
Citation: Mirzayev I, Gündüz AK, Özalp Ateş FS, Özcan G, Işık MU.
Factors affecting recurrence after surgical treatment in cases with ocular
surface squamous neoplasia. Int J Ophthalmol 2019;12(9):1426-1431
DOI:10.18240/ijo.2019.09.09
·Clinical Research·
Factors affecting recurrence after surgical treatment in cases with ocular surface squamous neoplasia
Ibadulla Mirzayev1, Ahmet Kaan Gündüz1, Funda Seher Özalp Ateş2, Gökçen Özcan1, Mehmed Uğur Işık3
1Department of Ophthalmology, Ankara University Faculty of Medicine, Ankara 06620, Turkey
2Department of Biostatistics, Ankara University Faculty of Medicine, Ankara 06620, Turkey
3Şanlıurfa Balıklıgöl State Hospital, Şanlıurfa 63100, Turkey
Correspondence to: Ahmet Kaan Gündüz. Department of Ophthalmology, Farilya Business Center 8/50, Ufuk Universitesi Cad, Çukurambar, Ankara 06510, Turkey. drkaangunduz@gmail.com
Received:
Abstract
AIM: To evaluate the risk factors leading to recurrence in patients with ocular surface squamous neoplasia (OSSN)
METHODS: The records of 112 patients with OSSN who underwent treatment and follow-up between February 1999 and August 2018 were reviewed retrospectively.
RESULTS: Totally 67 patients (59.8%) were male and 45 patients (40.2%) were female. The mean age at presentation was 63.7y (range 22-87y). Partial lamellar scleroconjunctivectomy (PLSC) was performed in 105 (93.7%) cases and enucleation was performed in 7 (6.3%) cases due to bulbus invasion as the first step treatment. Treatments used in addition to PLSC included cryotherapy in 78 eyes (74.3%), alcohol epitheliectomy in 57 eyes (54.3%) for presence of corneal involvement, and amniotic membrane transplantation in 17 eyes (16.2%) for ocular surface reconstruction. Topical mitomycin C was used in 10 patients (9.5%) and strontium-90 (Str-90) treatment in 4 (3.8%) patients because surgical margins were tumor positive at the histopathological examination. Postoperative histopathologic diagnoses were squamous cell carcinoma (52 cases), carcinoma in situ (44 cases), moderate conjunctival intraepithelial neoplasia (11 cases), and mild conjunctiva intraepithelial neoplasia (5 cases). At a mean follow-up of 20.1mo, tumor recurrence was observed in 21 (18.8%) cases. The rate of recurrence was found to be lower in cases that underwent supplemental cryotherapy compared to those that did not (P<0.001). There was no metastasis in any case.
CONCLUSION: In our series, the recurrence rate is 18.8% and overall globe salvage rate is 90.2% for OSSN at relatively short-term follow-up.
KEYWORDS: alcohol epitheliectomy; cryotherapy; mitomycin C; ocular surface squamous neoplasia; partial lamellar scleroconjunctivectomy; strontium-90
DOI:10.18240/ijo.2019.09.09
Citation: Mirzayev I, Gündüz AK, Özalp Ateş FS, Özcan G, Işık MU. Factors affecting recurrence after surgical treatment in cases with ocular surface squamous neoplasia. Int J Ophthalmol 2019;12(9):1426-1431
INTRODUCTION
Ocular surface squamous neoplasia
(OSSN), is a term used to describe cancerous epithelial lesions of the cornea
and conjunctiva and includes dysplasia, carcinoma in situ and invasive
squamous cell carcinoma[1]. Squamous cell
carcionoma is the fourth most common conjunctival tumour[2].
It is also the second most common malignant conjunctival tumor after malignant
melanoma[2]. The estimated incidence of OSSN, per 100
000 persons is
OSSN lesions are typically unilateral and slow growing. Their borders may or may not be well defined. Lloyd et al[10] reported that 79% of lesions are in the interpalpebral zone. Rarely, lesions can be seen at tarsal conjunctiva and fornix. Clinical staging according to American Joint Committee on Cancer (AJCC) 8th Edition is given in Table 1.
