
Citation: Yu T, Han XG, Gao Y, Song AP, Dang GF. Morphological and
cytological changes of meibomian glands in patients with type 2 diabetes
mellitus. Int J Ophthalmol 2019;12(9):1415-1419
DOI:10.18240/ijo.2019.09.07
·Clinical Research·
Morphological and cytological changes of meibomian glands in patients with type 2 diabetes mellitus
Tao Yu1, Xu-Guang Han2, Yang Gao1, Ai-Ping Song1, Guang-Fu Dang1
1Shandong Provincial Qianfoshan Hospital, the First Hospital Affiliated with Shandong First Medical University, Jinan 250014, Shandong Province, China
2Jinan Aier Ophthalmology Hospital, Aier Eye Hospital Group, Jinan 250014, Shandong Province, China
Correspondence to: Ai-Ping Song. Shandong Provincial Qianfoshan Hospital, the First Hospital Affiliated with Shandong First Medical University, No.16766 Jingshi Road, Jinan 250014, Shandong Province, China. 13793164931@163.com
Received:
Abstract
AIM: To observe the changes of microcellular structure of meibomian glands (MGs) in type 2 diabetes mellitus (DM), and to explore its correlation with the duration of diabetes.
METHODS: The study assessed 132 eyes of 132 patients with
type 2 diabetes mellitus (DM group) and 100 eyes of 100 non-diabetic
participants (NDM group). All patients underwent the examination of the
Keratograph
RESULTS: Compared with that in NDM group, the meiboscore was significantly higher (Z=-4.057, P<0.001), and there were more MGs dropout in DM group. With the prolongation of the course of diabetes, the absence of MGs aggravated and the MGs dropout score increased (r=0.596; P<0.001). LSCM showed that there were various cytological alterations in acinar cells of MGs with the progress of diabetes duration, such as expansion, atrophy or fibrosis of MG acinar units, decreased density of MG acinar units, deposition of lipid substances, infiltration of inflammatory cells, proliferation of fibrous tissues, etc. And the opening of the glandular duct changed from smooth at the beginning to narrow, blocked, fibrotic and so on. Compared with that in NDM group, the MGAUD in DM group was significantly lower (Z=-9.713; P<0.001), the MGALD and MGASD were significantly larger (Z=-9.751, -6.416; P<0.001). With the duration of diabetes, the MGAUD reduced, the MGASD increased (r=0.860, 0.364, P<0.001); but the MGALD had no correlation with diabetic duration (r=0.133, P=0.151).
CONCLUSION: With the progress of diabetes, the meibomian glandular acinar cells of diabetic patients show various manifestations. Those changes may result in the dysfuction of the MGs, tear film instability and dry eye symptoms in patients with type 2 DM.
KEYWORDS: diabetes; meibomian glands; tear films; keratograph; laser scanning confocal microscopy
DOI:10.18240/ijo.2019.09.07
Citation: Yu T, Han XG, Gao Y, Song AP, Dang GF. Morphological and cytological changes of meibomian glands in patients with type 2 diabetes mellitus. Int J Ophthalmol 2019;12(9):1415-1419
INTRODUCTION
Diabetes mellitus (DM) is a global disease and the prevalence is anticipated to continue to increase. The ocular complications of DM negatively impact the quality of life and carry an extremely high economic burden. Numerous studies have demonstrated that patients with diabetes are more likely to have ocular surface disorders, such as the dysfunction of lacrimal gland secretion, superficial punctate keratits, persistent epithelial defects, and decreased corneal sensitivity, etc[1]. Meibomian glands (MGs) are large sebaceous glands located in the tarsal plates of the eyelid that secrete a mixture of lipids and proteins called meibum onto the ocular surface, forming a superficial lipid layer that prevents the evaporation of tears[2]. Abnormalities in the MGs may cause instability of the tear film, resulting in either chronic irritation or damage to the ocular surface epithelium. Our previous study preliminarily observed some abnormal changes in the acinar glands of MGs in patients with type 2 diabetes[3]. However, due to the small number of samples and the lack of insufficient research, the study did not further classify these abnormal changes or analyze how these changes affect MG function with the course of diabetes.
