
Citation: Abdelkader M, Saleh S, Mokbel T, Abouelkheir H, Abd El Ghafar A. Beneficial effects of vincamine with thioctic acid and lutein on retinal and optic nerve functions in an opaque media. Int J Ophthalmol 2019;12(9):1450-1455
DOI:10.18240/ijo.2019.09.13
·Clinical Research·
Beneficial effects of vincamine with thioctic acid and lutein on retinal and optic nerve functions in an opaque media
Mona Abdelkader, Sameh Saleh, Tharwat Mokbel, Hossam Abouelkheir, Ayman Abd El Ghafar
Mansoura Ophthalmic Center, Mansoura Medicine Hospital, Faculty of Medicine, Mansoura University, Mansoura 35516, Egypt
Correspondence to: Sameh Saleh. Ophthalmology Center, Faculty of Medicine, Mansoura University, Mansoura 35516, Egypt. yohaahmed12@yahoo.com
Received:
Abstract
AIM: To detect whether the combination of vincamine, thioctic acid and lutein will improve the retina and optic nerve functions in cases of an opaque media with an optic nerve and/or a retinal defect or not.
METHODS: Totally 2000 patients (2000 eyes) of corneal opacities with defects in the optic nerve or/and the retinal functions were studied. Every patient received three types of drugs: thioctic acid with cyanocobalamine, vincamine, and lutein. The drugs were given daily for 3-12mo according to patient’s responses. Full field flash electroretinogram (ERG) and flash visual evoked potential (VEP) were done before treatment and at 1, 3, 6, and 12mo sequentially. Patients were followed up for 12mo.
RESULTS: In the 2000 eyes, 1000 eyes had both moderate optic nerve and retinal function defects; and 840 eyes out of the 1000 improved with the medical treatment. Another 500 eyes out of the 2000 eyes had extinguished retinal function with normal optic nerve function and only 125 eyes of them improved. The 290 out of the 2000 eyes had severe defects in optic nerve with normal retinal function and 130 of them improved. Another 210 eyes have mild optic nerve and retinal function defects and 194 improved.
CONCLUSION: The combination of vincamine, thioctic acid with cyanocobalamine, and lutein improved both retina and optic nerve functions mainly in mild and moderate defect than in severe cases.
KEYWORDS: retina; optic nerve; opaque media; thioctic acid with cyanocobalamine; vincamine; lutein
DOI:10.18240/ijo.2019.09.13
Citation: Abdelkader M, Saleh S, Mokbel T, Abouelkheir H, Abd El Ghafar A. Beneficial effects of vincamine with thioctic acid and lutein on retinal and optic nerve functions in an opaque media. Int J Ophthalmol 2019;12(9):1450-1455
INTRODUCTION
There are few studies on the effects of medical drugs on the retina and optic dysfunctions that trigger the author to study the effect of a specific combination of drugs on the retina and optic nerve functions. Also, oxidative stress participates in the pathogenesis of several retinal disorders and the role of oxidants remains unclear.
The retina is an extension of the brain, shaped from neural tissues and is connected to the brain properly by the optic nerve. Age-related macular degeneration (AMD) is one of the leading causes of severe central visual acuity loss in elderly people and seems to be an economic problem. Lutein is the most essential carotenoid present in certain selective tissues of the human body, mainly at the level of the retina, macula and lens. Antioxidant on the other hand removes free radicals and thereby terminates the chain reactions that can damage cells.
Antioxidants and lutein administration have been shown to improve visual function of vision-threatening eye diseases, AMD and diabetes[1-2]. Visual evoked potential (VEP) is a visually evoked electrophysiological signal extracted from visual cortex activity. It measures the electrical signal generated at visual cortex in response to visual stimulation. VEP depends on integrity of central vision at any level of visual pathway[3]. Full field flash electroretinogram (ERG) gives a mass-response of the retina to flashlight. The resultant response is the total response of the retina[4]. ERG gives information about treatment toxicity and safety[5].
The aim of this study was to evaluate the effect of a combined therapy that consisted of vincamine, thioctic acid with cyanocobalamine, and lutein on the visual function in cases of an opaque media with retina and/or optic nerve pathology.
SUBJECTS AND METHODS
Ethical Approval All subjects were carried out in accordance with the tenets of the Declaration of Helsinki (1989) of the world medical association. The study was approved by Mansoura International Review Board (IRB) Ethics Committee. Each patient signed a written consent before joining the study without any stipend.