Table 1 Clinical staging according to AJCC 8th Edition
Tx |
Primary tumor cannot be assessed |
T0 |
No evidence of primary tumor |
Tis |
Carcinoma in situ |
T1 |
Tumor (≤
|
T2 |
Tumor (>
|
T3 |
Tumor invades adjacent structures (excluding the orbit) |
T4 |
Tumor invades the orbit with or without further extention |
T
|
Tumor invades orbital soft tissues without bone invasion |
T4b |
Tumor invades bone |
T
|
Tumor invades adjacent paranasal sinuses |
T4d |
Tumor invades brain |
Pre-invasive OSSN lesions can be classified as mild, moderate or severe according to epithelial localization of dysplastic cells. The basal membrane is intact in these lesions. In mild dysplasia (CIN 1) dysplasia is confined to the lower one-third of the epithelium. In moderate dysplasia (CIN 2) the abnormal cells spread to the middle third of the epithelium. If there are atypical cells in all layers of epithelium and total loss of the normal cellular polarity, this condition defined as severe dysplasia (CIN 3) or carcinoma in situ.
There has been a paradigm shift in the treatment of OSSN. Topical chemotherapy replaced or supplemented surgical excision in selected cases. The medical management of OSSN involves the use of topical chemotherapeutic agents like mitomycin C (MMC) and 5-fluorouracil (5-FU) and topical/subconjunctival immunotherapy with interferon alpha-2b (IFNα2b)[11]. The first 2 are generally used for treatment of residual cells after surgical excision while the latter can be employed for primary treatment. MMC is an alkylating antimetabolite agent isolated from Streptomyces caespitosus. It inhibits DNA synthesis by producing free radicals[12]. 5-FU is a structural analogue of pyrimidinewhich inhibits DNA formation by blocking the enzyme thymidylate synthetase[13]. IFNα2b is an antimicrobial, antiviral and antineoplastic agent. It has been found to be effective for OSSN when used as a topical drop or given as perilesional/subconjunctival injections[14].
Surgical excision is the time-honored
treatment for OSSN. A ‘no touch’ technique, with a 4
Many surgeons prefer adjuvant cryotherapy to the limbus and conjunctival margins at the time of excision. Cryotherapy is thought to work by destroying the tumor cells and obliterating its microcirculation, resulting in ischemic infarction. Double freze-thaw technique is recommended.
The aim of this study was to evaluate the risk factors for recurrence following excision of OSSN lesions.
SUBJECTS AND METHODS
Ethical Approval The procedures used in this study conformed to the tenets of the Declaration of Helsinki. Institutional Ethics Committee approval was obtained. Informed consent was obtained from the subjects. We retrospectively reviewed the clinical and histopathology records of cases who were diagnosed with OSSN and managed on the Ocular Oncology Service from February 1999 to August 2018.
Demographic information (age and sex), laterality, ocular site involved, quadrantic location (superior, nasal, inferior, temporal), basal diameter (millimeters), treatment modality, histopathological diagnosis, recurrence rate, outcome of treatment and complications were evaluated.
The diagnosis in all cases was made clinically, based on examination with slit-lamp biomicroscopy and documented by anterior segment photography. For purposes of this study, tumors were restrospectively evaluated based on the clinical staging AJCC 8th Edition.
Treatment decision was made by one consultant ocular oncologist, depending on clinical appearance and
tissue diagnosis. Surgical treatment consisted of tumor excision using partial
lamellar scleroconjunctivectomy (PLSC) technique. In PLSC, limbus based pentagonal
or circular conjunctival incision was made 3
After surgical excision and pathologic confirmation of tumor positive margins MMC 0.02% eyedrops were self-administered by the patient 4 times daily for 2wk for 2 courses separated by 2wk. Strontium-90 (Str-90) local brachytherapy was similarly used in margin positive cases at a daily dose of 1800 cGy for 7d (total dose 12600 cGy). Follow-up examination were made of 1-3mo intervals initially and extended to yearly intervals gradually. Anterior segment photographs were taken at each visit.
