
Citation: Chairissy MD, Wulandari LR, Sujuti H. Pro-apoptotic and
anti-proliferative effects of Physalis angulata leaf extract on
retinoblastoma cells. Int J Ophthalmol 2019;12(9):1402-1407
DOI:10.18240/ijo.2019.09.05
·Basic Research·
Pro-apoptotic and anti-proliferative effects of Physalis angulata leaf extract on retinoblastoma cells
Marsha Dechastra Chairissy, Lely Retno Wulandari, Hidayat Sujuti
Department of Ophthalmology, Faculty of Medicine, Brawijaya University, Malang 65145, Indonesia
Correspondence to: Lely Retno Wulandari. Department of Ophthalmology, Faculty of Medicine, Brawijaya University, Malang 65145, East Java, Indonesia. lelyretnowulandari@gmail.com
Received:
Abstract
AIM: To investigate the effect of Physalis angulata leaf extract on apoptotic and proliferation of retinoblastoma cells. Despite several previous studies evidencing the anti-cancer potential of Physalis angulata; however, certain study that proves its benefits in retinoblastoma cancer cells has been limited.
METHODS: This study utilizes an in-vitro experimental study by applying Y79 human retinoblastoma cell line culture obtained from the American Type Culture Collection (ATCC; 10801 University Boulevard Manassas, VA 20110, USA). The cell was divided into 4 groups. Group I was the control group without the administration of Physalis angulata leaf extract. Whereas, group II, II and IV are engaged with 25, 50, and 100 µg/mL of Physalis angulata leaf extract respectively. After a 24h incubation, an examination with microtetrazolium (MTT) cell proliferation assay and Annexin V apoptosis detection was conducted. Statistical analysis was performed with the Tukey test.
RESULTS: Physalis angulata leaf extract improved apoptosis and significantly reduced the number of living cells in retinoblastoma cells, along with the increase in the given dose. Based on the Tukey test, a significant difference was found in the treatment group at 50 µg/mL (P=0.025) and 100 µg/mL (P=0.001) in the measurement of apoptosis. Proliferation measurements also indicated a significant decrease in the number of living cells in the 50µg/mL treatment group (P=0.004), and in the 100 µg/mL treatment group (P=0.000). Meanwhile, a dose of 25 µg/mL indicated insignificant difference in the two measurements. Improved apoptosis and decreased number of living cells occured at a dose of 100 µg/mL. Decreased number of living cells (in the measurement of proliferation) was due to the inhibited proliferation or improved apoptosis.
CONCLUSION: Physalis angulata leaf extract improve apoptosis in retinoblastoma cell culture, requiring further research to inhibit proliferation.
KEYWORDS: Physalis angulata; apoptotic; proliferation; retinoblastoma cells
DOI:10.18240/ijo.2019.09.05
Citation: Chairissy MD, Wulandari LR, Sujuti H. Pro-apoptotic and anti-proliferative effects of Physalis angulata leaf extract on retinoblastoma cells. Int J Ophthalmol 2019;12(9):1402-1407
INTRODUCTION
Retinoblastoma is suspected as a primary intraocular malignancy mostly occurring in children, with the worldwide general incidents ranging for 8000 children per year. It is unbeatable fact that developed countries have survived their high life expectancies; whereas, developing and poor countries have been suffered with the finding of less than 50%[1-3].
The pathogenesis of retinoblastoma is initiated with a disruption of cell cycle regulation leading to abnormal retinal cell growth. In retinoblastoma, mutations or inactivation of tumor suppression proteins include the retinoblastoma protein (pRB) and the transcription factor p53[4]. Both of the pRB and p53 allow the two main pathways of tumor suppression controlling cellular responses to oncogenic stimuli such as errors in cell division, DNA damage and incompatible mitogenic signals. Each of these pathways has certain regulators and effectors to stop the cell cycle or even induce apoptosis[5]. Inactivation of pRB prohibits cells to enter the proliferation phase resulting in uncontrolled DNA replication and imperfect replication results[6]. A mere pRB pathway is insufficient to induce tumor formation. Dysregulation in p53 pathway will also induce anti-apoptosis and excessive cell growth[7].
