Autophagy in
cell death is characterized by a large aggregation of autophagic vesicles and
no nuclear condensation. Autophagy refers to some degradable components, such
as protein and organelles, being encapsulated and transported for lysosomal degradation.
The amino acids and other small molecules produced by autophagic degradation
can be reused or can generate energy[8-9].
Under normal conditions, autophagy occurs at a fairly low level in many cells.
Autophagy is a controllable defense. When cells are exposed in physiological
stress stimuli (e.g., starvation, high temperature, external
stimulation, mutated protein aggregation or microbial invasion), autophagy can
be activated[10-11]. Previous
studies showed that autophagy, a cellular homeostasis-maintaining process,
plays an important role in response to many environments, but excessive
autophagy can directly lead to programmed cell death[12].
Autophagy
can play a protective or harmful role in different stages of pathological
processes. Many pathological processes have been found to be related to
autophagy, such as cancer, neurodegenerative diseases, and central nervous system
injury[13-14]. Autophagy has
been found to play a critical role in neuronal survival, the clearance of
senescent organelles and misfolded proteins in nervous system, and a protective
role in neurons[15-17].
However, autophagy can be one of modes of nerve cell death[18].
In visual system, autophagy participates in the pathological changes in RGCs
after optic nerve injury[19-23].
In animal models of optic nerve transection and intraocular hypertension,
autophagy was formed in RGCs, and high expression of autophagy-related
genes/protein indicated that autophagy was activated in RGCs after optic nerve
injury[19-22].
Although
autophagy has been demonstrated in photoreceptors in mouse and fly models[24-25], few studies have explored the
activation of autophagy in rats in vivo[26-27]. Our aim is to investigate the activation of
autophagy in the rat retina after ONC and to evaluate its relationship to RGC
apoptosis in vivo. In this study, we demonstrated that autophagy could
be activated after ONC in rats and has a relationship with apoptotic RGCs. By
using an ONC rat model, we investigated that the apoptosis of RGC might be
partially associated with the activation of autophagy in vivo. Thus,
autophagy modulation might provide a potential therapeutic target for the
amelioration of RGC degeneration in ONC.
MATERIALS AND METHODS
Ethical
Approval Adult Sprague Dawley rats (200
The animals
were randomly divided into seven groups (n=12 each): Sham, 1, 3, 5, 7,
14, and 21d after injury. The retinas from 6 of them at the indicated time were
used for westem blots (WB). The retinas from 3 of them were used for
immunohistofluorescence (IHF), and the others were used for transmission
electron microscopy.
The
Establishment of Optic Nerve Crush The rats were deeply anesthetized
with an intraperitoneal injection of a local anesthetic (10% chloral hydrate).
Analgesia was provided by subcutaneous administration of buprenorphine (0.1
mg/kg; Schering-Plough, Madrid, Spain). During and after surgery, the eyes were
covered with an ointment containing tobramycin (Tobrex; Alcon, S. A.,
Barcelona, Spain) to avoid corneal desiccation. Under a binocular surgical
microscope, a lateral canthotomy was used in the eye. An incision was performed
in the skin overlying the superior orbital rim, the supero-external orbital
contents were dissected, and the extraocular were sectioned. The nerve was
crushed
Western
Blot Protein pyrolysis and WB were
performed for individual retinas at different time points postoperatively, as
previously described[28]. Proteins were
electrophoresed on 10% SDS-polyacrylamide gel and were transferred to
polyvinylidene difluoride filters. After overnight incubation with antibodies
against Beclin-1 (1:800; Cell Signaling Technology, USA), LC3B (1:800; Sigma,
USA), p62 (1:5000; Abcam, USA) and GAPDH (1:2000; Santa Cruz Biotechnology,
USA) overnight at
Transmission
Electron Microscopy Blocks of
Immunohistofluorescence Retinal isolation and IHF were
detected as previously described[28]. The sections
were blocked and incubated with antibodies against LC3B (Sigma, 1:100), Brn
TUNEL
Staining TUNEL staining of fragmented DNA was
detected on whole retinal sections according to previous methods using an in
situ Cell Death Detection kit, POD (Roche Applied Science) and following
the manufacturer’s instructions. The retinal sections were fixed with 4%
paraformaldehyde for 1h and washed with 0.01 mol/L PBS (pH 7.0). Then, the
slides were incubated with permeabilization solution for 8min on ice and
subsequently added to citrate buffer for microwave irradiation for 3min,
followed by incubated with LC3B (Sigma, 1:100) for 4h on ice. The TUNEL
reaction mixture and 568 goat anti-rabbit IgG (1:200, Jackson Laboratory) were
incubated to the slides for 1h at
Statistical
Analysis The data were expressed as the
mean±SD and analyzed using the SPSS software (version 17.0, SPSS Inc, IL, USA).
