
Citation: Barsam AS, Gibbons A, McClellan AJ, Harbour JW, Smiddy WE. Follow the nevus: the cost-utility of monitoring for growth of choroidal nevi. Int J Ophthalmol 2019;12(9):1456-1464
DOI:10.18240/ijo.2019.09.14
·Investigation·
Follow the nevus: the cost-utility of monitoring for growth of choroidal nevi
Alexander S. Barsam1, Allister Gibbons1, Andrew J. McClellan2, J. William Harbour1, William E. Smiddy1
1Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Florida 33136, United States
2Texas Retina Associates, Ft. Worth, Texas 76104, United States
Correspondence to: William E. Smiddy. Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, 900 NW 17th Street, Miami FL 33136, United States. wsmiddy@med.miami.edu
Received:
Abstract
AIM: To develop a model to evaluate the cost-utility of choroidal nevi monitoring recommendations with varying clinical risk factors.
METHODS: A Markov model was created to evaluate the cost-utility in cost per quality-adjusted life-year ($/QALY) for monitoring patients with choroidal nevus. This probabilistic model was applied both to a hypothetically monitored and unmonitored group of patients beginning at different ages and with varying clinical risk factors of the nevus. Duration of screening was modeled for the remainder of the patients’ life expectancy. Best available clinical data on the prevalence and incidence of choroidal nevi/melanoma, and relative risk of nevus transformation were combined with the initial and downstream costs of screening, downstream costs of melanoma-related mortality, and QALY saved by monitoring, to estimate the best monitoring regimen. Main outcome measures were average $/QALY saved by consensus recommended monitoring scenarios for the duration of a patient’s remaining life expectancy in comparison with no follow-up, and the cost-utility of modified regimens.
RESULTS: The $/QALY of the recommended monitoring scenarios varied substantially based on nevus clinical risk factors, patient age, frequency of follow-up, and objective testing utilized. The $/QALY for the recommended monitoring scenario of a flat nevus without risk factors in a 60-year-old patient was $77 180. The $/QALY for monitoring a nevus with 3 clinical risk factors in a 60-year-old patient was $85 393. The $/QALY values for differently-aged patients were larger, and intermediate degrees of risk factors for nevus growth varied, depending largely upon the specifics of the modeled monitoring scenarios.
CONCLUSION: The average $/QALY of currently recommended monitoring scenarios fall within economically acceptable standards and could provide insight for formulating appropriate clinical strategies. Cost-utility could be enhanced by targeting higher risk groups and considering less frequent monitoring for the lower risk groups.
KEYWORDS: cost-utility; choroidal nevus; choroidal melanoma; screening; Markov modeling
DOI:10.18240/ijo.2019.09.14
Citation: Barsam AS, Gibbons A, McClellan AJ, Harbour JW, Smiddy WE. Follow the nevus: the cost-utility of monitoring for growth of choroidal nevi. Int J Ophthalmol 2019;12(9):1456-1464
INTRODUCTION
Choroidal melanoma is the most common primary intraocular malignancy among adults, but only occurs in about 6 per million in the United States[1]. The systemic survival rate has not improved despite more advanced and apparently porwerful methods of diagnosis and treatment[2]. One strategy for attempting to improve survival rates is to detect and treat choroidal melanomas at an earlier stage. Whereas randomized trials have compared different treatments[3-4] and assessed cost-utility of each treatment for already-diagnosed choroidal melanoma[5], cost-analyses for the monitoring of uveal nevi before malignant transformation have not been reported.
The prevalence of choroidal nevi in the US population is
high (~5%), and the rate of malignant transformation is low (~
The purpose of this study was to develop a model to assess the cost-utility of monitoring choroidal nevi for growth and transformation, taking into consideration different risk factor subsets, compared to an unmonitored course.
METHODS
Model Overview A Markov model was constructed to estimate the cost-utility of monitoring patients with a choroidal nevus. A schematic outline of the model is depicted in Figure 1[22].