One of the weaknesses of current retinoblastoma therapy is indicated to restrict the therapeutic index range allowing toxicity in normal tissue. In addition, a high level of recurrence causes a large number of treatment failures. At present, several concerned researches have been devoted by developing anti-tumor agents (one of which is pRB) to target the mechanisms which can suppress tumor development. The two main strategies that have been developed with the target of RB1 gene as cancer therapy are exploiting the RB1 gene mutation and reactivating tumor suppressor functions from RB1[8-10].
Physalis angulata is a plant, evidenced to have photochemical attributes addressing the anti-cancer potential[11]. One of the anticancer active substances successfully isolated from Physalis angulata leaf extract is physalin, which is a derivative of flavonoid glycosides. In addition, other ingredients were found, such as luteolin, carotenoids, and withanolides. In addition to anticancer effect, Physalis angulata leaf extract is also believed to have antiparasitic, anti-inflammatory, antimicrobial, anti-inflammatory, and immunosuppressive potential[12].
Several concerned studies have proven the anticancer potential of Physalis angulate[13-22]. However, there has not been any study that proves its benefits in retinoblastoma cancer cells.
MATERIALS AND METHODS
The design of this study involved an in vitro experimental in retinoblastoma cell line culture which was engaged with Physalis angulata leaf extract. The study was conducted at the Biomedical Laboratory of Faculty of Medicine in Brawijaya University, on October 2018.
The sample of this study was retinoblastoma cell line culture obtained from the American Type Culture Collection (ATCC; 10801 University Boulevard Manassas, VA 20110, USA). The cell culture was divided into four groups, including the control group (without exposure), treatment group 1 (with exposure to 25 µg/mL of Physalis angulata leaf extract), treatment group 2 (with exposure to 50 µg/mL of Physalis angulata leaf extract), and treatment group 3 (with exposure to 100 µg/mL of Physalis angulata leaf extract).
Inclusion criteria involved cell culture with characteristics of retinoblastoma cell line Y79 (ATCC® HTB18™) marked with multicellular cluster or grape-like cluster. Whereas, exclusion criteria involved cell culture without characteristic of retinoblastoma cell, ingrown cell culture during incubation period or cell culture with contamination from chemical material, micro-organism, or fungi experiencing an error during treatment.
Physalis angulata leaf extract is derived from extraction with absolute ethanol solvent by utilizing soxhlet extractor device. The liquid extract is diluted to acquire concentration with dose of 25 µg/mL, 50 µg/mL, and 100 µg/mL.
Proliferation and apoptosis of retinoblastoma cell involve the growth of retinoblastoma cell counted by microtetrazolium (MTT) cell proliferation assay method and by Flowcytometri Annexin V apoptosis detection kit (to reveal the percentage of living cell).
Research Procedure
The process of retinoblastoma cell
culture Retinoblastoma cell tissue was
washed 3 times with sterile phosphate-buffered saline (PBS) in laminar flow. The tissue was chopped
in a serum-free media to get a size of 1×
The extraction of Physalis
angulata leaf Totally
MTT proliferation assay procedure Various Physalis angulata extract
concentrations were added to 96 well-plates and were incubated in a incubator
with 5% CO2 at
Incubation
was performed at

Measurement
of Apoptosis using Annexin V Various Physalis angulata extract concentrations were added to 24 well-plates and were incubated in
incubator with 5% CO2 at
Statistical Analysis The normality test for each group was conducted with the Kolmogorov-Smirnov test and the homogeneity test of the data was performed with the Levene test. To determine apoptosis and retinoblastoma cell proliferation between each treatment, analysis with one way ANOVA was utilized. To compare data from each group, the Tukey test was applied, indicating significant result if P<0.05.
RESULTS
Primary Outcome From the results of statistical analysis, it was revealed that there was an effect of Physalis angulata leaf extract on retinoblastoma cell apoptosis in decreasing the number of living cells in the treatment groups with doses of 25, 50, and 100 µg/mL. The higher the dose, the higher the effect in increasing apoptosis and inhibiting the number of living cells in retinoblastoma cells (Table 1).