Subjects This study was carried out on patients attending the Outpatient’s Clinic of Mansoura Ophthalmic Center from February 2008 to July 2018. The study included 2000 patients (2000 eyes) of dense corneal opacities with defects in the optic nerve or/and the retinal functions. The choice of patients was randomized. About 300 (15%) patients had type 1 diabetes millitus, 500 (25%) patients had type 2 diabetes millus and 470 (23.5%) patients had hypertension. The subjects were divided into four groups. Group 1 comprised of 1000 patients (550 males and 450 females, aged 50±10y) with moderate defects in both optic nerve and retinal functions. Group 2 comprised of 500 patients (350 females, 150 males, aged 55±11y) with severe (extinguished) retinal function defect and normal optic nerve function. Group 3 comprised of 290 patients (200 male, 90 female aged 48±15y) that had severe optic nerve defects and normal retinal function and Group 4 comprised of 210 patients (135 females, 75 males, aged 55±9y) with mild defects in both optic nerve and retinal functions. Moderate defect means reduction of normal values by about 50%. Severe defect means reduction of normal values by more than 70%. Mild defect means the reduction of normal values by less than 30%. Patients with clear cornea, retinal detachment, vitreous hemorrhage, and glaucoma were exclusive.
Eye Examination All patients were examined on the first day before medical treatment after the first month, and this was done every three months for 12mo. The examination include visual acuity assessment, ultrasound to detect retinal detachment, vitreous haemorrhage, and any other surgical condition, ERG, and VEP. ERG and VEP were recorded using Roland Consult (Germany), and were done according to international standard for clinical electrophysiology and vision (ISCEV) standards[6-7].
Full Field Flash Electroretinogram After topical corneal anesthesia (benoxinate hydrochloride 4%), positive electrode [Dawson, Trick and litzkow (DTL) electrode] was placed just contact with corneal limbus; ground electrode was installed on the forehead; and negative electrode was placed near orbital rim temporarily. The recording was monocular. After dark adaptation for 20min, ERG was recorded in 5 steps: scotopic rod response, scotopic combined response, oscillatory potential, then light adaptation for 10min, following photopic cone response, and flicker response recording.
Flash Visual Evoked Potential Patients were instructed to wash
their hair with shampoo (for oil free scalp) on the day of the investigation to
reduce skin impedance for better recording of VEP. Standard silver chloride
electrodes of
Medical Treatment Each patient was administered the combination of lutein 6 mg (I Care capsule, Sigma industry), thioctic acid 600 mg with cyanocobalamine 500 mg (Thiotacid compound 600, Eva Pharma), vincamine 30 mg (Oxybral, Glaxo Company). Each patient was given these combination daily for 6 to 12mo according to ERG and VEP responses. When the improvement reached maximum, the combination was stopped and the patient continued on lutein only for the next 3mo
RESULTS
The study included 2000 patients (2000 eyes) with dense corneal opacity. Of them 1025 patients were female (51.25%) and 975 (48.75%) were male (Table 1). The causes of corneal opacities were included in Table 2.
Table 1 Demographic characteristics among groups
Groups |
Eyes |
Age (mean±SD), y |
Sex (F/M) |
Optic nerve function |
Retinal function |
Group 1 |
1000 |
50±10 |
450/550 |
Moderate defect |
Moderate defect |
Group 2 |
500 |
55±13 |
350/150 |
Normal |
Severe defect |
Group 3 |
290 |
46±15 |
90/200 |
Severe defect |
Normal |
Group 4 |
210 |
44±12 |
135/75 |
Mild defect |
Mild defect |
There were no statistical significant difference between groups in age and sex.
Table 2 Causes of corneal opacities among groups
Groups |
Trauma |
Bacterial infection |
Fungal infection |
Group 1 |
865 |
104 |
31 |
Group 2 |
144 |
356 |
0 |
Group 3 |
208 |
89 |
3 |
Group 4 |
57 |
134 |
9 |
In Group 1, patients have moderate reduction in the optic nerve and retinal function. There was improvement of both retina and optic nerve functions in 840 out of the 1000 eyes (84%). Among them 600 eyes (60%) of the 840 improved after 6mo, while the other 240 eyes (24%) improved after 9mo (Figures 1 and 2). There was an increase in the amplitudes of both scotopic rod and photopic cone responses and there was reduction in their latencies. There was also an increase in the amplitudes of both oscillatory potential and flicker responses.

Fiureg 1 ERG before and after treatment Scotopic ERG (A), oscillatory potential ERG (B), photopic ERG (C), and flicker ERG (D) which reduction in amplitude and delay in latency are improved after treatment.
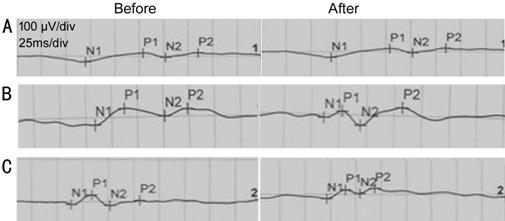
Figure 2 The change of P100 latency after treatment A: Severe delay in N2 and P2 latency did not improve after treatment; B: Severe delay in N2 and P2 latency improved with treatment; C: Moderate delay in N2 and P2 latency improved after treatment.