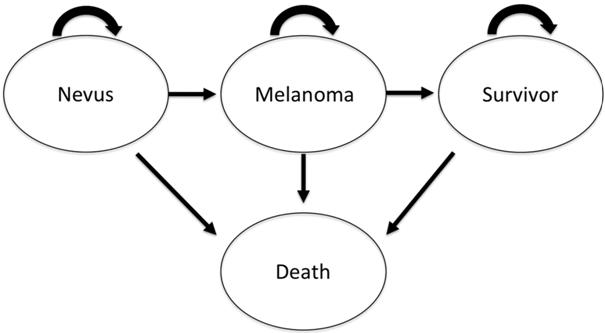
Figure 1 Schematic diagram of the Markov model beginning with a choroidal nevus in both the monitored and unmonitored group Each oval represents a different health state and arrows represent transition rewards as applied to passage from certain states to others.
A Markov model assumes individuals spend a certain amount of time in a specific ‘health state’, such as healthy, diseased, dead, etc. In this study, that state is a choroidal nevus that may even be an as yet undiagnosed small choroidal melanoma. Nevus to melanoma transformation is dependent on the probability of such an event occurring, which in this model mainly depended upon age and presence of risk factors[2,6,23]. When transformation occurs in this model, the affected individuals change health state to “Melanoma”, the patient is immediately treated with brachytherapy for a T1 melanoma or enucleation for a T3 melanoma. Melanoma-related mortality rates based on tumor stage are presented on each treatment branch arm, and impact downstream. Any of the groups can enter the absorbing state (death), although this is more likely in the melanoma state. Methods and Tables detail the assigned probabilities of these events.
In each cycle (in this study a year), they accumulate all costs and utilities corresponding to a certain health state, but a transition to and from other health states can occur, until reaching an absorbing state (in this study death). The model is run for as many cycles (called stages in the modeling literature) that usually correspond to years of life expectancy remaining at the time of nevus diagnosis. All calculations were performed and tabulated using TreeAge Pro 2018, R2 (TreeAge Software Inc., Williamstown, MA, USA).
The base case was a 60-year-old white patient with a choroidal nevus, with varying degrees of risk factors[16-19]. This age corresponds to the most common age for choroidal nevus referral to a tertiary center and also for the diagnosis of choroidal melanoma[23]. Because the actual life expectancy using Social Security Administration tables for a female is slightly longer than for a male, the duration of monitoring was weighted to encompass male and female data, as the prevalence of choroidal melanoma is nearly equally distributed between genders[23-24]. For comparison, monitoring was also modeled for a 20-, 40-, and 80-year-old patient, and for those with a higher risk for malignant transformation due to nevus characteristics.
The model consisted of a monitored (under a follow-up plan in accordance with how many accompanying risk factors were present, as defined below in the Monitoring Scenarios section hereafter referred to as “general consensus recommended monitoring guidelines”) and unmonitored groups[21]. The model assumed malignant transformation in the monitored group was detected and treated when the tumor was still (relatively) small and still classified as a T1 by the American Joint Committee on Cancer (AJCC) tumor staging[25]. In contrast, melanoma detection in the unmonitored group was assumed to occur only when tumor size reached the T3 classification. The majority of the data that correlates tumor size with mortality was based on the AJCC staging system (Table 1)[25].
Table 1 Nevus and tumor assumptions in melanoma monitoring model with mortality and health state utility assumptions
Parameters |
Value |
Source |
Avg. size of nevus (mm) |
||
Basal diameter |
0.75-6 |
5 |
Thickness |
flat |
5 |
Millimeter of growth = transformation |
||
Basal diameter |
0.5 |
37 |
Thickness |
0.5 |
37 |
Avg. tumor size (mm) |
||
Stage 1 (T
|
<3-9 base (or 9.1-12), <6 thick (or <3 what does OR mean) |
23 |
Stage 3 (T3b-d, T
|
12.1-15 (or 9.1-12) base & 6.1-15 thick (or 9.1-15) what does OR mean |
23 |
20-year melanoma-related death |
||
Stage 1 (T
|
8.00% |
23 |
Stage 3 (T3b-d, T
|
53.00% |
23 |
Health state utilities |
||
Post-enucleation |
0.81 |
26, 27 |
5-year VA loss post-brachytherapy |
0.9 |
25-27 |
Avg: Average; VA: Visual acuity; OR: Odds ratio.