Table 1 Mean of observation results of apoptosis and proliferation in retinoblastoma cell culture in the control group and treatment groups
Parameters |
Group |
n |
Mean±SD |
Min. |
Max. |
95%CI |
|
Lower bound |
Upper bound |
||||||
Apoptosis |
K |
6 |
0.86±0.18 |
0.63 |
1.15 |
0.67 |
1.05 |
P1 |
6 |
1.06±0.31 |
0.74 |
1.57 |
0.73 |
1.39 |
|
P2 |
6 |
1.33±0.17 |
1.09 |
1.52 |
1.15 |
1.51 |
|
P3 |
6 |
1.54±0.34 |
1.04 |
2.03 |
1.19 |
1.89 |
|
Proliferation |
K |
6 |
89.65±1.18 |
88.22 |
91.35 |
88.41 |
90.88 |
P1 |
6 |
87.84±1.01 |
86.41 |
88.88 |
86.76 |
88.90 |
|
P2 |
6 |
86.77±1.75 |
84.39 |
89.33 |
84.94 |
88.61 |
|
P3 |
6 |
84.80±1.01 |
82.95 |
86.05 |
83.74 |
85.86 |
|
Physalis angulata leaf extract increasing the retinoblastoma cell apoptosis, which was conducted on the one-way ANOVA test, indicated that the significance value for apoptosis was 0.001 (<0.05). In addition, the Tukey test identified the treatment groups marking differences in the treatment dose of 50 µg/mL (P=0.025), and 100 µg/mL (P=0.01); whereas, a dose of 25 µg/mL indicated insignificant difference (P=0.555; Figure 1).
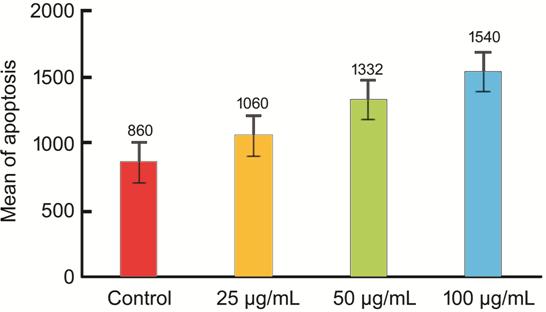
Figure 1 Apoptosis of retinoblastoma cell given Physalis angulata leaf extract.
Physalis angulata leaf extract fighting the retinoblastoma cell proliferation, which was conducted on the one-way ANOVA test, indicated that the significance value for proliferation was 0.000 (<0.05). In addition, the Tukey test identified the treatment groups marking differences in the treatment dose of 50 µg/mL (P=0.004), and 100 µg/mL (P=0.00); whereas, a dose of 25 µg/mL indicated insignificant difference (P=0.097; Figure 2).
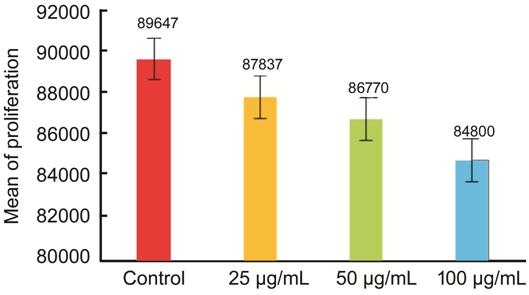
Figure 2 Proliferation of retinoblastoma cell given Physalis angulata leaf extract.
Secondary Outcome This research hypothesis states that: Physalis angulata leaf extract is likely to have pro-apoptotic effect compared to its anti-proliferative effect due to the two-fold apoptotic rate. Meanwhile, there was no decreased of living cell rate when cell proliferation was measured.
DISCUSSION
Several previous studies have proven the anticancer potential of Physalis angulata[13-22]. However, certain study dealing with benefits in retinoblastoma cancer cells remains limited. in this research, the researchers promote the novelty by studying the effect of Physalis angulata leaf extract on apoptosis and proliferation of retinoblastoma cell. In addition, the major mechanism of cancer damage is unbalanced condition between apoptosis and proliferation.
Apoptosis is a mechanism of programmed cell death while cell proliferation is a process which cells multiply by growing and splitting themselves into two. In tumor, there is an excessive proliferation process without the balance of apoptotic process.
The researchers performed apoptosis calculations by using Annexin V, as a quantitative method of calculating apoptosis, based on the phenomenon of phosphatidylserine released during apoptosis and the ability of Annexin V to bind to high affinity phosphatidylserine. In dead cells, the inner layer is extrinsically bound to Annexin V due to the loosing integrity of the plasma membrane, causing Annexin V to also bind the necrotic cells. In addition, to distinguish between dead cells and apoptotic cells, PI is added to cell suspension. Hence, dead cells and apoptotic cells are distinguished by double labeling of Annexin V and PI and analyzed with flow cytometry[8,23].
