
Citation: Abouelkheir HY, Badawi AE, Abdelkader AM, El-Kanishy A,
Saleh S, Abou Samra WA, Kasem MA, Mokbel T. Does the scleral encircling band
provide a protective effect against the progression of diabetic retinopathy? Int
J Ophthalmol 2019;12(9):1408-1414
DOI:10.18240/ijo.2019.09.06
·Clinical Research·
Does the scleral encircling band provide a protective effect against the progression of diabetic retinopathy?
Hossam Youssef Abouelkheir, Amani E Badawi, Amr M. Abdelkader, Amr El-Kanishy, Sameh Saleh, Waleed Ali Abou Samra, Manal Ali Kasem, Tharwat Mokbel
Mansoura Ophthalmic Center, Faculty of Medicine, Mansoura University, Mansoura 35516, Egypt
Correspondence to: Amr M. Abdelkade. Department of Ophthalmology, Mansoura Ophthalmic Center, Faculty of Medicine, Mansoura University, Mansoura 35516, Egypt. dramrabdelkader@gmail.com
Received:
Abstract
AIM: To evaluate the effect of scleral encircling bands on the development and progression of diabetic retinopathy (DR) in diabetic patients.
METHODS: The medical records of diabetic patients who underwent unilateral retinal detachment (RD) surgery using scleral buckle and encircling band were reviewed retrospectively. Both eyes of patients were included in the study: one eye in each patient had a scleral buckle with encircling band (the operated eye) and the other one is the non-operated eye. The demographic characters, duration of diabetes and period between surgery and the last recall visit were retrieved from each patient. All the cases underwent fundus photo and fluorescein angiography (when indicated) to confirm the DR staging.
RESULTS: Totally 25 patients fulfilled the inclusion and the exclusion criteria were become eligible for the study. A total of 50 eyes of 25 patients were enrolled in this analysis. The mean period of time passed from surgery with encircling band and the last reassessment visit was 12.5±2y. Even though DR could develop in the operated eyes, it was at a less degree of severity compared to the non-operated eyes of same patients (P=0.027).
CONCLUSION: Scleral encircling bands have protective effects against the development and progression of DR.
KEYWORDS: diabetic retinopathy; scleral encircling bands; retinal detachment
DOI:10.18240/ijo.2019.09.06
Citation: Abouelkheir HY, Badawi AE, Abdelkader AM, El-Kanishy A, Saleh S, Abou Samra WA, Kasem MA, Mokbel T. Does the scleral encircling band provide a protective effect against the progression of diabetic retinopathy? Int J Ophthalmol 2019;12(9):1408-1414
INTRODUCTION
Diabetic retinopathy (DR) remains one of the most important causes of blindness among population[1]. Its development and progression are influenced by the duration and type of diabetes besides many other systemic and ocular factors[2].
Aiello[3] reported that eyes with extensive chorio-retinal scars due to inflammatory conditions, trauma, etc showed a decreased prevalence and severity of DR. Depending on this observation, panretinal photocoagulation was used for the treatment of proliferative diabetic retinopathy (PDR). The most widespread explanation is that destruction of the ischemic retina by laser decreases the release of angiogenic factors[4].
Myopia has been reported to have a prophylactic effect against the development of DR[5]. There is an evidence that myopic eyes have reduced blood flow[6] and consequently associated with less damaged micro-circulation in eyes of diabetic patients[7].
From the same point of view, scleral buckling operations and encircling bands were found to decrease the blood flow velocity in the central retinal artery by 35%-50%[8-9], stated that encircling is the factor which is responsible for reducing the ocular blood flow after buckling procedures.
Although several studies have evaluated the protective and risk factors of DR progression, the impact of the scleral buckling on DR has not been elaborated. To the authors’ knowledge, it is the first report dealing with the association between buckling and the DR progress. The study hypothesis emerged from a striking observation that patients with a history of repaired retinal detachment (RD) with encirculing band, have less DR stages in the operated eye than the fellow eye.
