
Citation: He XG, Deng JJ, Yin Y, Zhang B, Xiong SY, Zhu JF, Zou HD,
Xu X, Wang L. Macular choroidal thickness in Chinese preschool children:
decrease with axial length but no evident change with age. Int J Ophthalmol 2019;12(9):1465-1473
DOI:10.18240/ijo.2019.09.15
·Investigation·
Macular choroidal thickness in Chinese preschool children: decrease with axial length but no evident change with age
Xian-Gui He1,2,3, Jun-Jie Deng2,3, Yao Yin2, Bo Zhang2, Shu-Yu Xiong2,3, Jian-Feng Zhu2, Hai-Dong Zou2,3, Xun Xu2,3, Ling Wang1
1Department of Maternal and Child Health, School of Public Health, Key Laboratory of Public Health Safety, Ministry of Education, Fudan University, Shanghai 200032, China
2Department of Preventative Ophthalmology, Shanghai Eye Disease Prevention and Treatment Center, Shanghai Eye Hospital, Shanghai 200040, China
3Department of Ophthalmology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai Key Laboratory of Ocular Fundus Diseases, Shanghai Engineering Center for Visual Science and Photomedicine, Shanghai 200080, China
Co-first authors: Xian-Gui He and Jun-Jie Deng
Correspondence to: Ling Wang. Department of Maternal and Child Health, School of Public Health, Key Laboratory of Public Health Safety, Ministry of Education, Fudan University, No.131 Dongan Road, Xuhui District, Shanghai 200032, China. lingwang@fudan.edu.cn
Received:
Abstract
AIM: To explore the distribution pattern of macular choroidal thickness (ChT) and its association with age as well as refractive status in Chinese preschoolers.
METHODS: School-based, cross-sectional study. A total of 550 healthy preschool children aged 3 to 6 years old from 6 kindergartens were enrolled. Comprehensive ocular examinations, including measurement of visual acuity, axial length, intraocular pressure and slit-lamp examination before cycloplegia, as well as refraction measurement and swept-source optical coherence tomography (SS-OCT) examination after cycloplegia, were performed. The macular ChT in each sector of the ETDRS grid was measured by the built-in software of SS-OCT.
RESULTS: The mean central ChT of the participants was 312±59 μm. The mean axial length and
spherical equivalent refraction were 22.36±
CONCLUSION: In preschool children, the ChT remains relatively stable with age, while a negative association between ChT and axial length existed. This will be helpful to elucidate the characteristics of ChT during the early refractive development.
KEYWORDS: preschool children; choroidal thickness; refractive error; optical coherence tomography
DOI:10.18240/ijo.2019.09.15
Citation: He XG, Deng JJ, Yin Y, Zhang B, Xiong SY, Zhu JF, Zou HD, Xu X, Wang L. Macular choroidal thickness in Chinese preschool children: decrease with axial length but no evident change with age. Int J Ophthalmol 2019;12(9):1465-1473
INTRODUCTION
The choroid, a highly vascular structure in the ocular wall, plays important roles in many aspects of the physiological function[1-2]. In addition, the choroid may play a role in the growth of eyeball and the changes of refractive status in animal models[3]. In spite of the great importance of the choroid, little is known about its exact functions and morphological changes during the process of emmetropization and the development of myopia in preschool children.
Previous studies in adults and school-age children showed that age[4-15], axial length (AL)[4,8,12,16-18], and spherical equivalent refraction (SER)[17-19] were major influencing factors of choroidal thickness (ChT). Adults with older age[4-6], longer AL[4,16,19], and greater myopia[4,16] were shown to have thinner choroid. Also, men have thicker choroid than women[4,20]. Similar to adults, school-age children with longer AL and higher degree of myopia were also reported to have smaller ChT[8,12,17-18]. In terms of age, however, results have not been constant among different studies. Reports showed that age was positively correlated with ChT in Caucasian children[7-9], while age was negatively correlated with ChT in Asian children[10-14]. A more recent research with large sample size performed by our study group showed that age was positively correlated with ChT for emmetropes and mild myopes with SER>-2.00 diopter (D), but not correlated with ChT for myopes with SER≤-2.00 D[15]. Additionally, the growth and development of eyeball in children also made the results different from adults. In the aspect of gender, previous studies also had controversial results. Most studies showed thicker choroid in girls than in boys[8-10,12,21], while some studies showed no difference between genders[7,17], or even reported opposite results[11].