In this study, we continued the previous study and observed the morphological and cytological changes of the MGs in type 2 DM and analyze its relationship with the duration of diabetes, so as to clarify the influencing factors and pathogenesis of the alterations of the MGs and to provide a basis for clinical treatment of such diseases.
SUBJECTS AND METHODS
Ethical Approval The study protocol was approved by the Ethics Committee of Qianfoshan Hospital Affiliated with Shandong First Medical University and adhered to the tenets of the Declaration of Helsinki. Before the examination, we have got written informed consents from all subjects.
Subjects In this comparative study, 132 eyes of 132 patients with type 2 DM (DM group; 64 males, 68 females; mean age 60.18±7.59y) and 100 eyes of 100 non-diabetic participants (NDM group; 52 males, 48 females; mean age 60.27±7.59y) were evaluated in Qianfoshan Hospital Affiliated to Shandong University during the period from October 2015 to November 2017. The mean duration of diabetes was 7.66±5.34y (range, 1-20y). The patients in DM group were those who had been diagnosed with diabetes in the Diabetes Department and were required to go to the Ophthalmology Clinic for fundus examination. And the participants in NDM group were those who came to the Optometry and Cataract Clinics complaining of poor vision. Exclusion criteria included ocular inflammation, Sjögren’s syndrome, long-term contact lens wear, continuous eye drops, a history of ocular trauma and surgery, and other treatments and systemic diseases that affect tear film quality and stability.
Keratograph
Laser Scanning Confocal Microscopy
Examination Laser scanning confocal microscopy
(LSCM) was performed using Heidelberg Retina Tomograph III–Rostock Cornea
Module (Heidelberg Engineering GmbH, Dossenheim, Germany) after Keratograph
Statistical Analysis The examination results of the right eye were analyzed using the Statistical Package for the Social Sciences version 17.0 (SPSS Inc, Chicago, III, USA). The Mann-Whitney U test was used for the parameters comparison among the two groups. The spearman correlation analysis was used to determine the correlation of diabetes duration and the indexes above. A P values less than 0.05 was considered to be statistically significant difference.
RESULTS
Keratograph
Table 1 Distribution of the MD score n (%)
Stage |
DM (n=132) |
NDM (n=100) |
0 |
51 (38.63) |
67 (67) |
1 |
29 (21.97) |
18 (18) |
2 |
25 (18.94) |
10 (10) |
3 |
21 (15.91) |
5 (5) |
4 |
6 (4.55) |
0 |
DM: Diabetes mellitus group; NDM: Non-diabetic participants group.
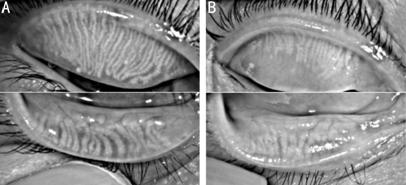
Figure 1 MGs dropout were showed by
Keratograph
LSCM showed that the cell structure
of the MG in DM group presented different forms with the progress of diabetes
duration. In the early, the morphology of MG in DM group were roughly similar
with NDM group. Most MG bubbles were round or oval, its shape were regular, and
the density of gland bubble was high. The epithelial cells which present
uniform density and medium reflection existed in the periphery of the acini.
There was a uniform grey dark space and occasional highlight reflective
material in the internal of acinus cavity (Figure
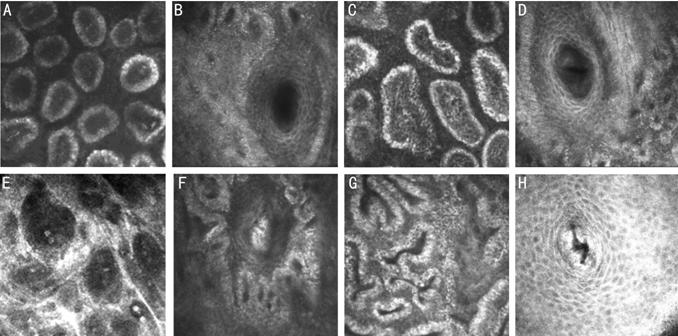
Figure 2 The alterations of MGs evaluated by LSCM in DM group A: Most MG bubbles were round or oval, its shape were regular, and the density of gland bubble was high; B: The structure of MG ductal wall was clear and smooth; C: MG bubble became expansion, a few of acinar presented atrophy, the shape became irregular, and the density of gland bubble decreased; D: The ductal wall became thicker, and the opening of the gland duct became narrower; E: Most of the acinar cells became expansion and deformation, and the cytoderm became thinning; F: The structure of ductal wall were disappeared and cornified, and there were fat deposition in the internal of ducts; G: The atrophic acinars became strea and the cell form were very irregular; H: The structure of ductal wall were disappeared and the opening of the gland duct was atrophic and cornified.