Statistical Analysis Statistical analyses were performed using SPSS for Windows 11.5 (SPSS Inc, Chicago, IL, USA). Kolmogorov-Smirnov test was used to assess the assumption of normality. The continuous variables that do not have normal distribution were expressed as median (min-max). Also, categorical variables were summarized as counts (percentages). For non-normally distributed continuous variables, differences between groups were tested using Mann-Whitney U test. Lastly, relationships between categorical variables were determined by Pearson Chi-square/Fisher exact test while relationships between continuous variables were determined by Spearman correlation analysis. A two-sided P-value<0.05 was considered as statistically significant.
The relationship between recurrence and age, sex, laterality, site of ocular involvement, mean basal tumor diameter, tumor epicenter, AJCC clinical stage, histopathologic diagnosis, tumor positive surgical margins, use of adjuvant treatments including cryotherapy, AE, AMT, postoperative MMC drop, and Str-90 brachytherapy, length of follow up were evaluated.
RESULTS
Demographic Features Totally 67 patients (59.8%) in our series were male and 45 patients (40.2%) was female. The mean age at presentation was 63.7y (range 22-87y). The 50 cases (44.6%) had involvement of the right eye and 62 cases (55.4%) had involvement of the left eye. Demographic data and tumor characteristics are listed in Table 2.
Table 2 Clinical and demographic characteristics in 112 patients with OSSN n (%)
Parameters |
Values |
Mean age (range, y) |
63.7 (22-87) |
Gender |
|
F |
45 (40.2) |
M |
67 (59.8) |
Affected eye |
|
Right |
62 (55.4) |
Left |
50 (44.6) |
Ocular site involved |
|
Bulbar conjunctiva |
112 (100) |
Tarsal conjunctiva |
1 (0.9) |
Cornea |
57 (50.9) |
Fornix |
2 (1.8) |
Caruncle |
2 (1.8) |
Orbital involvement |
5 (4.5) |
Mean basal tumor diameter (range, mm) |
8.6 (3-25) |
Tumor epicenter |
|
Temporal |
52 (46.4) |
Nasal |
54 (48.2) |
Inferior |
3 (2.7) |
Superior |
3 (2.7) |
AJCC stage (clinical) |
|
Tis |
59 (52.7) |
T1 |
1 (0.9) |
T2 |
8 (7.1) |
T3 |
39 (34.8) |
T4 |
5 (4.5) |
Treatment Modalities PLSC was performed in 105 cases
(93.8%) as first treatment (Figure 1). The 7 cases (6.2%) underwent enucleation
as the first treatment due to bulbus invasion (Figure 2). Treatments used in
addition to PLSC included cryotherapy to the surgical margins at the time of
tumor removal in 78 patients (74.3%), AE in 57 patients (54.3%) due to presence
of corneal involvement (Figures 1, 3 and 4), and AMT in17 patients (16.2%) for
ocular surface reconstruction (Figures 3 and 4). Adjuvant therapy
was provided with topical MMC in 10 cases (9.5%) and with Str
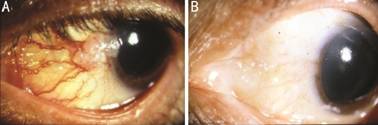
Figure 1 OSSN with corneal invasion A: Preoperative anterior segment photograph of a gelatinous OSSN with corneal invasion; B: Postoperative photograph at 14mo after PLSC, cryotherapy, and AE demonstrating a well-healed ocular surface and no tumor recurrence.
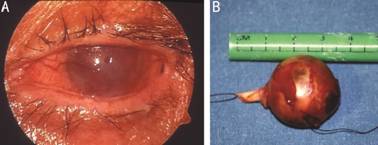
Figure 2 Diffuse OSSN with intraocular invasion A: Preoperative anterior segment photograph of OSSN with intraocular involvement; B: Gross photograph of the enucleated eye.