To observe and record the relation between the encircling band and progression of DR and to find out if there a protective effect of an encircling band similar to that seen in myopia, we thought that our aim could be achieved by comparing both eyes of the same diabetic patient: the operated eye with encircling band and the other non-operated eye over a sufficient period of time.
SUBJECTS AND METHODS
Ethical Approval The aim of the study was explained
to the recruited patients and informed consent was obtained from all patients.
The study was approved by the Ethics Committee Of Faculty of Medicine, Mansoura
University (IRB “R/
Study design A retrospective observational cohort study.
Subjects The medical records of all patients who underwent surgical repair of unilateral rhegmatogenous retinal detachment (RRD) using scleral buckle and an encircling band technique at the surgical retina unit (Mansoura Ophthalmic Center, Mansoura University, Egypt) were reviewed retrospectively. The review period of extended for cases operated from January 2001 through December 2008.
In the beginning, we started to collect data of patients who were initially diagnosed with RRD with only one eye and treated by the scleral buckle with the encircling band procedure. All patients should have a known history of diabetes at the time of presentation with no evidence of any changes related to DR by that time.
An exclusion list was put to exclude patients with one or more of the following conditions if found in either eye: glaucoma, myopia with refraction more than -6 D, anisometropia, amblyopia, optic atrophy, history of ocular injury or intraocular surgery and different chorio-retinal atrophic conditions that may affect the retinal blood flow.
Methods
Surgical procedure Two main surgeons (Abouelkheir HY , El-Kanishy A) and one assistant (Abdelkader AM) performed the surgical procedures of the selected patients according to each RD condition. The scleral buckle (silicone tire 279, Labtician Ophthalmics, Inc., Oakville, Ontario, Canada) was used as a tamponade for all visible retinal break with a silicone circling band (240, Labtician Ophthalmics, Inc., Oakville, Ontario, Canada).
Data collections A process of recalling the patients with scheduled appointments to come back for a reassessment of their retinal condition was carried out in the period between June 2018 and September 2018. Recalled patients were underwent to a comprehensive ocular and systemic history taking and re-examination with an exclusion of patients developed any ocular disease found in the exclusion list in either eye during the period since the time of the RD repair and the last recall visit in 2018. In addition, we excluded patients who had undergone any kind of treatment related to DR for instance, laser photocoagulation, intra-vitreal steroid, anti-vascular endothelial growth factor (VEGF) or vitrectomy as we cannot comfortably judge on the correlation between the effectiveness of treatment and stage of DR by the time of reassessment. Besides recording the demographic characters, the following data were retrieved from each patient: duration of diabetes, the duration between surgery and the last recall visit. Slit-lamp biomicroscopy examination of both anterior and posterior segments and a detailed fundus examination with the indirect ophthalmoscope and +90 D lens was performed. All the cases clinically diagnosed to have DR underwent fundus photo and fluorescein angiography (when indicated) to confirm the diagnosis and DR staging using Retinal Camera (TopoconTRC-50 DX, Hasunuma-cho, Itabashi-ku, Tokyo, Japan) by an expert medical retina specialist (Badawi AE).
Diabetic retinopathy staging The documentation of the stage of DR in both eyes was based on International Clinical Diabetic Retinopathy Disease Severity Scale[10]. Table 1 exhibits the definitions and gradings of different DR stages.
Table 1 DR staging (International Clinical Diabetic Retinopathy Disease Severity Scale)[10]
DR stage |
Ophthalmoscopic findings |
No retinopathy |
No apperent abnormalities |
Mild NPDR |
Only microaneurysms |
Moderate NPDR |
More than just microaneurysms, but less than severe NPDR. |
Severe NPDR |
Any of the following without any PDR signs: 1) more than 20 intraretinal haemorrhages in each of 4 quadrants; 2) venous beading in 2 or more quadrants; 3) prominent irma in 1 or more quadrant. |
PDR |
One or more of the following: 1) neovascularization; 2) vitreous/preretinal haemorrhage. |
NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy; IRMA: Intraretinal microvascular abnormalities.