When it comes to the preschool children, however, the associations between ChT and other ocular or systemic parameters may also be different. Increased age, increased AL[22], relatively constant SER[22], mixed with the growth and development of the ocular wall in preschool children, make the distribution pattern of ChT in preschool children complicated, and different results are expected when comparing to the adults and school-age children. However, so far, not a single study has ever focused solely on the distribution pattern of macular ChT in preschool children. Results from a small portion of the preschool children mixed with a majority of the school-age children can be found in some previous studies[7-8,12-14,23-24], but the age-specific ChT of preschool children and its association with other ocular or systemic parameters were not available. To make up for the vacancy, our study aimed to explore the distribution pattern of ChT in the macular region in preschool children with different age, genders, refractive status and AL. Through this study, we tried to illuminate the associated factors of ChT and the potential development process of ChT in the macular region in Chinese preschool children. These results will also be compared to the results from our previous in school-age children, which may be helpful to elucidate the change of macular ChT in children and adolescents during the early refractive development.
SUBJECTS AND METHODS
Ethical Approval Ethical approval was obtained before the study from the Institutional Review Board of Shanghai General Hospital, Shanghai Jiao Tong University, and the study conformed to the tenets of the Declaration of Helsinki. The study protocol was explained to the children as well as their guardians in advance. Written informed consent was obtained from guardians before the study, and assent was obtained from the children.
Settings and Participants This was a cross-sectional study performed in January 2017. All the measurements took place at the six selected kindergartens in Jiading District in Shanghai, China. Healthy Chinese children aged between 3 and 6 years old were enrolled. Those with amblyopia (best corrected visual acuity<0.63), strabismus, fundus diseases, congenital cataracts or glaucoma were excluded from the study.
Ocular Examinations One ophthalmologist, two public
health physicians, five optometrists, and two nurses formed the examination
team. Ocular examinations included visual acuity, AL, intraocular pressure
(IOP) and slit-lamp examination before cycloplegia, as well as refraction
measurement and optical coherence tomography (OCT) examination after cycloplegia.
Visual acuity was tested by an ETDRS chart at a 4-m distance. Children who wore
glasses were asked to undergo the visual acuity assessment with and without
glasses, rspectively. AL was measured by IOL Master (version 5.02, Carl Zeiss
Meditec, Oberkochen, Germany), and IOP was measured by a non-contact tonometer
(model NT-4000, Nidek Inc., Fremont, CA, USA). Refraction and corneal radius of
curvature (CR) were obtained by an autorefractor (KR-8900, Topcon, Tokyo,
Japan). One drop of 0.5% proparacaine (Alcaine, Alcon) was administered in each
eye first, and then two drops of 1% cyclopentolate (Cyclogyl; Alcon, Fort
Worth, TX, USA), with each administered 5min apart. Light reflex and pupil
diameter were examined at least half an hour after the administration of the
last drop of cyclopentolate. Cycloplegia was considered complete with the
absence of light reflex and pupil diameter more than
Choroidal Thickness
Measurements SS-OCT (DRI OCT Triton, Topcon,
Tokyo, Japan) was used to measure the macular ChT of the right eyes. Age, AL,
SER and CR were input into the software to correct magnification factors before
image collection. To minimize diurnal variation of the ChT, the SS-OCT
examinations were conducted by one experienced examiner between 10:
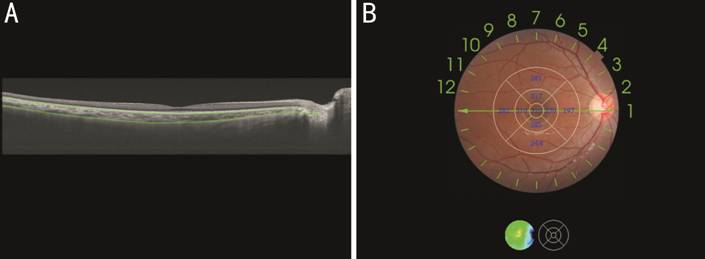
Figure 1 Thickness map of the macular choroid obtained by swept-source optical coherence tomography A: Measurement of macular choroidal thickness (ChT): the definition of ChT was the vertical distance between Bruch’s membrane (the superior green line) and the choroidal-scleral interface (the inferior green line); B: ETDRS grid was used to show ChT (μm) of each sector in the macular region.