Clinical data from several sources were utilized to define the health state utilities for subjects in the monitored group compared to those unmonitored[5,26-28]. Since this study did not involve human research, it did not fall under the oversight of the institutional review board (IRB) of the University of Miami (Miami, FL, USA).
Determining Costs The costs of monitoring strategies were calculated using the third-party payer perspective using direct medical costs, consulting the 2018 Medicare fee schedules form the Centers for Medicare and Medicaid Services (CMS) and Hospital Outpatient Prospective Payment System (OPPS) using non-facility values[29]. The following current procedural terminology (CPT) codes were used: 92004-new outpatient comprehensive eye exam, 92014-established outpatient comprehensive eye exam, 92250-fundus photography, 76512- B-scan ocular ultrasound, and 92134- retina optical coherence tomography.
Costs of treatment of melanoma at stages 1 and 3 were abstracted from examination and testing frequencies in previously published reports[5]. For the enucleation arm, the initial cost of an ocular prosthesis was set at the reimbursement rate for V2627 of $1437 dollars, and it was assumed to be replaced every 5-years at the same cost. All melanoma survivors, irrespective of the stage, were assumed to undergo follow-up every year until death[30]. In order to accurately reflect downstream costs saved by monitoring, end-of-life costs for treatment of metastatic cancer were incorporated from a published report estimating the average cost of care for a patient dying of metastatic choroidal melanoma and other causes of death[31]. All costs were converted to 2018 US dollars. Costs and utilities were discounted 3% per cycle (annum) as previously recommended[32].
The threshold for cost effectiveness is generally equated with the “willingness to pay” (WTP), which varies between countries. Technically, it is based on a multiple of the per-capital gross domestic product (GDP) per disability- associated life years (DALYs), a (macroeconomic) metric of lost productivity to society. The quality-adjusted life-year (QALY) is a (microeconomic) metric of how an illness has affected an individual, but it loosely parallels the value for a DALY. Hence, the value of $50 000/QALY, (the per-capita GDP for the US), has been a resilient benchmark, although $100 000/QALY or even $150 000/QALY has now been considered as an acceptable WTP[33]. Different WTP cutoffs were calculated with the sensitivity analysis for the readers benefit.
Determining Health State Utility Values Vision utility was based on published time trade-off values obtained from the Ophthalmic Utility Research Study Group[26-27]; the unaffected eye was assumed to have vision no worse than 20/25. Mean utility value after enucleation was approximated for a patient with NLP vision and a normal fellow eye[27-28]. The mean visual acuity among those detected early in monitoring assumed visual utility as reported after treatment with plaque brachytherapy. This has been reported as resulting in a loss of at least 5 lines of vision after 5y in approximately 33% of patients treated for small-uveal melanomas. We assumed a normal fellow eye, yielding a utility value of was 0.88[34]. The unmonitored group’s mean visual acuity assumed all patients were treated with enucleation and had a final utility of 0.79.
Quality Adjusted Life Years QALYs were calculated by multiplying the patient’s utility in a series of health states by the patient’s number of cycles spent in that state[26]. The QALYs saved were calculated by monitoring the difference in QALYs between the monitored and unmonitored groups (incremental utility). The difference in total costs between the monitored and unmonitored group (incremental cost) was then divided by the incremental utility (QALYs) saved by monitoring, which generated a cost per QALY ($/QALY). The throughput of the Markov model resulted in the information necessary to calculate this measure in several clinical scenarios.