With the progress of the diabetes
duration, gland bubble became expansion, a few of acinar presented atrophy, the
shape became irregular, and the density of gland bubble decreased. The
epithelial cells presented highlight reflection, part of structure of the
epithelial cell was not clear. There was a highlight reflective space which
consider for eyelid fat deposition in the internal of acinus cavity. There was
uneven brightness of signal which consider for the presence of inflammatory
cells in the interstitial of gland bubble (Figure
In the late duration of diabetes and
diabetic retinopathy, MGs appeared a variety of changes. Most of the acinar
cells became expansion and deformation, and the cytoderm became thinning. There
was uneven high brightness density which consider for pathologic lipid
deposition in acinar chamber. The cellular structure became disorder and
disappear in some cytoderm. There were a few of fibrous tissue and inflammatory
cells surround the gland bubbles. Some acinars became atrophy (Figure 2E). The
structure of ductal wall were disappeared and cornified. There were fat
deposition in the internal of ducts (Figure
Otherwise a few atrophic acinars
were strea. The form were very irregular, and there were uniform gray liquid
dark space in the bubble chamber. The cellular structure of the bubbles wall
became disorder and there were a few of inflammatory cells surround the gland
bubbles (Figure
Compared with the NDM group, the MGAUD in DM group was significantly lower while the MGALD and MGASD were significantly larger (Table 2). MGAUD reduced with the duration of diabetes, and MGAUD was significantly negatively correlated with the duration of diabetes (r=-0.860, P<0.001); MGALD has no correlation with diabetic duration (r=0.133, P=0.151). MGASD was significantly positively correlated with the duration of diabetes (r=0.364, P<0.001).
Table 2 Parameters between the two groups
Parameters |
DM group (n=118) |
NDM group (n=100) |
P |
Z |
MGAUD, U/mm2 |
79.42±24.68 |
115.38±19.05 |
0.000 |
-9.713 |
MGALD, μm |
91.21±22.35 |
58.01±14.99 |
0.000 |
-9.751 |
MGASD, μm |
45.87±11.68 |
31.34±34 |
0.000 |
-6.416 |
DM: Diabetes mellitus group; NDM: Non-diabetic participants group; MGAUD: Meibomian gland acinar unit density; MGALD: Meibomian gland acinar longest diameter; MGASD: Meibomian gland acinar shortest diameter.
DISCUSSION
In our study, we observed the morphological and cytological alternations of MGs in all of the subjects to evaluate the function of the MGs. Keratograph meibography showed that 61.36% of the patients in DM group had MG dropout, compared with 33% in the NDM group. And the degree of dropout increased significantly and gradually with the progress of diabetic duration. The dropout represents atrophy and injury of the MGs. This suggests that MG function is affected by the chronic course of diabetes. LSCM revealed that most of the morphology of acinar cells were regular and the density of acinar untis were high in NDM group, while there were more abnormal cytological alterations of acinar cell in DM group. Those anomalies showed many different manifestations with the progress of diabetes. Most of them were characterized by dilation, irregular morphology and decreased density of the MG acinar units. There were a lot of highlight reflections inside the acinar cytoderm and around the acinar cell, which was different from the general population and considered inflammatory cells infiltration. This suggested that inflammatory factors might also play a role in the progression of pathological changes. Besides, there were a lot of uneven highlight reflections which considered for pathologic lipid accumulated in the aciner chamber. It was found in patients with long course of diabetes, some of the acinar cytoderm became thinner and was surrounded by a mass of fibrous tissues, and a few acinar cells became atrophic and surrounded by a much larger number of inflammatory cells. We also found that there were many abnormalities in the opening of glandular tubes in diabetic patients, such as thickening, keratinization and constriction of the tube wall, which could cause the contraction and diastolic dysfunction of the ducts, and even the lipid blockage in the ducts. The stasis of lipid would cause the expansion of the acinar units, disorder and even disappearance of the acinar cytoderm, compression of the intercelluar mass which would be eventually replaced by the fibrous tissue.