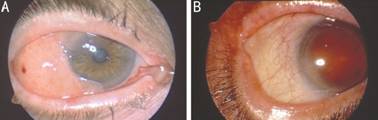
Figure 3 Extensive OSSN requiring amniotic membrane transplantation for ocular surface reconstruction A: Preoperative anterior segment photograph of OSSN with corneal invasion; B: Postoperative photograph at 18mo after PLSC, cryotherapy, alcohol epitheliectomy, and amniotic membrane transplantation showing no residual tumor or recurrence.
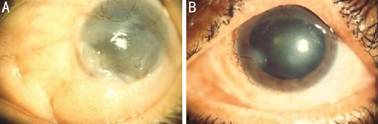
Figure 4 Limbal stem cell deficiency after excision of widespread OSSN and amniotic membrane transplantation A: Preoperative anterior segment photograph of OSSN with corneal invasion in the left eye; B: Postoperative photograph at 12mo after PLSC, cryotherapy, AE, and amniotic membrane transplantation showing possible limbal stem deficiency at 8 o’clock position and no tumor recurrence.
Table 3 Initial treatment methods used in patients with OSSN n (%)
Initial treatment methods |
Values |
PLSC+cryotherapy+AE |
45 (42.9) |
PLSC+cryotherapy |
17 (16.2) |
PLSC |
10 (9.5) |
PLSC+cryotherapy+AE+AMT |
7 (6.7) |
PLSC+topical MMC |
5 (4.8) |
PLSC+AMT |
5 (4.8) |
PLSC+cryotherapy+AMT |
5 (4.8) |
PLSC+topical Str-90 |
4 (3.8) |
PLSC+cryotherapy+topical MMC |
2 (1.9) |
PLSC+AE |
2 (1.9) |
PLSC+cryotherapy+AE+topical MMC |
2 (1.9) |
PLSC+AE+topical MMC |
1 (0.9) |
Enucleation |
7 (6.3) |
OSSN: Ocular surface squamous neoplasia; PLSC: Partial lamellar scleroconjunctivectomy; AE: Alcohol epitheliectomy; AMT: Amniotic membrane transplantation; MMC: Mitomycin C; Str-90: Strontium-90 brachytherapy.
Histopathologic Diagnoses Postoperative histopathologic diagnoses were squamous cell carcinoma in 52 cases (46.4%), carcinoma in situ in 44 cases (39.3%), moderate conjunctival intraepithelial neoplasia in 11 cases (9.8%) and mild conjunctival intraepithelial neoplasia in 5 cases (0.4%).
Follow-up, Ocular Complications, Recurrence, Globe Salvage Rate, and Metastasis At a mean of 20.1 (median: 7.0, range: 1-144) mo follow-up there were no significant ocular surface complications. Mild limbal stem cell deficiency was seen in 5 cases (4.5%, Figure 4) and dry eye related symptoms in 14 patients (12.5%) after topical chemotherapy and Str-90 brachytherapy. Recurrence was observed in 21 cases (18.8%). The recurrence rates for <6, 6-12, 12-24mo and >24mo follow-up periods were calculated as 7.1%, 4.5%, 3.6% and 3.6%, respectively. Recurrence rate was 11.5% in PLSC+cryotherapy group (9/78) vs 44.4% in the noncryotherapy group (12/27). The rate of recurrence in cases undergoing PLSC and cryotherapy was found to be statistically lower than in the group undergoing PLSC without cryotherapy (P<0.001). There was no statistically significant relationship between AE, MMC, AMT, Str-90 and tumor recurrence (all P values >0.05). Second and third recurrences were seen in 7 (33.3%) and 2 (28.6%) cases, respectively. During follow-up period, 3 eyes with recurrence were enucleated. One case required exenteration due to orbital invasion. Overall, 11 out of 112 eyes underwent enucleation (9.8%) or exenteration (0.9%). The globe salvage rate was 90.2%. None of cases developed metastasis.
DISCUSSION
OSSN represents a spectrum of diseases ranging from mild dysplasia to invasive squamous cell carcinoma involving the conjunctiva and cornea. OSSN most commonly occurs in elderly males. In our study, the majority of cases presented during 6th to 7th decade (mean age: 63.7y) with a marked male preponderance. Similar findings have been reported in other studies[16-17]. Studies from Africa reported female preponderance and HIV positivity were associated with OSSN[18]. Several factors might effect recurrence rates after surgical excision of OSSN including tumor size, tumor location, tumor positive surgical margins at histopathologic examination, use of adjuvant treatments in addition to surgical excision, and length of follow-up.