Statistical Analysis An online database system was used for real-time examination data input with logic automatically checked. Data from auto-refraction and SS-OCT examinations were output from the instrument directly to the online system. The data set output from the online system were then checked by a staff member to ensure that there was no discrepancy. Statistical software (IBM SPSS Statistics Inc., Version 22.0, Chicago, IL, USA) was used for statistical analyses. Only the data from the right eyes were used finally.
As the number of 3-year-old children was quite small (n=14), these children were analyzed together with the 4-year-old children. In the analysis according to their refraction, children were divided into four groups, namely myopic group (SER≤ -0.50 D), emmetropic group (-0.50 D<SER<+0.50 D), mild hyperopic group (+0.50 D≤SER<+3.00 D) and moderate hyperopic group (SER≥+3.00 D). In addition, to analyze the relationship between ChT and AL, these children were further divided into four groups according the quantile of AL. The mean±standard deviation (SD), 25th percentile, 50th percentile and 75th percentile were calculated to discribe the distribution of continuous variables. Kolmogorov-Smirnov tests were used to examine the data distribution. To assess intergroup differences, t-tests or analysis of variance were adopted for those normally distributed data, while Kruskal Wallis tests were adopted for those abnomally distributed data. Bonferroni method was use for post-hoc tests. Simple linear regression and muliple linear regression analyses were used to determine the independent factors of ChT. A P<0.05 was considered statistically significant (two tailed).
RESULTS
General Characteristics of the Particpants and Difference Between Gender and Age Totally 590 preschool children aged 3 and 6 years old were enolled in the study and 40 of them were excluded due to incomplete data or unreliable measurement results, leaving a total of 550 (93.2%) preschool children in the final analysis. Among the 550 preschool children, there were 274 (49.8%) boys and 276 (50.2%) girls. The number of children that was 3, 4, 5 and 6 years old was 14, 108, 208 and 220, respectively.
The general characteristics of the included children and the differences between boys and girls were shown in Table 1. The mean height, weight, AL, CR and AL/CR of boys were larger than girls (all P<0.01) and the mean SER of girls was larger than boys (both P<0.001). No significant difference was observed in uncorrected visual acuity (UCVA) and IOP between genders.
Table 1 General characteristics of the included children and the differences between genders
Characteristics |
Total |
Boys |
Girls |
Pa |
Height, cm |
111.81±6.54 |
112.71±6.6 |
110.92±6.36 |
0.002 |
Weight, kg |
20.11±3.7 |
20.79±4.05 |
19.43±3.18 |
<0.001 |
UCVA |
0.68±0.16 |
0.69±0.17 |
0.68±0.15 |
0.269 |
IOP, mm Hg |
15.46±2.78 |
15.46±2.77 |
15.46±2.79 |
0.987 |
SER, diopter |
1.51±0.83 |
1.37±0.79 |
1.66±0.85 |
<0.001 |
AL, mm |
22.36±0.72 |
22.67±0.65 |
22.04±0.65 |
<0.001 |
CR, mm |
7.83±0.25 |
7.88±0.25 |
7.78±0.25 |
<0.001 |
AL/CR |
2.86±0.09 |
2.88±0.09 |
2.84±0.09 |
<0.001 |
UCVA: Uncorrected visual acuity; IOP: Intraocular pressure; SER: Spherical equivalent refraction; AL: Axial length; CR: Corneal radius of curvature. aDifference between boys and girls was tested using t-tests, P<0.05 was considered statistically significant.
