
Citation: Xu L, Yu RJ, Ding XM, Li M, Wu Y, Zhu L, Chen D, Peng C, Zeng CJ, Guo WY. Efficacy of low-energy selective laser trabeculoplasty on the treatment of primary open angle glaucoma. Int J Ophthalmol 2019;12(9):1432-1437
DOI:10.18240/ijo.2019.09.10
·Clinical
Research·
Efficacy of low-energy selective laser trabeculoplasty on the treatment of primary open angle glaucoma
Li Xu1,2, Ru-Jing Yu1,2, Xu-Ming Ding1,2, Mao Li1,2, Yue Wu1,2, Li Zhu1,2, Di Chen1,2, Cheng Peng1,2, Chang-Juan Zeng1,2, Wen-Yi Guo1,2
1Department of Ophthalmology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China
2Shanghai Key Laboratory of Orbital Diseases and Ocular Oncology, Shanghai 200011, China
Correspondence to: Wen-Yi Guo. Department of Ophthalmology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine; Shanghai Key Laboratory of Orbital Diseases and Ocular Oncology, Shanghai 200011, China. wyguo@163.com
Received:
Abstract
AIM: To investigate the efficacy of low-energy selective laser trabeculoplasty (SLT) on the treatment of primary open angle glaucoma (POAG) patients.
METHODS: Outpatients with POAG who underwent 360-degree SLT using an initial energy of 0.3 mJ (total energy of 30-40 mJ) were reviewed retrospectively from September 2011 to January 2018.
RESULTS: Eight-six eyes of 44 POAG patients underwent
360-degree SLT using initial energy of 0.3 mJ and were followed up regularly.
The total energy used was 32.5±2.5 mJ (23-40 mJ, 105±6 spots). The average
pretreatment intraocular pressure (IOP) was 19.8±
CONCLUSION: Low-energy SLT is safe and effective for POAG patients during a 2-year follow-up. Younger POAG patients may obtain better results after low-energy SLT treatment.
KEYWORDS: primary open angle glaucoma; selective laser trabeculoplasty; low energy
DOI:10.18240/ijo.2019.09.10
Citation: Xu L, Yu RJ, Ding XM, Li M, Wu Y, Zhu L, Chen D, Peng C, Zeng CJ, Guo WY. Efficacy of low-energy selective laser trabeculoplasty on the treatment of primary open angle glaucoma. Int J Ophthalmol 2019;12(9):1432-1437
INTRODUCTION
Primary open angle glaucoma (POAG) is a chronic optic neuropathy, that is usually associated with increased intraocular pressure (IOP) and leads to progressive loss of retinal ganglion cells and the optic nerve fiber layer[1-2]. The elevation of IOP is caused by increased production of aqueous humor and/or the blockage of the trabecular meshwork (TM) region where the aqueous humor drains out.
In POAG, elevated IOP is the major and the only modifiable risk factor for the development and progression of glaucoma. The progression of POAG can be prevented by lowering the IOP[3]. The IOP reduction management options for POAG include medications, laser treatments, and anti-glaucoma surgery[3]. Anti-glaucoma medication is often considered as an initial management of POAG, and prostaglandin analogs (PGAs) have been reported to be widely used[4]. However, using too much anti-glaucoma medication may bring about side effects and may reduce compliance with the treatment[5-7].
Selective laser trabeculoplasty (SLT) was first described in 1995 by Latina and Park[8]. Based on the concept of selective photothermolysis, SLT utilizes a laser to target the pigmented TM cells selectively without damaging the neighboring nonpigmented cells or structures of the TM[8-10]. SLT, which is usually performed with a 532-nm Nd:YAG laser, has been reported to be safe, effective and repeatable for IOP lowering treatment in POAG patients[11-15].
In the traditional procedure for SLT, the energy should be adjusted at different zones of the angle to match the amount of pigmentation. As the operator may attempt to do this several times during the adjustment, some areas of TM may receive extra laser energy. In this study, we used a low and stable energy setting to simplify the laser procedure of SLT. We hypothesized that low-energy SLT could be safe, effective and convenient in Chinese POAG patients. Furthermore, we investigated the relationship between the efficacy of SLT and the baseline characteristics of patients to determine whether we could predict the outcome.