Differences among the groups were analyzed with one-way analysis of variance
(ANOVA), followed by Tukey’s post hoc multiple comparison tests. P
values of <0.05 were considered statistically significant.
RESULTS
The
Expression of Autophagy-Related Proteins LC3, p62/ Beclin
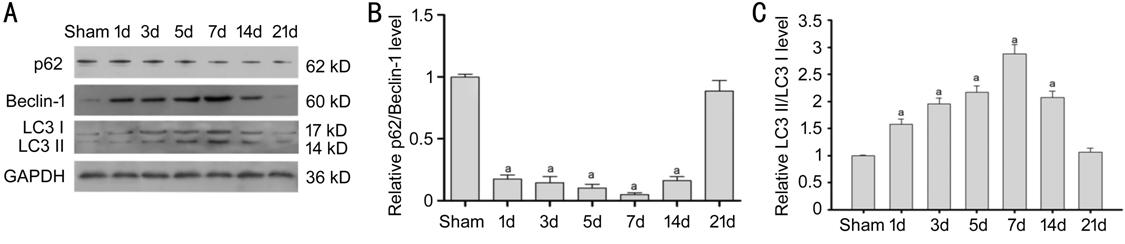
Figure 1 The
expression of LC3, Beclin-1 and p
The
Observation of Autophagosomes in the Retina After ONC Using Transmission
Electron Microscopy Under transmission electron
microscope, we observed that there was little or no bilayer membrane
autophagosomes in the sham retinas. However, the number of autophagosomes
increased in the retinal tissue after ONC (Figure 2), indicating that retinal
autophagy was activated after ONC.
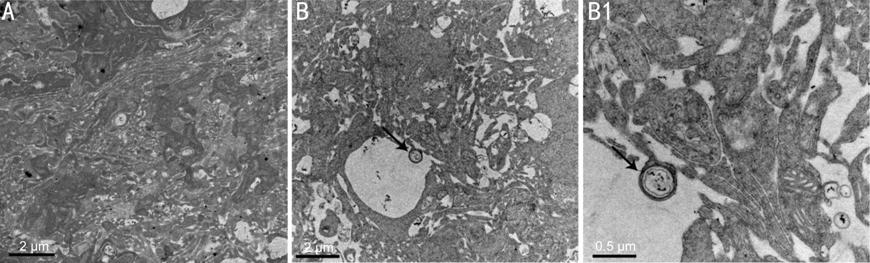
Figure 2
Electron microscopy analysis of representative RGCs from the corresponded 7
day-sham and 7 day-injured retinas A: Normal retinal ultrastructure; B:
The ultrastructure of the retina 7d after ONC. Bar=2 μm. B1: Indicate the
enlargement of autophagosomes in diagram B. Bar=0.5 μm.