In Group 2, there were severe reductions in retinal function with normal optic nerve function. There was an improvement in only 125 eyes (25%) patients. Seventy-four eyes improved after 9mo (74/125) and 51 eyes (51/125) improved after 12mo (Table 3). In Group 3, there was a severe reduction in the optic nerve with normal retinal function. There was an improvement of 130 eyes (44.8%) out of 290 eyes. Thirty eyes improved after 6mo (30/130), 43 eyes (43/130) improved after 9mo and 57 eyes (57/130) improved after 12mo. There was an increase in the amplitude of P100 with a reduction in latency (Table 4).
Table 3 Full field flash electroretinogram changes in Group 2 after treatment
ERG |
Before treatment |
After treatment |
P |
Scotopic |
|||
Rod amplitude |
45±12 |
55±7 |
0.001 |
Latency |
80±6 |
69±4 |
0.004 |
Combined |
|||
Amplitude of b wave |
125±20 |
188±25 |
0.008 |
Latency |
45±9 |
40±6 |
0.005 |
Oscillatory potential |
|||
Amplitude |
15±8 |
20±4 |
0.001 |
Latency |
37±3 |
25±5 |
0.01 |
Photopic |
|||
Amplitude |
40±8 |
55±10 |
0.007 |
Latency |
39±4 |
31±2 |
0.002 |
Flicker |
|||
Amplitude |
42±9 |
58±11 |
0.005 |
Latency |
69±5 |
60±4 |
0.003 |
Table 4 Full field flash electroretinogram changes in Group 3 after treatment
ERG |
Before treatment |
After treatment |
P |
Scotopic rod |
|||
Amplitude |
10±11 |
40±10 |
0.008 |
Latency |
88±6 |
73±7 |
0.005 |
Combined |
|||
Amplitude |
90±19 |
150±29 |
0.004 |
Latency |
50±6 |
40±5 |
0.002 |
Oscillatory potential |
|||
Amplitude |
6±4 |
17±7 |
0.008 |
Latency |
40±9 |
30±4 |
0.009 |
Photopic |
|||
Amplitude |
15±6 |
39±13 |
0.005 |
Latency |
43±3 |
39±2 |
0.007 |
Flicker |
|||
Amplitude |
20±5 |
43±5 |
0.006 |
Latency |
70±14 |
66±10 |
0.008 |
In Group 4, there was a mild reduction of both optic nerve and retina functions. There was improvement in 194 out of 210 (92.3%) patients. The improvement took place after 6mo (Tables 5 and 6).
Table 5 Visual evoked potential P100 latency changes among groups
Groups |
Before treatment |
After treatment |
P |
Group 1 |
101±45 |
125±8 |
0.005 |
Group 2 |
110±5 |
110±2 |
0.9 |
Group 3 |
177±16 |
140±5 |
0.001 |
Group 4 |
125±9 |
116±4 |
0.008 |
Table 6 ERG changes in Group 4 after treatment
ERG |
Before treatment |
After treatment |
P |
Scotopic rod |
|||
Amplitude |
50±7 |
68±10 |
0.005 |
Latency |
70±6 |
60±7 |
0.007 |
Combined |
|||
Amplitude |
188±21 |
210±11 |
0.006 |
Latency |
40±5 |
35±3 |
0.004 |
Oscillatory potential |
|||
Amplitude |
22±5 |
26±4 |
0.007 |
Latency |
30±7 |
21±3 |
0.004 |
Photopic |
|||
Amplitude |
50±6 |
60±10 |
0.006 |
Latency |
29±7 |
25±5 |
0.008 |
Flicker |
|||
Amplitude |
50±9 |
60±6 |
0.005 |
Latency |
60±6 |
53±4 |
0.003 |
Complication of Medical Treatment Fifty patients (50/2000, 2.5%) complained about abdominal disturbance like nausea, vomiting, but this symptom was controlled by digestion regulator drug (domperidone, motilium capsule, Gansing company). Other patients were well tolerated.
DISCUSSION
This study shows that oral supplementation with a new combination of lutein 6 mg, vincamine 30 mg, thioctic acid 600 mg and cyanocobalamine 500 mg daily to patients with retina and optic nerve pathologies increased both retina and optic function when measured using full field flash ERG and VEP. There is an increasing possibility of the effect of macular pigment on visual performance which is concentrated within foveal and macular pigment that is composed of lutein[8], and oxidative damage is one of the pathogenesis of several retinal and optic nerve pathologies. These motivated the author to study the effects of the combination of vincamine, thioctic acid, cyanocobalamine and lutein on the visual function in an opaque media with retinal and/or optic nerve pathology.