Statistical Analysis Both eyes of each patient were
enrolled (to exclude any bias from the systemic factors) with a total number of
50 eyes. The eyes with an encircling band (the operated eyes) were one group,
and the other eyes (non-operated eyes) were the second group. The collected
data were analyzed by IBM SPSS (statistical package for social science)
software package version 20.0. Chi-square test was used for categorical
analysis between the two groups. The Fisher exact test was selected if the
expected count in any cell was five or less. Mann-Whitney and Wilcoxon tests
were used to measure the mean rank of DR severity in the two groups. In this
study, the study sampling were calculated using a computer program (G power
RESULTS
The 25 patients (13 males and 12 females) fulfilled the inclusion and the exclusion criteria have become eligible for the study. A total of 50 eyes of 25 patients with diabetes were enrolled in this analysis. At the time of recall assessment, the mean age was 61.8±5.2y. The mean duration of diabetes was 14.9±2.6y and the mean period of time passed from surgery with an encircling band and the last reassessment visit was 12.5±2y. Table 2 demonstrates the demographic and descriptive findings of the study participants.
Table 2 Demographic data, duration of diabetes, period between surgery and reassessment, and stage of DR in the study eyes
Stage of DR in the operated eyes |
Stage of DR in the non-operated eyes |
Period between surgery and reassessment visit (y) |
Duration of diabetes (y) |
Sex |
Age at the reassessment visit (y) |
Patient’s ID |
None |
Mild NPDR |
12 |
14 |
Male |
66 |
1 |
None |
None |
11 |
12 |
Male |
61 |
2 |
None |
Mild NPDR |
14 |
15 |
Female |
56 |
3 |
None |
None |
10 |
11 |
Male |
49 |
4 |
Mild NPDR |
Severe NPDR |
15 |
17 |
Female |
65 |
5 |
None |
Moderate NPDR |
14 |
16 |
Female |
63 |
6 |
None |
None |
11 |
12 |
Female |
60 |
7 |
Mild NPDR |
Severe NPDR |
13 |
18 |
Male |
59 |
8 |
None |
None |
10 |
13 |
Male |
57 |
9 |
Severe NPDR |
PDR |
14 |
19 |
Female |
67 |
10 |
None |
None |
10 |
12 |
Male |
56 |
11 |
None |
None |
12 |
14 |
Male |
68 |
12 |
Severe NPDR |
PDR |
16 |
21 |
Female |
72 |
13 |
PDR |
PDR |
15 |
20 |
Male |
69 |
14 |
None |
None |
11 |
13 |
Male |
64 |
15 |
None |
Mild NPDR |
11 |
14 |
Female |
63 |
16 |
None |
Moderate NPDR |
14 |
16 |
Female |
67 |
17 |
None |
None |
10 |
12 |
Male |
60 |
18 |
None |
None |
10 |
11 |
Male |
54 |
19 |
Moderate NPDR |
Moderate NPDR |
15 |
17 |
Female |
62 |
20 |
Mild NPDR |
PDR |
16 |
19 |
Female |
65 |
21 |
None |
Mild NPDR |
12 |
14 |
Female |
59 |
22 |
None |
Mild NPDR |
11 |
15 |
Male |
61 |
23 |
None |
None |
12 |
13 |
Female |
58 |
24 |
None |
Mild NPDR |
13 |
15 |
Male |
64 |
25 |
NPDR: Non-proliferate diabetic retinopathy; PDR: Proliferative diabetic retinopathy.
There was a statistically significant difference supporting the decreased incidence of development of DR in the operated eyes with encircling band compared with the non-operated eyes (P=0.027, Table 3). Analysis of the 50 eyes showed that DR with different stages was found in 22 (44%) of eyes while 28 eyes (56%) showed no evidence of any diabetic changes.
Table 3 The severity of DR at the time of reassessment among the 2 groups
Group |
Mean rank |
P |
Operated eyes |
21.38 |
0.027 |
Non-operated eyes |
29.62 |
|
Mann-Whitney and Wilcoxon tests. |
||
Among those 22 eyes that showed DR changes, only 7 (31.8%) eyes had surgery with an encircling band and 15 (68.2%) eyes were non-operated. Figure 1 demonstrates the distribution of DR stages in the operated and non-operated eyes.