Differences among age groups for general characteristics were displayed in Table 2. IOP, AL and AL/CR were significantly larger in older age group and the UCVA of the older age group was better. No difference was observed in SER and CR among different age groups.
Table 2 Differences of general characteristics among age groups
Parameters |
Age, y |
Minimum, 25th, 50th, 75th, maximum |
Pa |
|||
Total (n=550) |
3-4 (n=122)b |
5 (n=208) |
6 (n=220) |
|||
Central choroid, μm |
312±59 |
314±59 |
312±60 |
312±59 |
163, 267, 309, 356, 487 |
0.920 |
In T, μm |
322±58 |
321±57 |
319±59 |
324±57 |
155, 278, 321, 365, 483 |
0.678 |
In S, μm |
310±57 |
313±57 |
309±59 |
309±55 |
146, 267, 308, 348, 483 |
0.844 |
In N , μm |
266±57 |
269±59 |
266±58 |
266±55 |
128, 225, 265, 305, 452 |
0.825 |
In I, μm |
298±58 |
298±58 |
299±60 |
298±58 |
154, 256, 292, 339, 478 |
0.972 |
Out T, μm |
311±51 |
306±48 |
309±53 |
316±52 |
166, 271, 310, 348, 457 |
0.167 |
Out S, μm |
291±53 |
293±50 |
289±56 |
292±52 |
108, 252, 289, 328, 467 |
0.713 |
Out N, μm |
197±48 |
198±51 |
196±48 |
197±46 |
64, 161, 196, 226, 369 |
0.914 |
Out I, μm |
271±50 |
270±49 |
271±51 |
272±49 |
158, 236, 267, 307, 415 |
0.912 |
Average thickness, μm |
276±48 |
275±47 |
274±49 |
277±47 |
153, 238, 276, 308, 432 |
0.856 |
UCVA |
0.68±0.16 |
0.58±0.13 |
0.66±0.14 |
0.76±0.15 |
0.1, 0.63, 0.63, 0.8, 1.25 |
<0.001 |
IOP, mm Hg |
15.46±2.78 |
14.55±2.5 |
15.48±2.62 |
15.97±2.94 |
9, 14, 15, 17, 29 |
<0.001 |
SER, diopter |
1.51±0.83 |
1.44±0.9 |
1.56±0.82 |
1.51±0.81 |
-2, 1, 1.5, 2, 4.75 |
0.496 |
AL, mm |
22.36±0.72 |
22.16±0.65 |
22.32±0.71 |
22.5±0.74 |
20.6, 21.83, 22.37, 22.86, 24.73 |
<0.001 |
CR, mm |
7.83±0.25 |
7.82±0.25 |
7.82±0.26 |
7.84±0.25 |
7.12, 7.64, 7.83, 7.99, 8.77 |
0.739 |
AL/CR |
2.86±0.09 |
2.83±0.08 |
2.85±0.1 |
2.87±0.09 |
2.48, 2.81, 2.85, 2.9, 3.36 |
0.001 |
In T, In S, In N, In I: Temporal, superior, nasal and inferior section of the inner circle of the ETDRS grid; Out T, Out S, Out N, Out I: Temporal, superior, nasal and inferior section of the outer circle of the ETDRS grid; UCVA: Uncorrected visual acuity; IOP: Intraocular pressure; SER: Spherical equivalent refraction; AL: Axial length; CR: Corneal radius. aStatistical significance was tested using analysis of variance, P<0.05 was considered statistically significant. bFourteen 3-year-old children were included in this group.