Monitoring was assumed to diagnose 100% of documentable growth with the tumor classified as T1 by AJCC classification[25]. Sensitivity analyses considering lower rates were also calculated. QALYs in the unmonitored group were determined with the assumption that the tumor would reach size classified as a T3 tumor before diagnosis and treatment. If the patient was determined to have died secondary to their melanoma, the model assumed their death occurred at the year of diagnosis for utility and cost calculation purposes. A half-cycle correction was applied to the model. Melanoma survivors were assumed to have an annual follow-up after treatment for the remainder of their life.
Monitoring Scenarios Monitoring was modeled after published recommendations as referenced above and depended upon the number of accompanying risk factors. (Table 1)[19-20]. Specifically, monitoring of a flat nevus with no clinical risk factors consisted of a comprehensive new patient eye examination with a fundus photograph taken at the initial visit followed by a 6-month interval examination, with the patient monitored annually thereafter for the remaining life expectancy (RLE) with no further imaging. The arm of analysis pertaining to monitoring high-risk lesions was more stringent; thus, when there were one or more clinical risk factors, monitoring with examinations is done every 6mo, and B-Scan and optical coherence tomography (OCT) of the lesion are done annually.
The $/QALY was calculated for monitoring of a nevus with 0, 1, 2, or 3 risk factors in each of the two arms (monitored and unmonitored). All nevi with more than 3 risk factors were assumed to be referred an ocular oncologist from the onset, so this was not modeled.
A sensitivity analysis for surveillance frequency was modeled with a range of examination and testing frequencies. This consisted of calculating costs for only an initial B-scan ocular ultrasound (B-scan) and OCT in the screening process, as well as calculating costs for annual B-scan and OCT. This was done to provide a range of cost estimates that might reflect variations in common office practice. Analysis was extended to incorporate preferred practice patterns into the unmonitored group’s costs, where a visit to the ophthalmologists would happen every 3y (range 2-4) in patients under the age of 40, and every 2y (range 1-3) in patients aged 60 or older[30].
Age-adjusted Melanoma Incidence and Survival The incidence of malignant transformation was based on data analysis from the Surveillance, Epidemiology, and End Result (SEER) Program database of the National Cancer Institute to estimate the risk of malignant transformation of a choroidal nevus[2,6,23]. Annual age-specific incidence rates of choroidal melanoma for each 5-year age group were utilized and were calculated in a prior study by comparing nevus prevalence data to melanoma prevalence from the SEER data (Table 2)[6].
Table 2 Age-adjusted incidences and lifetime cumulative incidences of nevus malignant transformation with associated clinical risk factors
Parameters |
Value |
Source |
Age-adjusted incidence |
||
20-year-old |
0.00071% |
5 |
40-year-old |
0.00468% |
5 |
60-year- old |
0.01907% |
5 |
80-year-old |
0.02729% |
5 |
Cumulative incidence until RLE |
||
20-year-old (RLE: 59.32y) |
0.78% |
5, 37 |
40-year-old (RLE: 40.35y) |
0.73% |
5, 37 |
60-year-old (RLE: 22.84y) |
0.59% |
5, 37 |
80-year-old (RLE: 8.86y) |
0.14% |
5, 37 |
Increased risk from clinical risk factors (median HR) |
||
1 clinical risk factor |
2.50 |
38 |
2 clinical risk factors |
3.00 |
38 |
3 clinical risk factors |
4.00 |
38 |
RLE: Remaining life expectancy; HR: Hazard Ratio.
These incidence rates act as a cumulative incidence over the course of screening and assume that all choroidal melanomas arose from a preexisting nevus[35], and were used to calculate the percentage of nevi that transform to melanoma in the model.