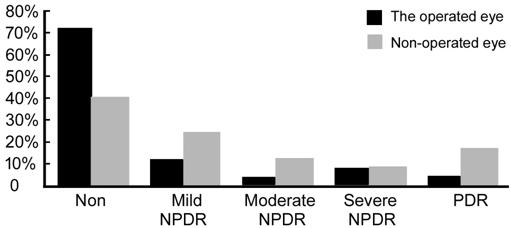
Figure 1 DR stages in both operated and non-operated eyes.
Despite there was a significant decrease in the whole DR incidence in the operated eyes, there was a significant difference between the operated eyes and the fellow eyes in “no retinopathy and PDR” stages mainly. In the operated eyes with encircling bands: 18 (72%) eyes showed no evidence of DR versus 10 (40%) in the non-operated eyes (P=0.019) while the PDR stage was recorded in 1 (4%) eye in the operated eyes versus 4 (16%) eyes in the non-operated group (P=0.022, Table 4).
Table 4 DR stage distributions among the two groups and its significance n (%)
Stage |
Non-operated eyes |
Operated eyes |
P |
Non |
10 (40) |
18 (72) |
|
Mild NPDR |
6 (24) |
3 (12) |
0.693 |
Moderate NPDR |
3 (12) |
1 (4) |
0.712 |
Severe NPDR |
2 (8) |
2 (8) |
1.000 |
PDR |
4 (16) |
1 (4) |
|
NPDR: Non-proliferate diabetic retinopathy; PDR: Proliferative diabetic retinopathy. Chi-square test (Fisher test), aP˂0.05.
Figures 2 and 3 illustrate that even though DR could develop in the operated eyes, it was definitely at a less degree of severity compared to the non-operated eyes of the same patients.
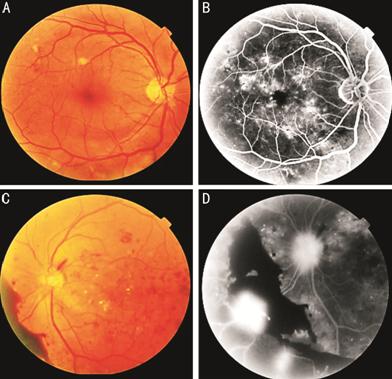
Figure
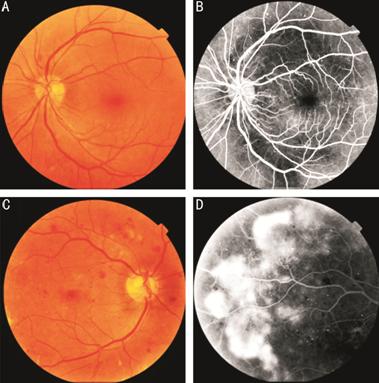
Figure
DISCUSSION
In spite, the bothersome drawbacks of the scleral buckling procedure, it is still commonly used as a primary approach for RRD repair[11]. Indentation of the globe with axial length alteration and a refractive shift is one of the frequent side effects of the scleral buckling[12-13].
To study the influence of encircling band on DR, we retrospectively compared both operated and non-operated eyes of the same diabetic patient to adjust all the general and systemic risk independent factors that may affect the development and progression of DR. The current results demonstrated obviously that the scleral buckling procedure affected the DR progression after the surgery. There was a significantly decreased incidence of development of DR in the operated eyes with encircling bands compared to the non-operated eyes. Furthermore, our findings showed that even though DR could develop in eyes with encircling bands, it remained at a less stage of severity compared to the non-operated eyes of the same patients (asymmetric DR), as well as the DR severity was correlated with the date of operation.
Predominantly, DR progresses in a symmetrical manner in both eyes over long periods of time, making it extremely exceptional to occur asymmetrically[5]. Several definitions of asymmetric DR have been described: unilateral non-proliferate diabetic retinopathy (NPDR) or unilateral PDR with no retinopathy in the fellow eye, NPDR in one eye and PDR in the fellow eye, and advanced diabetic retinal changes in one eye and low-risk PDR in the other eye[14].