Distribution Pattern of ChT in the ETDRS Grid in Different Age, Gender, Refractive Level and Axial Length Groups As indicated by Table 2, the mean central foveal ChT was 312±59 μm and the mean ChT of the whole ETDRS grid was 276±48 μm. The choroid was thickest in the temporal sectors, followed by the superior sectors, and then the inferior sectors and was thinnest in the nasal sectors in all age groups. In addition, the ChT of sectors in the parafoveal region was larger than the corresponding sectors in the perifoveal region, especially in the nasal part and the inferior part. In the horizontal direction, the ChT gradually decreased from the temporal sectors to the nasal sectors. In the vertical direction, the difference of ChT between the superior sector and the inferior sector was larger in the perifoveal region than that in the parafoveal region. Figure 2 showed the distributions of ChT in different age groups in the central foveal region. The ChT was normally distrbuted in all age groups in the central foveal region and in the whole ETDRS grid. Similar results were found in other sectors of the ETDRS grid as well. Table 2 showed the comparative results of ChT among different age groups. No significant difference was observed among different age groups in all sectors (all P>0.05).
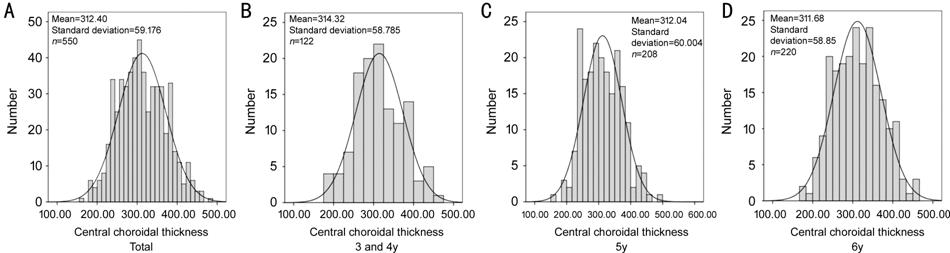
Figure 2 Age-specific distributions of choroidal thickness A: The distribution of central choroidal thickness (ChT) for the whole participants; B: The distribution of central ChT for 3 and 4-year-old children; C: The distribution of central ChT for 5-year-old children; D: The distribution of central ChT for 6-year-old children. The bars of each figure represented the real distributions of central ChT, while the curve of each figure represented the normal distribution with the same mean and standard deviation.
In terms of the differences between genders, boys had thinner choroid than girls in the superior sectors both in the parafoveal (P=0.019) and perifoveal (P=0.026) regions. In the rest of the sectors, no significant difference was observed between genders. The general distribution pattern of choroid was very similar between genders. Differences between genders in central ChT were shown in Figure 3.

Figure 3 Differences between genders in central choroidal thickness The blue symbols represented boys and the red symbols represented girls.
Differences among refractive groups were displayed in Figure 4. No statistical significant difference was observed among these refractive groups (all P>0.05), though the ChT of each sector seemed to be smaller in myopic children.
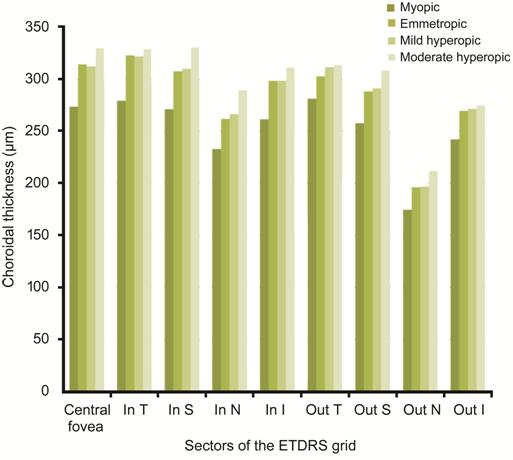
Figure 4 Choroidal thickness of different refractive groups In T, In S, In N, In I: Temporal, superior, nasal and inferior section of the inner circle of the ETDRS grid; Out T, Out S, Out N, Out I: Temporal, superior, nasal and inferior section of the outer circle of the ETDRS grid.