The Distribution
of LC
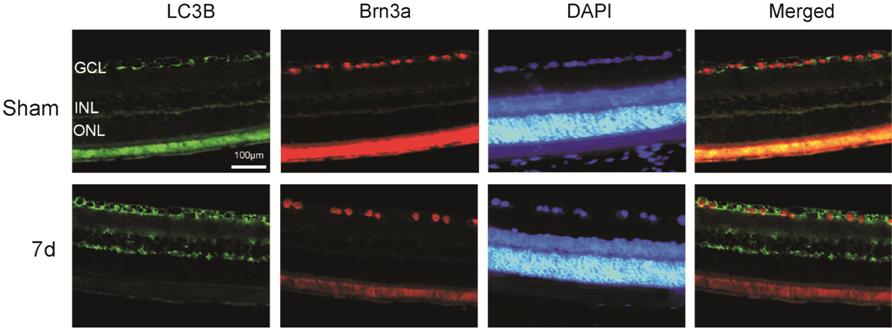
Figure 3
Immunofluorescence analysis of the association between LC3 and the RGC marker,
Brn
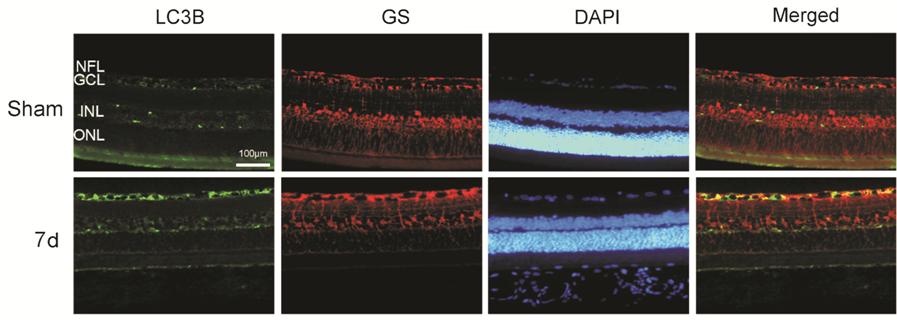
Figure 4
Immunofluorescence analysis of the association between LC3 and the Müller cell
marker, GS, was conducted in corresponded 7 day-sham and 7 day-injured
retinas NFL: Nerve fiber layer; GCL:
Ganglion cell layer; INL: Inner nuclear layer; ONL: Outer nuclear layer;
Bar=100 μm.
The
Relationship Between Autophagy and RGC Apoptosis After ONC To further confirm the relationship
between autophagy and RGC apoptosis after ONC, double-staining of LC3B and
TUNEL were performed. There were few LC3+ cells or TUNEL+ cells in the ganglion
cell layer (GCL) of the sham group (Figure 5). In addition, the co-localization
of LC3+ and TUNEL+ cells increased markedly at 7d. Notably, not all the TUNEL+
cells overlapped with the LC3+ cells, and some TUNEL+ cells had no LC3+
signals. This suggested that part of RGC apoptosis after ONC might be related
to the activation of autophagy.

Figure 5
Immunofluorescence and TUNEL staining analysis of the association between
autophagy and RGC apoptosis GCL: Ganglion cell layer; INL: Inner
nuclear layer; ONL: Outer nuclear layer; Bar=100 μm.
DISCUSSION
Optic nerve
injury is a critical cause of visual impairment in the world. Here, we
addressed the role of autophagy in retinal neurodegeneration after optic nerve
injury in vivo. First, by using the model of ONC in rats that induces
RGC apoptosis and by testing autophagic proteins and autophagosomes in vivo,
we found that autophagy was rapidly activated in the retina after ONC. Further,
we demonstrated that autophagic activation might be related to the regulation
of RGC apoptosis. Altogether, these data showed autophagy in retinal cells
after ONC in rats.