The protective effect of myopia and elongated axial length against the development of PDR has been shown in many studies[5,7,15]. This could explicate the association between myopia and asymmetrical DR. The authors supposed that, the scleral buckling with longer axial length, as a result, confers the similar protection as in myopia. This effect may be attributed to the low ocular blood flow in myopic eyes[6,16]. Refractive and axial myopic eyes seem to have narrow retinal vessels, increased branching in both venules and arterioles, and less tortuosity in the arterioles[5].
Additionally, myopic eyes suffer more frequently from total posterior vitreous detachment (PVD) (50% vs 12.2% in emmetropic eyes)[17] which reduces the risk of PDR and progression of neovascularization, while the incomplete PVD is reported to be associated with more severe DR. The vitreoretinal traction acts as vitreous scaffold that causes the proliferation of new vessels, thickening, and progression of the fibrous bands[18].
However, a large study did not disclose the association between myopia and with the incidence of DR, but it considered myopia as a protective factor against the progression to PDR in younger-onset diabetes patients only[19]. The pathological microvascular alterations in early stages of DR are mainly perivascular and intravascular such as microaneurysm formulation and thickening in the capillary basement membrane, while in advanced stages, the pathology is foremost extravascular like proliferation and exudation[20]. In myopic eyes, reduced blood flow could decrease the extravasation of blood elements that act as a promoter for macrophages to potentiate the proliferation consequences. This explains the protective role of myopia in delaying the vision-threating stages rather than its impact on the disease incidence[5,21]. The authors assumed the same theory that the myopic shift and AL alterations occurring after the scleral buckling provides such protection against DR progression.
Reduced ocular blood flow with
scleral buckle was observed in many studies[22-24]. Regillo et al[8] used color Doppler imaging to study blood flow changes after scleral buckling
with encircling bands and their results showed a decrease of average blood flow
velocity in the central retinal artery by 35% on the first operative day and by
50% one week postoperatively. In two different studies[9,25], the central retinal artery peak systolic and end
diastolic velocities were (6.64 and
Retinal hypoxia has been known as the main contributor of the induction of VEGF and PDR development[31]. Reduction in oxygen demand and consumption in eyes with longer axial length was established that might mitigate the consequence of hypoxia in diabetic patients[32]. Several studies have speculated a lower concentration of VEGF in the aqueous of elongated eyes[33-35], thus clarifies the inverse relationship between PDR prevalence and longer axial length. Our current findings supported their findings, where there was a significant decrease the PDR incidence in the operated eyes. Further, scleral buckling reduces the probability of PVD occurrence as well as a progression of previous one via vitreous base support and shorting the distance between the vitreous base and the retina. Likewise, the buckling decreases vitreous traction and epiretinal membranes[36] and thus promotes an extra protection against the proliferation. In addition, panretinal photocoagulation was found to decrease ocular blood flow[37-38]. These studies reveal the major role of ocular blood flow in DR. Encircling, through reduction of the retinal blood flow, appears to interfere with the main hemodynamic change associated with development and progression of DR.
This study showed decreased DR severity in eyes with formerly successful scleral buckling with encircling bands. Scleral buckling at the equator during diabetic vitrectomy may improve the results and help regression of the proliferative retinopathy. However, the limitation of this study includes absence of another control group of patients who did not undergo surgery at all due to the retrograde nature of the study and insuffcient use of carotid doppler to role out ocular ischemia syndrome. Studies with larger sample size including independent control groups are needed for summation of more outcomes including computation of ocular blood flow and considering the interindividual variance.
In conclusion, encircling bands have protective effects against the development and progression of DR. Depending on this observation we think that applying an encircling element at the equator during diabetic vitrectomy may improve the results and help regression of the proliferative retinopathy. However, in order to confirm this practical application, a separate study should be done with large numbers of eyes and long term follow up to get more information about this procedure.
ACKNOWLEDGEMENTS
Conflicts of Inerest: Abouelkheir HY, None; Badawi AE, None; Abdelkader AM, None; El-Kanishy A, None; Saleh S, None; Abou Samra WA, None; Kasem MA, None; Mokbel T, None.
REFERENCES