Relative-risk of Malignant Transformation of a Nevus The increase in
relative risk (RR) of malignant transformation based on the clinical
characteristics of a nevus (i.e., the number of risk factors) is
incorporated into the model. Several clinical features have been identified:
thickness over
Sensitivity Analysis Uncertainty was evaluated with one-way and probabilistic sensitivity analyses (PSA) using second-order Monte Carlo simulations repeated 10 000 times. The cost of yearly monitoring was varied as to incorporate different strategies, more or less extensive (as described above) varying the number of yearly visits (1 to 2) and varying the ancillary testing associated at each visit (none, to yearly B-scan, fundus photography, and OCT of the lesion). The yearly cost of the non-monitored group was varied from one visit every 4y and up to one visit every year.
The cost of treatment for melanoma, enucleation and brachytherapy were varied to their maximum and minimum according to published costs[5]. The cost of end-of-life care for melanoma and other causes of death was also varied based on published reports[5,31]. The appendix includes a more complete range of values used in sensitivity analysis.
RESULTS
Cost-utility of Recommended Monitoring Respective cost-utility ratios demonstrate that the $/QALY of following a choroidal nevus with the general consensus recommended guidelines vary substantially based on the presence of clinical risk factors for nevus transformation and patient age (hence, expected duration of follow-up; Figure 2).
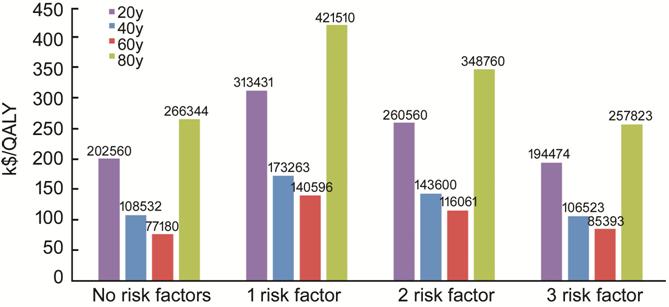
Figure 2 Graph representing the $/QALY of the recommended monitoring strategy for a nevus with zero to three clinical risk factors Monitoring was modeled for each age group’s RLE according to general consensus recommended guidelines depending on the risk of malignant transformation. The Methods section details specific screening for each clinical scenario.
The mean $/QALY for monitoring a benign nevus without risk factors in a 60-year-old patient was $77 180 (Figure 2); monitoring of an 80-year-old patient with a flat nevus was 3.5 times more expensive per QALY than monitoring a 60-year-old. Screening of a 20- and 40-year-old was 2.6 and 1.4 times more expensive per QALY. In contrast, recommendations in monitoring a nevus in a 60-year old with 3 risk factors yielded an average $/QALY of $85 393, and $116 061 and $140 596/QALY for a nevus with 2 and 1 clinical risk factors respectively in the base case (Figure 2). The variation in modeled costs was also calculated for less stringent monitoring guidelines (Table 3).
Table 3 Recommended versus less frequent monitoring regimens in a 60-year-old patient in low risk (no additional risk factors) and high risk (3 risk factors)
High versus low risk patient |
Cost-utility average (range) in $/QALY |
Monitoring of a 60-year-old patient with a high-risk nevus |
|
Recommended monitoring: biannual f/u and annual OCT+B-scan after initial fundus photo |
85393 (75686-88588) |
Less frequent monitoring: initial fundus photo, OCT+B-scan, then only biannual f/u clinical exam |
52247 (50201-56404) |
Monitoring of a 60-year-old patient with a low-risk nevus |
|
Recommended monitoring: initial photo, 2 clinical exams in 1st year, annual f/u thereafter |
77180 (71463-80381) |
Less frequent monitoring: initial photo, 2 clinical exams in 1st year, every other year f/u thereafter |
5519 (Cost saving -8719) |
$/QALY: US$ per quality-adjusted life year; f/u: Follow-up examination; OCT: Optical coherence tomography; B-scan: B-scan ocular ultrasound. Range corresponds to the maximum and minimum one-way sensitivity analysis.
Cost-Utility of Alternative Monitoring Regimens If the regimen for monitoring a patient with high-risk nevus (3 risk factors) is “relaxed” to just an initial fundus photo, ultrasound, and OCT, and followed only clinically every 6mo thereafter (with no additional testing) the average $/QALY of was reduced to $52 247 (Table 3). Similarly, if the regimen for the patient with a low-risk is “relaxed” to obtaining testing only if growth is suspected clinically, the average cost/QALY is reduced to only $5519.