From the data analysis in this study, normal data distribution was obtained and the variance homogeneity test of the data with the Lavene test was found to be significant marking a homogeneous data variant. Since the variant of the data is normal and homogeneous, the Tukey test was applied to determine the different effects of Physalis angulata leaf extract on retinoblastoma culture cell among the treatment groups. Analysis of the data with the Tukey test indicated a significant difference (P<0.05), such as in the control group and the treatment groups with a dose of 50 µg/mL (P=0.025) and a dose of 100 µg/mL (P=0.01). Meanwhile, there was no significant difference (P=0.555) between the control group and the treatment group with a dose of 25 µg/mL. Thus, it is concluded that the administration of Physalis angulata leaf extract can increase apoptosis in retinoblastoma cells. From the results of the analysis, the dose of 100 µg/mL affects the greatest increase in apoptosis compared to the other doses.
This is in line with the research of Handayani[13] which states that the ethanol extract of Physalis angulata can inhibit the growth of breast cancer cells by reducing the expression of c-Myc protein, increasing the wild p53 protein and Apaf-1 protein, decreasing the number of mitosis, and increasing the number of apoptosis. This is in line with the research conducted by Ooi et al[14] which asserts that Physalis angulata extract can induce apoptosis in T-47D human breast cancer cells through c-myc-, p53-, and caspase-3-dependent pathways.
Many methods in calculating cell proliferation, such as the method of direct counting with tryphan blue and the MTT assay method. Direct counting is a simple method for assessing the integrity of cell membranes with less sensitive ability. The basis of enzymatic testing in MTT assay is performed to measure the ability of living cells based on mitochondrial activity from cell culture. In this study, living cells are measured with the MTT assay method due to several advantages, such as: relatively faster, sensitive, and more accurate when compared to direct counting method. Beside the two methods above, cell proliferation may be measured by examining one or more specific markers within a cell such as Brdu, Ki-67 or PCNA.
From the data analysis in this study, normal data distribution was obtained and variance homogeneity test with the Levene test was found to be significant marking a homogeneous data variant. Because the variant of the data is normal and homogeneous, the Tukey test was applied to determine the different effects of Physalis angulata leaf extract on retinoblastoma culture cell among the treatment groups. Analysis of the data using the Tukey test indicated a significant difference (P<0.05), such as in the control group and in the treatment groups with a dose of 50 µg/mL (P=0.004) and a dose of 100 µg/mL (P=0.00). Meanwhile, there was no significant difference (P=0.097) between the control group and the treatment group with a dose of 25 µg/mL. It is concluded that the administration of Physalis angulata leaf extract potentially reduced the number of living cells from retinoblastoma cells. From the results of the analysis, the dose of 100 µg/mL reduced the biggest number of living cells compared to the other doses. The decrease in the number of living cells is due to inhibited cell proliferation, which is in accordance with several other studies stating that Physalis angulata extract can reduce cell proliferation[15-16]. However in this study, there is a possibility of the decreasing number of living cells due to the increasing number of apoptosis. The research by Darma et al[15], reported that Physalis angulata has cytotoxic activity in uterine cancer cells through modulation of expression of p53 which causes cessation of cell proliferation. In a study conducted by Fitria et al[16], it was stated that Physalis angulata plays a role in regulation of proliferation, cell cycle and apoptosis of MDA-MB 231 breast cancer cell.
Physalin and withanolides contained in Physalis angulata extract are suspected to provide the anti-cancer mechanism. Based on research, physalin and withanolides have anti-inflammatory, anti-cancer and anti-oxidant effects. There are 6 types of Physalin in Physalis angulata extract, such as physalin A, B, D, F, J and N. The highest content of physalin inhibits the flowers and leaves. Rengifo-Salgado and Vargas-Arana[12] have found several new Withanolides, namely physagulin A, B, C and D which were obtained from methanol extract of Physalis angulata fresh leaves and stems.