Table 3 demonstrated the differences among AL groups. Significant difference was observed in all sectors. Further post-hoc tests indicated that the first quantile group (AL ranges from minimum to 25th) had significantly thicker choroid than the third quantile group (AL ranges from 50th to 75th) and the fourth quantile group (AL ranges from 75th to maximum) in all sectors (all P<0.05), while no difference was observed between the latter two groups.
Table 3 Differences of macular choroidal thickness among axial length groups mean±SD, μm
Parameters |
AL |
Pa |
|||
Min-25th, n=138 |
25th-50th, n=137 |
50th-75th, n=141 |
75th-max, n=134 |
||
Central choroid |
333±56 |
316±59 |
300±58 |
301±58 |
<0.001 |
In T |
340±56 |
325±59 |
309±56 |
311±56 |
<0.001 |
In S |
330±54 |
313±57 |
299±54 |
298±57 |
<0.001 |
In N |
286±56 |
270±58 |
254±53 |
255±56 |
<0.001 |
In I |
316±57 |
301±60 |
286±55 |
290±57 |
<0.001 |
Out T |
323±50 |
315±52 |
301±48 |
304±52 |
0.001 |
Out S |
308±49 |
293±55 |
283±50 |
280±54 |
<0.001 |
Out N |
211±48 |
198±47 |
188±44 |
191±49 |
<0.001 |
Out I |
282±49 |
273±52 |
264±47 |
266±49 |
0.009 |
In T, In S, In N, In I: Temporal, superior, nasal and inferior section of the inner circle of the ETDRS grid; Out T, Out S, Out N, Out I: Temporal, superior, nasal and inferior section of the outer circle of the ETDRS grid. aStatistical significance was tested using the analysis of variance, P<0.05 was considered statistically significant.
Associated Factors of Macular Choroidal Thickness, Spherical Equivalent Refraction and Axial Length To explore the relationship between ChT and other ocular parameters, simple linear regression analysis were performed. In the central foveal region, ChT was positively correlated with SER (r=0.134, P=0.002) and negatively correlated with AL (r= -0.218, P<0.001) and CR (r=-0.100, P=0.018). Additionally, central ChT was not correlated with age, UCVA and IOP. Scatterplots for central ChT by age, SER, AL and CR were shown in Figure 5. Similar results were also discovered in other sectors of the ETDRS grid.
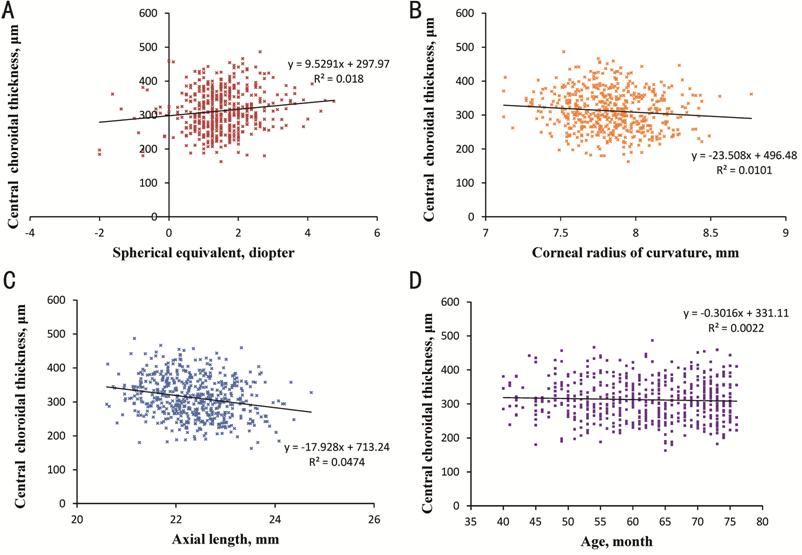
Figure 5 Relationship between central choroidal thickness and ocular parameters A: The relationship between central choroidal thickness and spherical equivalent; B: The relationship between central choroidal thickness and corneal radius of curvature; C: The relationship between central choroidal thickness and axial length; D: The relationship between central choroidal thickness and age.