For all risk factor levels, monitoring a 60-year-old patient had the lowest $/QALY compared to the other modeled age groups. Cost-utility was further optimized in this age group when incorporating AAO preferred practice patterns that call for an examination at least every other year after the age of 60 (mostly by adding costs to the unmonitored group), where monitoring a nevus with 3 risk factors with the recommended objective testing decreases the $/QALY to $56 908. When a less frequent monitoring regimen is also applied to what is recommended for a nevus with 3 clinical risk factors in a 60-year-old patient, a baseline fundus photo, OCT, and B-scan, an intermediate exam at 6mo with annual follow-up examinations (with no additional testing), resulted in $14 477/QALY.
Sensitivity Analysis Following the general
consensus recommended monitoring guidelines for a nevus without risk factors in
a 60-year-old patient and varying costs as described in the appendix for
maximum and minimum, also varying follow-up visits of the unmonitored group
from yearly to every 4y, and survival rates by 5% each way, 83% of the
iterations were within the willingness to pay (WTP) threshold of $150 000/QALY
and 17% of the iterations were above the $150K WTP (Figure 3). PSAs were
performed with 10 000 second-order parameters varying costs and events as
described in the text. The ellipsoid enclosed the values within the 95%
confidence interval. The diagonal line is the WTP limit, to which everything
below is within the predefined $/QALY $150 000 mark. The horizontal line at “
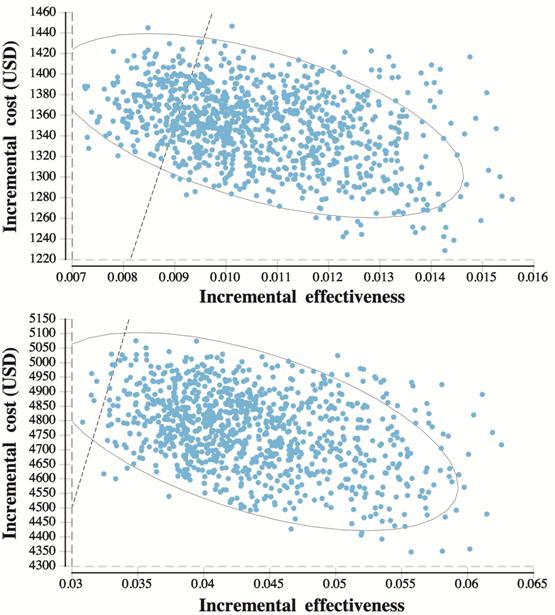
Figure 3 Probabilistic Sensitivity Analyses (PSA) of the incremental cost-effectiveness of entering subjects with a nevus into a monitoring protocol versus usual ophthalmic care A: For the low-risk nevus in a 60-year-old patient models were within the WTP 91% of the time and over the WTP threshold 9% of the time; B: For the high-risk nevus (3 risk factors present) in a 60-year-old patient models were within the WTP 99% of the time and over the WTP threshold 1% of the time.
There were no scenarios in the general recommended monitoring guidelines that were purely cost-saving compared to not monitoring. The model was also executed for lower WTP thresholds, finding that monitoring regimens did not meet a WTP threshold below $50K in any of the iterations (Table 4).
Table 4 Percentage of modeled scenarios below various WTP thresholds
High versus low risk |
If WTP $50000 |
If WTP $100000 |
High risk |
||
Over WTP (not cost-effective) |
100% |
98% |
Under WTP |
0 |
2% |
Low Risk |
||
Over WTP (not cost-effective) |
100% |
68% |
Under WTP |
0 |
32% |
WTP: Willingness to pay.