Different studies have shown that an increase in serum lutein elevate lutein density in the retina[9-11]. Lutein is a dietary carotenoid derived from green vegetables and yellow fruits. It was hypothesized that it protects against eye disorders by its physical blue light filtration properties and local antioxidant activity (It protects against light-induced oxidative damage)[12-13]. Also, lutein has anti-inflammatory antiangiogenic properties and free radical scavenging[14-15]. The macular carotenoids lutein has neuroprotective and visual performance benefits once deposited in retinal tissues[16]. Sustained lutein consumption, either through diet or supplementation contribute to reducing burden several ocular diseases[17-18].
Xu and Lin[19] found that malondialdehyde content increased in the retina of rat induced by photic injury, and the increase was protected significantly by lutein treatment. The activities of peroxide dismutase and glutathione peroxidase was decreased in rats treated with lutein. The expression of c-fos protein was significantly higher in the retina of blue light injury than that in lutein-treated groups. Neuron nitricoxide synthase was not significant different between treated and untreated groups[19].
Alpha-lipoic acid is both water and fat soluble that permit to work in every cell in the body in contrast to other antioxidant eithet water or fat soluble[20]. Alpha-lipoic acid is a necessary cofactor for mitochondrial α-ketoacid dehydrogenases, and thus serves a critical role in mitochondrial energy metabolism. It elicits a unique set of biochemical activities with potential pharmacotherapeutic value against a host of pathophysiologic insults[21]. It is capable of quenching reactive oxygen species and reactive nitrogen species such as hydroxyl radicals, peroxyl radicals, superoxide, hypochlorous acid and peroxynitrite, thus they prevent singlet oxygen-induced DNA damage[22]. It also, elicits an array of cellular actions, ranging from a potent antioxidant to a metal chelator to a mediator of cell signaling pathways[20-21].
Thiotacid acid may be transformed during metabolic process to its reduced form. Both reduced and oxidized forms have strong antioxidant and detoxification effect. They protect the cell against free radicals. Also, they scavenge superoxide radical and hydroxyl radical and prevent lipid peroxidation. Thiotacid acid protects the neurons from the ischemic damage by the prevention of the accumulation of free radicals[23]. Cyanocobalamine (vitamin B12) is important for nucleic acid synthesis and essential for nerves.
Yildirim and Emmez[24] showed that thioctic acid protects from ischemia-reperfusion injuries to the retina in general and to ganglion cells in particular. He said that increased lipid level in retina is responsible for the high vulnerability of eye to oxidative stress and peroxidative damage of membrane lipids allow severe damage. Thus, lipid peroxidation may be the major biochemical alteration in many diseases..He suggested that exogenous thioctic acid delays the ischemia-reperfusion injury process of eye by means of its free radical scavenging effects and inflammation[24]. Thioctic acid may cause allergy and rash in some cases[20]. In our study, there was no case recorded.
Vincamine is an indole alkaloid that has neuroprotective attributes and enhancement of vascular blood flow[23]. Vincamine is natural alkaloid derived from the plant vinca minor.Vincamine affects aggeration of blood and plasma and change viscosity of whole blood. Its vasoactive property increases selectively cerebral blood flow. Vincamine is nootropic supplement and peripheral vasodilator. It is able to increase blood flow and oxygen of the brain. Although the mechanism of action has not been fully elucidated, it has been established that it has a relatively high affinity for human muscarinic receptors[25]. One of drawback of vincamine is that it is poor soluble in water and intestinal tract[25].
The use of this combination will be beneficial beacause of the combined effects of the increased visual performance of using lutein, the antioxidant effect and the protection from free radical by using thioctic acid, the vasodilator effect and the protection from ischaemic stress by using vincamine and an increase in amino acid formation by the use of vitamin B12. The combined drugs were tolerated with the patients. There were only mild gastric disturbances in 2.5% that was controlled.
In this study, there was an increase in the retina and optic nerve functions as evaluated by ERG and VEP. There was an improvement of rod and cone functions and an increase in optic nerve function and retino-cortical transmission in most of the patients with mild reduction of retinal and optic nerve dysfunction (95%) in Group 4 and a moderate reduction in retina and optic nerve dysfunctions (90%) in Group 1. There was also an improvement in the minority of patients with a severe reduction in retinal function (20%) in Group 2 and severe reduction of optic nerve function (66.6%) in Group 3.
In summary, the combination of vincamine, thioctic acid and lutein are effective in the improvement of both the retina and optic nerve dysfunctions especially in mild and moderate reduction of retinal function.
ACKNOWLEDGEMENTS
Conflicts of Interest: Abdelkader M, None; Saleh S, None; Mokbel T, None; Abouelkheir H, None; Abd El Ghafar A, None.
REFERENCES