To further determine the independent factors of ChT in the ETDRS grid, multiple linear regression analysis were followed. Table 4 displayed the results of central ChT. In the central foveal region, ChT was negatively correlated with AL (β=-21.184, P<0.001) and positively correlated with weight (β=1.502, P=0.041). One millimeter increase in AL was associated with a 21.2-μm decrease in the central ChT. Similar results were found in other sectors of the ETDRS grid. The overall R2 of the model was 0.061 (P<0.001).
Table 4 Independent factors of central ChT
Independent variables |
Unstandardized coefficients B |
Standardized coefficients β |
t |
Pa |
R2 |
Constant |
755.208 |
- |
9.425 |
<0.001 |
0.061 |
AL |
-21.184 |
-0.258 |
-5.685 |
<0.001 |
|
Weight |
1.502 |
0.093 |
2.044 |
0.041 |
ChT: Choroidal thickness; AL: Axial length. aP<0.05 was considered statistically significant.
DISCUSSION
To our knowledge, this is the first study to explore the age-specific macular ChT and the associated factors in Chinese preschool children with SS-OCT. No difference was observed in all sectors among the age groups and no difference was observed in most of the sectors between genders. No statistical significant difference was observed among refractive groups, though the ChT of each sector seemed to be smaller in myopic children. Among different AL groups, ChT was thicker in the first quantile group than the third quantile group and the fourth quantile group in all sectors. AL and weight were the independent factors of central ChT.
Our current study indicated that the ChT in the macular region was almost constant in children aged 3 and 6 years old and age was not correlated with ChT in any sectors of the ETDRS grid. All the previous studies did not further stratify the preschool children according to their age and the detailed relationship between age and ChT in preschool children was unclear. Results from those few studies that have ever mentioned the ChT in preschool children were also not consistent. Read et al[8] reported that the ChT of the children aged between 4 and 6y was 312±62 μm, which was significantly thinner than those aged between 7 and 9y (337±65 μm, P<0.05) and bordered on significance compared to those aged between 10 and 12y (341±61 μm, P=0.08), indicating that age was positively correlated with ChT. Zengin et al[23] also reported that the subfoveal ChT in children aged between 4 and 7y was 306.8±42.0 μm and age was positively correlated with subfoveal ChT in the whole study population (ranging from 4 to 23y). However, some other studies showed opposite results. Nagasawa et al[12] reported that the mean ChTs of children aged between 3 and 5, 6 and 9, 10 and 15 years old were different in the central and inner ring of the ETDRS grid (P=0.0011 and 0.0191), suggesting a thinner ChT in older children in the central and inner ring. Park and Oh[14] reported that the subfoveal ChT was 370.6 μm and 324.2 μm for children aged 7y or younger and 7y or older, respectively, and the subfoveal ChT was negatively correlated with age in the whole study population. Differences among these studies maybe due to racial difference, as the former two studies were carried out in Australia and Turkey, respectively, while the latter two were performed in Japan and Korean, where the prevalence of myopia is high in school age children.
In termes of the relationship
between gender and ChT, our current study showed no significant difference
between genders in most of the sectors or the whole area of the ETDRS grid. So
far, this is the first study to explore the difference of ChT between genders
in preschool children. This result was consistent with some of the previous
studies carried out in school-age children[7,17]. However, some previous studies reported difference
between genders in school-age children. Li et al[26] reported significant difference between genders in the emmetropic children
According to our study, no statistical significant difference was observed among these refractive groups (all P>0.05), though the ChT of each sector seemed to be smaller in myopic children. These results suggested that ChT may decrease in myopes, even at a very young age. Previous studies carried out in school-age children also showed thinner choroid in myopic children when compared to hyperopic ones[17-18]. These consistent results from both the preschool and school-aged children indicated that choroidal thinning may occur once myopia onset, regardless of children’s age. Moreover, the ChT of children with moderate hyperopia seemed thicker than mild hyperopes, though without statistical significance. None of the previous studies has ever finely stratified the SER in hyperopes and explore the ChT in these refractive groups in preschool children and our results can make up for the vancancy in this field.