Following the recommended monitoring guidelines for a 3-risk factors nevus in a 60-year-old patient and varying costs also varying follow-up visits of the control group from 0 to yearly, survival rates by 5% each way, and varying the bi-annual follow-up to include all ancillary testing or no testing at all, 99% of the iterations were within the WTP threshold. As for the no-risk factor subgroup, there were no scenarios following the general consensus recommended guidelines for high-risk nevi that met even the highest WTP threshold of $150 000/QALY.
Another sensitivity analysis was performed to evaluate the impact of a lower sensitivity effect of determining tumor growth. A lower rate might be from growth that could not be detected by clinical examination, or if there were some cases that experienced malignant transformation without apparent growth. If the sensitivity rate of determining transformation from a nevus to a Stage 1 melanoma in the monitored group was 90% or 75%, the $/QALY increased from $85 393 to $125 430 and $148 603 respectively.
DISCUSSION
This study reports the first cost-utility analysis of existing general consensus recommended monitoring guidelines, as well as modified regimens and varied cohorts, for monitoring a choroidal nevus. Applying the general consensus recommended monitoring guidelines for a low-risk or high-risk nevus to the age group with the highest transformation rates (60-year old) confers a screening within the $150 000/QALY, a value which has come to be the upper limit of the economic WTP standard for what is considered cost-effective in the US[33]. As would be expected, the cost-utility depends upon the time for melanoma transformation, the duration of the follow-up (largely dependent upon incident age), as well as the RR (overall incidence) of transformation. Thus, subgroups with lower (or higher) risk of malignant transformation in the overall population of patients with a nevus (based on nevus characteristics and patient age) may fall above (or below) this threshold. The current study roughly stratified the monitored group into low and high-risk cases with separate, more or less stringent monitoring regimens, as is the current clinical practice. Understanding cost implications for such subgroups might be important for the health policymaker when devising or endorsing various screening strategies. These findings support the value of recent AAO preferred practice patterns calling for annual or every other year eye exams for all patients over the age of 65, which decreases the incremental $/QALY of screening suspicious lesions by approximately 30%[30].
The current study model further demonstrates how the frequency of follow-up, the choice of objective testing (OCT and B-scan), and the RR of the subgroup frames a range of cost-utility. One of the most important factors impacting cost-utility for monitoring any disorder, a choroidal nevus included, is the risk of transformation to the diseased state-in this context, choroidal melanoma. All other variables equal, it is more cost-effective to follow a high-risk nevus (monitor annually as opposed to not at all). In such cases even more frequent follow-up regimens confer a favorable cost-utility. The paucity of natural history studies assessing nevi transformation limits the accuracy of calculating the cost-utility, but studies are consistent in identifying the much higher RR with 2 or more clinical risk factors[21].
A limitation of modeling the cost-utility of monitoring nevi with different characteristics is that not all clinical risk factors are equal in their RR for transformation, and clinicians must consider the nature of each unique risk factor. These data on growth is too fragmented to include in the current model. For example, tumor thickness is a much stronger predictor of growth than the other risk factor[38]. These specific rates could be helpful when deciding which patients to follow more frequently and would likely alter cost-utility. This information could also be used when determining both frequency and modality of regimens for nevi with 1 or more concerning risk factors. In order to make these presumptions to predict tumor growth based on the aforementioned risk factors, the model assumes that published prevalence data on choroidal nevi encompass nevi with zero clinical risk factors[6].
The cost-utility of nevus monitoring is inherently optimized in the 60-year-old age group because of the higher incidence of choroidal melanomas (presumably due to higher transformation rates) coupled with substantial RLE inherent in this subset of patients[6,23]. Although an 80-year-old patient has a higher age-adjusted transformation rate and a shorter monitoring duration, they have a shorter life expectancy, leading to a lower cumulative incidence of melanoma and less QALYs saved than the 60-year-old cohort, making nevus monitoring less cost-effective in the older age group. In contrast, the age-adjusted transformation rates are lowest near age 20, making total costs of monitoring more expensive by the time the cumulative incidence catches up to that of a 60-year-old presenting with a nevus. Our model took the third-party payer perspective, thus did not include age-specific data that would capture lost revenue from work, etc. (societal perspective), but this might demonstrate the value of monitoring, especially in younger patient cohort.