In our study,
the mean tumor basal diameter was
We found that tumor location was not related to recurrence. Galor et al[21] showed that nasal tumor location was associated with a decreased risk of tumor recurrence (P=0.0008). The recurrence rate after surgical excision with tumor positive surgical margins was reported as 56%[22]. Blasi et al[23] reported that the recurrence rate after surgical excision was 72% at a mean follow-up of 11mo despite the fact that positive surgical margins were found in only 3 cases (7%). In our study, there was no significant relationship between surgical margin positivity and recurrence rate (P=0.440). This may be due to the extensive use of cryotherapy after surgical exision. Galor et al[21] found that the rate of recurrence in cases with positive surgical margin was higher than those with negative margins (P=0.008). These findings suggest that recurrence can also seen in cases with tumor free margins or the pathologic examinations might have been incomplete or in error.
Because of the high recurrence rate with surgical excision alone, surgery is combined with adjuvant therapies such as cryotherapy, AE, and postoperative topical chemotherapy. The rate of recurrence in cases undergoing PLSC and cryotherapy was found to be lower than in cases undergoing surgery without cryotherapy. Peksayar et al[24] reported that the recurrence rate after excision and cryotherapy in OSSN was 9%. Our results confirm the effectiveness of cryotherapy as an adjuvant therapy for OSSN. The rate of recurrence in cases undergoing PLSC and cryotherapywas similar (11.5%) in our series and was found to be lower than in cases undergoing PLSC without cryotherapy (44.4%, P<0.001). Galor et al[21] similarly reported that treatment with adjuvant cryotherapy significantly decreased the risk of tumor recurrence. A retrospective study by Sudesh et al[25] showed a 7.7% recurrence rate when excision was used with cryotherapy, compared to 28.5% for simple excision. However, Maudgil et al[26] reported that there was no significant difference in recurrence between patients receiving adjuvant cryotherapy and those who did not.
In our study, the overall recurrence rate was 18.8% at mean follow-up of 20.1 (median: 7.0, range: 1-144)mo. Several studies reported recurrence rates ranging from 13% to 37% in OSSN[16,26-28]. In the study by Kim et al[16], the recurrence rate was 37% at a mean follow up 30mo showing that longer follow-up may be associated with a higher recurrence rate.
We performed enucleation in 7 cases (6.3%) as first step treatment due to intraocular tumor invasion. The 5 of these 7 cases were stage T4 and 2 of these were stage T3. Ali et al[29] reported that extensive OSSN invading the orbit was the commonest indication for an exenteration in a tertiary care center in South India. In the study by Meel et al[30] 7.0% of OSSN cases required exenteration. In our study, only 1 case (0.9%) required exenteration.
Although conjunctival OSSN is regarded as a low grade malignancy, McKelvie et al[31] showed that two of their 26 patients (7.7%) with recurrent OSSN developed and died of metastatic disease following orbital exenteration. In our study, none of the cases developed metastasis during follow up. Overall, the metastasis rate is <1% in patients with OSSN[32].
In conclusion, OSSN is common malignant ocular surface tumor. It has the potential to cause ocular surface destruction, orbital invasion, and rarely metastases. Different treatment methods have been proposed ranging from surgical excision to topical chemotherapy alone. Our series reflects the results of a surgery based series. At relatively short-term follow-up, we achieved no recurrence in 81.2% of cases after surgery. Globe salvage rate was 90.2%. Our data show that adjuvant cryotherapy may be beneficial in reducing recurrence after surgical excision. Although not on a statistically significant level, AE has also been found to be helpful in managing corneal OSSN lesions.
ACKNOWLEDGEMENTS
Conflicts of Interest: Mirzayev I, None; Gündüz AK, None; Özalp Ateş FS, None; Özcan G, None; Işık MU, None.
REFERENCES