A negative correlation between AL
and ChT has been confirmed by studies both in adults and school-age children[4,8,12,16-18]. Our current study in preschool children also showed
similar relationship. For the three age groups, the AL was significant longer
in children with older age, while no significant difference was observed among
the three age groups in the ChT. This suggested that the growth and development
of the choroid during childhood may counteract the effect of axial elongation
and thus maintain the ChT at a steady level. Also, the growth and development
of choridal tissue may slow down the axial elongation by regulating the scleral
growth factors or by buffering the mechanical stress[3,29]. Combining the theories of previous studies[3,29] and the changes of ChT shown in Figure
6 (a combination of our current study on preschoolers and our previous study on
school-age children[15]) together, we speculated
that 6-year-old could be a cut point where the choroidal growth and development
slow down and can no longer compensate for choroidal thinning caused by axial
elongation, thus the choroid gradually get thinner (Figure
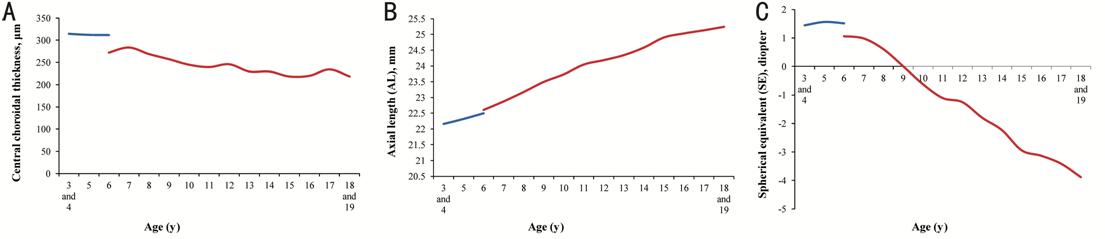
Figure 6 Change of central choroidal thickness, axial length and spherical equivalent with age Line graphs to display the changes of central choroidal thickness (A), axial length (B) and spherical equivalent (C) with age. The blue lines represented preschool children and the red lines represented school-age children. In all the three figures, 3- and 4-year-old children were merged into one group for analysis.
There are several limitations in the current study. Firstly, this is a cross-sectional study, the casual relationship can not be determined. To further confirm our results, longitudinal studies are needed in the future. Secondly, the number of children with myopia and higher degree of hyperopia is small and the changes of ChT as well as other ocular parameters can not be fully observed in these groups. More preschool children should be included in future studies. Thirdly, the ocular and systemic parameters included in the regression model can only explain about 6% of the variance in ChT. More variables, like lens power, should be included in future studies to futher illuminate the influence factors of ChT in Chinese preschool children. Last but not least, diurnal variations may influence the measurement of choroidal thickness. Therefore, we tried to finish the OCT examination as soon as possible. How, the influence of diurnal variations was inevitable. In our future studies, the diurnal window will be controlled more strictly.
To conclude, our current study focused on the ChT of preschool children and explored its relationship with age, gender, SER and AL. Among them, AL was the most important influencing factor. Longer AL was associated with thinner ChT in Chinese preschool children, though such effect may be counteracted by the growth and development of the choroid, remaining the ChT in preschool children stable. ChT may decrease in myopes, even as preschoolers. Age and gender did not have significant influence on the ChT of preschool children. To confirm our speculation, logitudinal studies with larger sample size should be conducted. This will be helpful to elucidate the changes of macular ChT in children during the early refractive development and early progress of myopia.
ACKNOWLEDGEMENTS
Foundations: Supported by Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (No.2017YQ019); Key Discipline of Public Health-Eye health in Shanghai (No.15GWZK0601); Overseas High-end Research Team-Eye health in Shanghai (No.GWTD2015S08).
Conflicts of Interest: He XG, None; Deng JJ, None; Yin Y, None; Zhang B, None; Xiong SY, None; Zhu JF, None; Zou HD, None; Xu X, None; Wang L, None.
REFERENCES