Our previous research found that the tear film rupture time was significantly shorter in DM group than that in NDM group, and 68.64% of patients in DM group expressed with the dry eye symptoms, and the proportion was 30% in NDM group[3]. The DM group suffered more severe ocular discomfort and tear film instability. The stability of the tear film is affected by the lipid layer of the tear film. The MGs secret lipids, which are the main constituent of the lipid layer. Therefore, the changes of MGs can give rise to the abnormality of tear film. These findings supported that due to the abnormality of the morphology and structure of MGs in diabetic patients, the stability of the tear film changed, resulting in the discomfort of ocular surface.
The mechanisms producing these morphological and cytological changes in the MGs in the type 2 DM were still unclear. It has been confirmed that the androgen and estrogen receptors protein expressed in acinar epithelial cell nuclei and these hormones influenced the lipid profile within this tissue[10-11]. Previous studies indicated that the androgens could regulate the production of these lipids and androgen deficiency might lead to MG dysfunction, altered lipid profiles in MG secretions, tear film instability, and evaporative dry eye[11]. Other studies showed that the estrogen receptors might play a role in modulation of the lipid layer of the tear film, and their activity may be linked to MG dysfunction and dry eye syndrome[12]. According to clinic date, the androgen and estrogen levels of diabetic patients were significantly lower than those of normal people[13]. Lower levels of two sexual hormones in diabetic patients may lead to MG dysfunction.
Changes in MGs may be influenced by neuropathological mechanisms. Studies have showed that the MGs were richly innervated by diverse nerve fiber types[14]. The immunohistochemical staining suggested a largely parasympathetic origin for this innervation, with relatively smaller contributions from sympathetic and sensory sources. These findings also suggested that MG secretion is under the control of diverse neurotransmitter-neuromodulator mechanisms. It has been demonstrated in animal models that any injury or disease of the nerves can result in dry eyes[15]. Neuropathy, as a common complication of diabetes mellitus[16], may lead to MG dysfunction through abnormal innervation.
Studies have shown that the secretion and distribution of MGs are mainly dependent on the blink movements[17]. Lipid is delivered to the tear film in the up-phase of the blink. The lipid layer comes to a stop well after completion of the blink and remains relatively immobile until it is compressed in the down-phase of the blink that follows. Delivery of lipid to the marginal reservoirs is mainly the result of continuous secretion, under neural and hormonal control, supplemented by lid action. They probably also provide the chief route for meibum excretion[18]. However, diabetic patients generally exhibited decreased corneal sensitivity and blink movement[19], which will lead to less lipid excretion and blockage of glandular duct, further resulting in MG dysfuction.
Finally, our study presents some limitations. First of all, MGs changes related with diabetes were multifactorial, so that we could not cover all confounding factors. Secondly, we only evaluated morphologic changes in the MGs and had not involved the changes in the composition of lipids.
In conclusion, our study found that there were more morphological and cytological alterations of acinar and glandular ducts of MGs in patients with diabetes, and the abnormalities of MGs became serious with the progress of diabetes duration. Keratograph and LSCM provide a method for doctors to understand the microstructure and pathophysiological characteristics of the MGs of patients with diabetes, which can help doctors diagnose, treat and evaluate these diseases.
ACKNOWLEDGEMENTS
Conflicts of Interest: Yu T, None; Han XG, None; Gao Y, None; Song AP, None; Dang GF, None.
REFERENCES