Another study conducted by
Boonsombat et al[17] mentioned that
physalins B, D and F isolated from Physalis angulata indicated strong
cytotoxicity against various tumor cell lines, including KB (nasopharyngeal
Ca), A431 (epithelial carcinoma), HCT-8 (Duodenum Ca), PC-3 (prostate Ca), and
ZR751 (breast Ca), with half maximal effective concentration (EC50)
<4 mg/mL. In a study conducted by Hsu et al[18],
physalin B isolated from Physalis angulata had selective cytotoxicity
for melanoma cells. Physalin B can induce melanoma cell apoptosis through NOXA,
caspase-3, and mitochondria-mediated pathways. Wu et al[19] reported that physalin F induces cell apoptosis via the mitochondrial pathway mediated by reactive oxygen species (ROS), and it
suppresses NF-κB activation in renal cancer cells. Damu et al[20] on his study mentioned that biological evaluation of
withangulatins B-H (1-7), and new minor physalin, such as physalin W, together
with 14 identified compounds (withaphysanolide, withaphysalin A,
dihydrowithanolide E, physaprun A, physanolide A, and physalins B, D, F, G, I,
J, T, U, and V) isolated from Physalis angulata plants, provide
extensive cytotoxic activity against human cancer cells. In addition, angulatin
B, physalins D and F indicate strong cytotoxic activity against various human
cancer cell lines with EC50 values ranging from 0.2-1.6 mg/mL.
Another study mentioned that withanolides physangulidines A, B and C isolated
from Physalis angulata presented significant antiproliferative activity
against DU
Another suspected mechanism on the effect of Physalis angulata extract as pro-apoptosis and anti-proliferation agent is the content of Physalis angulata extract which has an influence on cyclooxygenase. In a study conducted by Kalsum et al[24], Physalis angulata extract had a large effect on decreasing the levels of cyclooxygenase (COX)-1, COX-2 and PLA-2. In a study by Sutrisna et al[25], it was found that Physalis angulata extract could reduce COX-2 activity in breast cancer cells (MCF-7).
However, the studies about COX-2
expression in retinoblastoma provide the controversy. In a study conducted by
Wang et al[26] about the COX-2 expression
in the normal human eye and its expression pattern in the selected eye tumor
including retinoblastoma, it was found that there was weak to medium expression
of COX-2. That result is different with study by Suoza Filho et al[27], that found the overexpressed of COX
From the results of this study, the researchers assume that Physalis angulata leaf extract is more likely to have pro apoptotic effect than anti-proliferation. Therefore, Physalis angulata leaf extract is more suitable as an adjuvant chemoradiation therapy which is anti-proliferation in its future use. However, accurate data remains limited due to the need of further research with better cell quality and a longer incubation period (48 or 72h). In addition to obtain better proliferation data, it is recommended to synchronize cells for 48h before proliferation measurements are carried out. The result from this study is expected to be the basis for further research to discover the potency of Physalis angulata as an anticancer agent of retinoblastoma.
The limitation of this study lies on the very small amount of cell density, due to slower growth of retinoblastoma cells. There are several possible reasons such as: 1) the utilized cell was in the fourth passage (not optimal) due to genetic instability and phenotypic shift during the previous culture process; 2) there is an error during the storage process or in the previous treatments, in liquid nitrogen, 3) the number of cells which were frozen is insufficient during previous breeding. Thus, when the thawing process was carried out, the number of cells obtained is insufficient despite the 2wk process (the half-life for the retinoblastoma cell is 40h); 4) the lack of the dose range used in this study; therefore, further research with a larger replication group and more cell density and a longer incubation period (48 or 72h) is required to obtain better results and to find out the effective doses as well as the toxic doses of Physalis angulata leaf extract. Besides, the chemical structure of Physalis angulata leaf was not specifically separated due to the lack of available facilities, time and expensive cost. In the future, future research is advised to separate the chemical structure of the object and its effect on apoptosis and proliferation in normal cells.
Further research is also suggested to perform reexamination with better condition and better cell quality. Further research on the effect of Physalis angulata leaf extract on proliferation using Ki-67, BrdU, or other more sensitive and more specific measurement methods is also recommended to conduct 48-hour synchronization before proliferation measurement to get more accurate proliferation rate.
In addition, further research is advised to explore the active substances in Physalis angulata leaf extract separately (by using periodic titration to isolate the active substances) to find out the direct effect on retinoblastoma cell culture. Finally, further research to determine the effective dose and the toxic dose and the effect of Physalis angulata leaf extract on normal cell culture is strongly advisable.
ACKNOWLEDGEMENTS
Conflicts of Interest: Chairissy MD, None; Wulandari LR, None; Sujuti H, None.
REFERENCES