SUBJECTS AND METHODS
Ethical Approval Outpatients with POAG who underwent SLT were reviewed retrospectively at the Department of Ophthalmology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine from September 2011 to January 2018. It was certified that during this research, all applicable institutional regulations concerning the ethical use of human subjects were followed. All examinations, follow-up and treatments were performed by the same ophthalmologist (Dr. Guo WY). All the patients signed written informed consent. The participants did not receive any stipend.
Diagnosis and Outcome Measures The patients were included if they
met the following criteria: age ≥18y, an elevation of IOP (>
Detailed ophthalmic examinations including IOP measurement, gonioscopy, slit-lamp biomicroscopy, and funduscopic examination were performed before SLT. A systemic and ocular medical history was also recorded for each patient. After laser treatment, the patients were seen for follow-up regularly. IOP, C/D and complications were observed at each follow-up time point. IOP was the main outcome parameter and was compared to the pretreatment IOP. All the IOPs in this study were measured using Goldmann applanation tonometry immediately after topical anesthesia by 0.4% oxybuprocain.
Laser Procedure SLT was performed with a Latina goniolens and a q-switched frequency-doubled 532 nm Nd:YAG laser (Ellex SoloTM, Ellex Medical Pty. Ltd., Adelaide, SA, Australia). Before treatment, one drop of 0.4% oxybuprocain was applied to each eye. A fixed spot size of 400 μm and pulse duration of 3ns were implemented. Nonoverlapping 87-120 spots were applied to 360 degrees of the angle at approximately mid-height of the TM. The initial energy level was set at 0.3 mJ, and the energy level was stable during the treatment. The total number of spots and total amount of energy used for each eye were recorded. Postoperative medications after SLT included pranoprofen eye drops 4 times daily for one week. After SLT, the patients were followed up regularly (1, 3, 6mo, 1, and 2y). Patients’ pretreatment prescriptions for glaucoma were modified at follow-up visits according to their IOP measurements when necessary.
The definitions of SLT success were:
IOP≤
Statistical Analysis All statistical analyses were performed using SPSS (Version 22.0, IBM Corporation, Armonk, NY, USA). All the data were summarized using means and standard deviations (SD) or medians and interquartile ranges (IQR). Categorical data were analyzed using the Chi-square test, and values in the drug reduction group and drug retention group were compared using an independent samples t-test. The surgical success rate was calculated with Kaplan-Meier survival curve analysis. A P value less than 0.05 was considered statistically significant.
RESULTS
Low-energy SLT was performed on 86
eyes from 44 POAG patients (35 males and 9 females) included in this study.
Prior to SLT, all the eyes were being treated with glaucoma medications (1 to 3
drugs). The total energy used was 32.5±2.5 mJ (23-40 mJ, 105±6 spots). The
median age of SLT treatment was 38.31y. The average number of pretreatment
medications was 1.5±0.7. The average pretreatment IOP was 19.8±
Table 1 Baseline (pretreatment) demographic and clinical data
Pretreatment findings |
SLT (n=86) |
Age of SLT (y), median (IQR) |
38.31 (31.98, 47.73) |
Sex |
|
Male |
68 (35 patients) |
Female |
18 (9 patients) |
Follow-up duration (mo), median (IQR) |
29.54 (22.11, 45.13) |
IOP (mm Hg), mean±SD |
19.8±3.9 |
C/D, median (IQR) |
0.80 (0.70, 0.90) |
Energy (mJ), mean±SD |
32.5±2.5 |
Number of pretreatment glaucoma medications, mean±SD |
1.5±0.7 |
Eighty eyes were followed up for
longer than 1y, and 64 eyes were followed up for more than 2y. The average
pretreatment IOP was 19.8±
Table 2 Evaluation of IOP and cumulative proportion of SLT success
Parameters |
Pre-SLT |
1mo |
3mo |
6mo |
1y |
2y |
Eyes (n) |
86 |
86 |
86 |
86 |
80 |
64 |
IOP (mm Hg) |
19.8±3.9 |
16.9±3.3 |
16.5±3.3 |
17.1±3.4 |
16.6±3.5 |
16.5±2.8 |
Cumulative proportion of SLT success (%) |
/ |
80.2 |
77.9 |
73.3 |
61.7 |
55.2 |
P |
<
|
<
|
<
|
<
|
<
|
aIndependent samples t-test; SLT: Selective laser trabeculoplasty.