Many studies
have found that retinal or optic nerve injury derived from various causes can
induce autophagic activation in mice and Ganges River monkeys[32]. In the optic nerve transection injury model, the
number of green fluorescent-labeled GFP-LC3 positive cells in the retinal GCL
increased, and the level of autophagy-related gene Atg5 mRNA increased
significantly, indicating that autophagy was activated rapidly[19]. In the mouse model of retinal ischemia-reperfusion
injury caused by an acute increase in intraocular pressure (IOP), the autophagy
at 12h and 24h in retinal neurons was obviously activated after the injury[20]. In the Ganges River monkey chronically high IOP
model, the expression levels of LC3B II/LC3B I and Beclin-1 protein were
detected. Meanwhile, autophagic bodies were observed using transmission
electron microscope. The results showed that the autophagic flow increased
after the increase in chronic IOP[21]. In rat
models of chronic ocular hypertension, autophagy was activated after the
increase in IOP, and IOP increased early. Autophagic bodies were detected in
the plexiform layer (IPL). After that, autophagic bodies decreased in the IPL
and increased in GCL, indicating that autophagy in the RGCs was activated after
optic nerve injury[22]. Our study showed that the
retinal autophagy-related protein LC3 increased significantly 1d after ONC in
rats and peaked at 7d, and the p62/Beclin-1 ratio decreased, a finding that was
consistent with previous finding[33]. In
addition, we used immunofluorescence and observed LC3 co-localization with RGCs
in the GCL after ONC and co-localization with Müller cells in GCL, indicating
that autophagy was activated in RGCs and Müller cells in retina after optic
nerve injury. The autophagic activation of Müller cells after optic nerve
injury has not been reported, but in central nervous system injuries, such as
spinal cord injury models and traumatic brain injury models, the
autophagy-related gene Beclin-1 is expressed in neurons, astrocytes, and oligodendrocytes,
which was consistent with our results.
There was
still controversy regarding the activation of autophagy in order to promote
neuronal survival or apoptosis after nerve injury in the central nervous
system. In the rat model of persistent middle cerebral artery occlusion,
autophagy was found to be significantly activated in the ischemic area, there
was a reduction in the obstruction area after autophagic inhibition, and there
was alleviation of cerebral edema and neurological symptoms[34].
However, in two studies of brain trauma, autophagy was found to be activated,
and autophagy exerted opposite effects. One found that autophagy protected
neurons in the early stage of the injury[35], and
the other found that autophagy could lead to the death of neurons[36]. Thus, autophagic activation after nerve injury has
different effects on different researchers and different models of neurons,
which may be related to the time and extent of the injury. Some scholars
believe that mild to moderate activation of autophagy plays a protective role
in ischemic and hypoxic neurons. Over-activation of autophagy may destroy the
dynamic balance of cell metabolism and lead to neuronal death[35-37].
There have
been few studies on the effects of autophagy in the fate of RGCs in retinal
optic nerve injury. In the model of retinal ischemia-reperfusion injury induced
by ocular hypertension, autophagy was activated, and the apoptosis of RGCs was
increased. The death of RGCs was reduced in responses to autophagic inhibitors[20]. Autophagy has been shown to activate and induce RGC
death in chronic ocular hypertension[22], whereas
inhibition of autophagy decreases RGC apoptosis[20],
suggesting that autophagy may promote loss of retinal neurons, consistent with
our results. However, some scholars have also reported that in serum-free
culture conditions, the application of drugs to inhibit autophagy will reduce
the viability of RGCs[23]. In this study, the
results of the TUNEL assay showed that autophagic activation in RGCs was
accompanied by apoptosis of RGCs after ONC, indicating that autophagic
activation may be related to RGC apoptosis in this model.
In light of
the fact that autophagic activation might change the fate of RGCs after ON
injury, we hope to offer greater protection of the optic nerve through the
study of autophagy regulation in future research.
ACKNOWLEDGEMENTS
Authors’
contributions: Kang LH and
Hu N designed the experiments. Zhang S and Jiang S performed the experiments.
Kang LH performed the data analysis and wrote the paper. Hu N revised the
paper. All authors read and approved the final manuscript.
Foundation: Supported by Science and Technology
Project of Nantong, China (No.MS22015002).
Conflicts of
Interest: Kang LH, None; Zhang S, None; Jiang S, None; Hu N, None.
REFERENCES