These findings suggests that a potential cost-saving strategy might include less frequent monitoring for a clinically benign nevus without risk factors (or with fewer risk factors), as well as utilizing less frequent objective testing in their screening process. While the risk of lessening any screening procedure is missing an event of disease, other oncologic screening regimens have also grappled with this to ensure that certain modifications that are medically acceptable[39]. This cost-saving strategy is only possible because of the substantially decreased incidence of a transformation event in these more commonly benign lesions.
Future studies might be able to incorporate newer genetic testing techniques that might allow more precise risk stratification of patients to optimize the value of monitoring regimens tailored to specific subgroups, as has been done for defining metastatic risk of established melanomas[8,40]. That is, the spectrum of clinical benefit derived from nevus screening is also determined by the magnitude of lost utility that monitoring prevents. For example, if screening and early treatment could prevent not only tumor growth but also differentiation and development of chromosomal abnormalities, such as loss of chromosome 3, even more clinical value is derived, though the costs of such testing would have to be incorporated into the model[41-42]. On the other hand, pundits could argue that if metastasis occurs when a melanoma is small, while still appearing to be a suspicious choroidal nevus, the value of monitoring declines[37,43]. Regardless of when this phenomenon occurs, mortality rates have remained relatively unchanged over the last few decades.
Long-term mortality rates significantly impact the
calculated $/QALY in this analysis, as the less vigorously monitored group
incurs greater end of life-costs with a decreased life expectancy; it should be
mentioned that the model utilizes the most encompassing mortality data
available regarding choroidal melanoma. Numerous studies have estimated the 5-
and 10-year mortality rates of uveal melanoma, although results have varied due
to variable study design and the use of different size classifications of the
uveal tumor. The AJCC mortality data were used in the current model because it
was a unified attempt to join the clinical prognostic factors from previous
works into an all-encompassing classification system, where posterior uveal
melanoma was graded according to tumor basal diameter and thickness[44]. Several assumptions were made in the model including
the assumption that all choroidal melanomas arise from a preexisting nevus.
While clinical and histopathological evidence exist to support this phenomenon[10-11], there are also cases of
choroidal melanoma arising de novo[9]. De novo
cases would not be detectable at the nevus stage, so these would lessen the
cost-utility of screening, but probably too few to substantially affect the
results of this model. Furthermore, the model rests on the assumption that in
clinical practice documented rapid tumor enlargement signifies transformation
of a choroidal nevus to a choroidal melanoma. This model incorporates the same
assumptions following that documented growth (of >
The current study modeled plaque radiotherapy treatment with its incumbent visual acuity losses. It is predictable that alternative treatments with better treatment or side effect profiles would also alter the cost-utility of screening. While there is a trend towards earlier treatment of small uveal melanomas, even with alternative therapies, such as transpupillary, thermotherapy, local resection, or charged particle irradiation, focus was placed on the two most frequently employed treatments of plaque radiotherapy and enucleation[48].
In conclusion, this study is the first to model cost-utility for monitoring choroidal nevi and demonstrates a favorable marginal cost-utility for the general consensus recommended procedure for a choroidal nevus in the age group that presents with the highest rate of melanoma. We recognize the quandary in applying cost-utility to clinical practice, since the consequence of missing malignant transformation is severe, so it should be emphasized that cost-utility is just one factors impacting clinical monitoring decisions. Still, considering targeted and more precise methods for higher risk groups, modified regimens for lower risk groups, and development of new treatment modalities with better mortality and morbidity profiles might enhance cost-utility and clinical outcomes.
ACKNOWLEDGEMENTS
Conflicts of Interest: Barsam AS, None; Gibbons A, None; McClellan AJ, None; Harbour JW, None; Smiddy WE, None.
REFERENCES