Table 3 Comparison between baseline characteristics and clinical outcome in SLT success group and SLT failure group
Pretreatment findings |
SLT success (n=50) |
SLT failure (n=36) |
P |
Age of SLT (y), median (IQR) |
37.67 (31.62, 43.88) |
41.28 (32.15, 55.53) |
|
Sex |
|||
Male |
40 |
28 |
0.803b |
Female |
10 |
8 |
|
Follow-up duration (mo), median (IQR) |
28.31 (18.87, 45.86) |
33.42 (23.16, 44.74) |
|
IOP (mm Hg), mean±SD |
20.4±3.9 |
19.1±3.9 |
|
C/D, median (IQR) |
0.85 (0.70, 0.90) |
0.80 (0.70, 0.90) |
|
Number of pretreatment glaucoma medications, mean±SD |
1.7±0.8 |
1.3±0.5 |
|
aIndependent samples t-test; bChi-square test. SLT: Selective laser trabeculoplasty. IQR: Interquartile ranges. C/D: Cup-to-disc ratio.
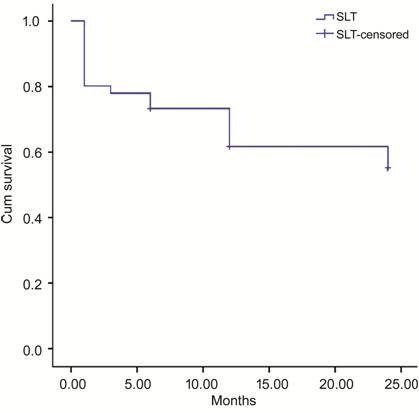
Figure
After SLT, 59 eyes maintained the
pre-SLT medications and were defined as the drug retention group, and 27 eyes
required reduced drugs and were defined as the drug reduction group. In the
drug retention group, the pre-SLT IOP was 20.1±
Table 4 Evaluation of IOP after SLT (drug retention group and drug reduction group)
Parameters |
Pre-SLT |
1mo |
3mo |
6mo |
1y |
2y |
Drug reduction |
||||||
Eyes (n) |
27 |
27 |
27 |
27 |
23 |
19 |
IOP (mm Hg) |
19.2±4.4 |
16.1±2.6 |
16.5±3.1 |
16.8±2.9 |
16.0±2.6 |
16.3±2.4 |
P |
/ |
|
|
|
|
|
Drug retention |
||||||
Eyes (n) |
59 |
59 |
59 |
59 |
57 |
45 |
IOP (mm Hg) |
20.1±3.7 |
17.3±3.6 |
16.6±3.5 |
17.2±3.6 |
16.9±3.8 |
16.5±2.9 |
P |
/ |
<
|
<
|
<
|
<
|
<
|
aPaired-samples t-test.
Table 5 Comparison between baseline characteristics and clinical outcome in drug reduction group to drug retention group
Pretreatment findings |
Drug reduction (n=27) |
Drug retention (n=59) |
P |
Age of SLT (y), median (IQR) |
35.14 (29.79, 42.39) |
41.28 (32.88, 51.93) |
|
Sex |
|||
Male |
19 |
49 |
0.180b |
Female |
8 |
10 |
|
Follow-up duration (mo), median (IQR) |
29.54 (18.88, 47.43) |
27.86 (22.30, 44.74) |
|
IOP (mm Hg), mean±SD |
19.2±4.4 |
20.1±3.7 |
|
C/D, median (IQR) |
0.80 (0.70, 0.90) |
0.80 (0.80, 0.90) |
|
Number of pretreatment glaucoma medications (mean±SD) |
1.9±0.9 |
1.4±0.5 |
|
aIndependent samples t-test; bChi-square test.
DISCUSSION
Glaucoma is the second leading cause of blindness in the world[16]. Because the drainage pathway of the TM region is blocked, high pressure builds up in the eye and causes optic nerve damage, leading to chronic vision loss in patients with POAG.
As mentioned, the progression of POAG can be prevented by lowering the IOP[3]. A laser beam is applied to burn out some areas of the TM tissue near the base of the iris[17]. This can increase the efficiency of fluid outflow. Laser trabeculoplasty is used in the treatment of POAG. Argon laser trabeculoplasty (ALT) uses an argon laser, and SLT uses an Nd:YAG laser. As the power of the Nd:YAG laser is much lower than that of the argon laser and the Nd:YAG laser can selectively target melanocytes, SLT usually causes less thermal damage in the TM than ALT[18-19] and has been used more widely.
As SLT is widely used in the
treatment of POAG, the efficacy of SLT was recently reported in several
studies. It was reported to be safe and effective for the treatment of POAG. In
2017, Aptel et al[20] reported that SLT
treatment could reduce the absolute IOP value but did not modify the circadian
rhythm of IOP in French POAG patients during a 6-months follow-up after
a complete wash-out of the medical treatment. The initial energy level of SLT
in this study was set at 0.7 mJ[20]. The
pretreatment IOP was 22.1±
Traditionally, the initial energy of SLT was set at 0.7-0.8 mJ, and then adjusted according to the response until champagne bubbles formed. At this point, the laser energy was reduced by 0.1 mJ for the treatment. The energy level was further adjusted at different zones of the angle to match the amount of pigmentation in different areas. The operator may attempt several times to find the suitable energy of SLT at every different area of the angle, which could be time-consuming and could introduce extra damage to the TM. In our study, we set a lower initial laser energy level at 0.3 mJ to investigate the effect and safety of SLT on the treatment of Chinese POAG patients. As the energy level was stable during the treatment, the time duration of SLT was reduced. Our results showed well-controlled IOP after low-energy SLT within 2y. The simplified laser procedure may explain the high success rate of low-energy SLT. No severe complications were observed in any of the patients in our study. This indicated the safety of low-energy SLT (initial energy at 0.3 mJ) for POAG patients.
In POAG, anti-glaucoma medication is often considered as an initial management[3]. However, every anti-glaucoma medication may have local and systemic side effects. For example, PGAs may cause conjunctival hyperemia, elongation, and iris darkening; they also have abortive potential, which is their most dangerous side effect[5]. In addition, using too much anti-glaucoma medication may reduce the compliance with the treatment. In our study, all eyes were treated with medications (1 to 3 drugs) before SLT treatment, and the average number of pretreatment medications was 1.5±0.7. After SLT treatment, the drug treatment of 27 eyes was reduced and the IOPs were lower than those before treatment. However, the post-SLT IOPs in the drug retention group were significantly lower than those before treatment. These findings demonstrate the effectiveness of low-energy SLT.
In our study, patients in the SLT success group and in the drug reduction group were younger than those in the SLT failure group and in the drug retention group, respectively. Lower pretreatment IOP was found in the drug reduction group than that of drug retention group. This indicates that the cases at earlier stages of POAG may obtain better results after low-energy SLT treatment. As POAG is often painless and is only indicated by progressive visual field loss and optic nerve damage (increased C/D on fundus examination), early diagnosis of POAG is difficult and thus indicates the importance of regular ophthalmic examination.
A limitation of this study is the lack of a control group that underwent a standard SLT with high and variable energy.
In summary, our study showed good control of IOP after low-energy SLT treatment in POAG patients. Additionally, this procedure was safe for the patients. As nearly half of the patients included received follow-up for less than 2y, further studies and investigations are needed to validate the long-term effect of SLT on POAG patients. Younger patients were more likely to experience SLT success and to reduce anti-glaucoma medication after low-energy SLT. Further studies should be performed to investigate the mechanisms that lead to better results of SLT in young patients.
ACKNOWLEDGEMENTS
Foundations: Supported by National Nature Science Fundation (No.81670845), Research Foundation of Shanghai Science and Technology Committee (No.14411960600); The Science and Technology Commission of Shanghai (No.17DZ2260100).
Conflicts of Interest: Xu L, None; Yu RJ, None; Ding XM, None; Li M, None; Wu Y, None; Zhu L, None; Chen D, None; Peng C, None; Zeng CJ, None; Guo WY, None.
REFERENCES