
Citation: Liu WS, Li YJ. Comparison of conbercept and ranibizumab
for the treatment efficacy of diabetic macular edema: a Meta-analysis and
systematic review. Int J Ophthalmol 2019;12(9):1479-1486
DOI:10.18240/ijo.2019.09.17
·Meta-Analysis·
Comparison of conbercept and ranibizumab for the treatment efficacy of diabetic macular edema: a Meta-analysis and systematic review
Wei-Shai Liu, Yan-Jie Li
Department of Ophthalmology, the First Hospital of Shanxi Medical University, Taiyuan 030000, Shanxi Province, China
Co-first authors: Wei-Shai Liu and Yan-Jie Li
Correspondence to: Yan-Jie Li. Department of Ophthalmology, the First Hospital of Shanxi Medical University, No.85th Jiefang South Road, Yingze District, Taiyuan 030000, Shanxi Province, China. liyanjie-0311@126.com
Received:
Abstract
AIM: To evaluate the efficacy of intravitreal injection of conbercept (IVC) and ranibizumab (IVR) in patients with diabetic macular edema.
METHODS: Reviewers have searched 12 databases, including PubMed, Medline, EMBASE, Web of Science, Springer, ScienceDirect, OVID, Cochrane Library, ClinicalTrials.gov, cqVIP, WanFangdata and China National Knowledge Infrastructure (CNKI), up to December 28, 2018. RevMan 5.3 (Cochrane Library Software, Oxford, UK) was employed for statistical analysis. Fixed and random effects models were applied to assess heterogeneity. Odds ratio (OR) was applied for dichotomous variables; weighted mean difference (WMD) was applied for continuous variables. The confidence interval (CI) was set at 95%. Central macular thickness (CMT) and best-corrected visual acuity (BCVA) were employed to analyze the improvement of DME patients. Inclusion criteria for picking out studies were retrospective studies and randomized controlled trials (RCTs) that compared IVC and IVR for the treatment of diabetic macular edema.
RESULTS: Four retrospective studies and five RCTs were included with a total of 609 patients. No statistically significant difference was observed in mean CMT and mean BCVA in the baseline parameters [BCVA (WMD: -0.48; 95%CI: -1.06 to 0.10; P=0.1), CMT (WMD: -0.83; 95%CI: -15.15 to 13.49; P=0.91). No significant difference was found in the improvement of BCVA and adverse event (AE) in IVC group, compared with IVR group after treatment of loading dosage [the 1st month BCVA (WMD: 0.01; 95%CI: -0.26 to 0.27; P=0.96), the 3rd month BCVA (WMD: -0.04; 95%CI: -0.14 to 0.06; P=0.46); the 6th month BCVA (WMD: -0.24; 95%CI: -1.62 to 1.14; P=0.73)], AE (OR: 0.84; 95%CI: 0.38 to 1.84; P=0.66)]. A slight difference was found in the effectiveness rate (OR: 1.70; 95%CI: 0.97 to 2.96; P=0.06), There were statistically significant differences between IVC and IVR treatment in terms of CMT [1st month CMT (WMD: -19.88; 95%CI: -27.94 to -11.82; P<0.001), 3rd month CMT (WMD: -23.31; 95%CI: -43.30 to -3.33; P=0.02), 6th month CMT (WMD: -74.74; 95%CI: -106.22 to -43.26; P<0.001)].
CONCLUSION: Pooled evidence suggests that both IVC and IVR are effective in the therapy of diabetic macular edema and affirms that IVC presents superiority over IVR therapy in regard of CMT in patients with diabetic macular edema, but no statistically significant difference with regard to visual improvement. Relevant RCTs with longer-term follow-up are necessary to back up our conclusion.
KEYWORDS: diabetic macular edema; central macular thickness; best-corrected visual acuity; conbercept; ranibizumab
DOI:10.18240/ijo.2019.09.17
Citation: Liu WS, Li YJ. Comparison of conbercept and ranibizumab for the treatment efficacy of diabetic macular edema: a Meta-analysis and systematic review. Int J Ophthalmol 2019;12(9):1479-1486
INTRODUCTION
Diabetic retinopathy (DR) is one of the most common and severe ocular complications of diabetes mellitus (DM)[1]. The prevalence rate of DM in China was as high as 5.49% in adults aged 35-74y[2]. Diabetic macular edema (DME) is the extracellular accumulation and exudation in the macula due to the leakage of the blood-retina barrier[3]. About one in ten patients with DR have DME, if untreated, which may result in visual loss and blindness, bringing about heavy burdens to patients and society[4-5]. Corticosteroid is revealed effective in the therapy of DME, but results are often unsatisfactory thanks to the common side effects of ocular hypertension and cataract, which may need for surgery after about one year[6]. Focal/grid laser has been recommended by the Early Treatment Diabetic Retinopathy Study (ETDRS) for the administration of DME, in order to lower the possibility of visual loss[7]. However, laser photocoagulation, often with limited visual gain, is barely satisfactory, which was proven to be less effective than antiangiogenic therapy[8]. Studies have shown that the occurrence of DME is related to the increased level of vascular endothelial growth factor (VEGF) released from malfunctional Müller cell, which plays a crucial role during the process of pathologically vascular leakage of the inner blood-retina barrier in the sensory retina in diabetic patients with DME, due to retinal ischemia and hypoxia[3,9]. Therefore, antiangiogenic therapy has been believed a standard cure for treatment of DME and has largely substitute laser photocoagulation in some regions[10].
Ranibizumab (also known for Lucentis; Novartis, Basel, Switzerland), a 48 kDa recombinant antigen-binding fragment that binds to all isoforms of VEGF-A, was widely used in wet age-related macular degeneration (wAMD) patients, also in patients with DME[11-12]. Conbercept (also known for Lumitin and KH902, Chengdu Kang Hong Biotech Co, Ltd., Sichuan, China), a 143 kDa recombinant anti-VEGF fusion protein. It has been produced by the expression system of Chinese hamster ovary (CHO) cells, is alike in structure to aflibercept (also known for Eylea, Regeneron Pharmaceuticals, Eastview, NY, USA), which combines placental growth factor (PIGF) and all isoforms of VEGF-A as well as VEGF-B[13-15]. In addition, the conbercept was reported to have a stronger binding affinity for VEGF than that of ranibizumab, due to the engineered Fab fragment with the additional fourth extracellular domain of VEGFR-2[16]. As far as clinical practice is concerned, some patients with DME who were nonresponsive to intravitreal ranibizumab and bevacizumab therapy were still effective with the treatment of conbercept[17]. A newly updated Meta-analysis for DME management suggests that intravitreal aflibercept injection may show superiority to ranibizumab and bevacizumab in DME subjects at one year in terms of visual gain and anatomic reconstruction[18]. Although conbercept was authorized in December 2013 by the China State Food and Drug Administration (CFDA) for the therapy of wAMD, the drug has not yet received marketing authorization for the treatment of DME. This Meta-analysis was designed to contrast the efficacy of intravitreal injection of conbercept (IVC) with efficacy of intravitreal injection of ranibizumab (IVR) for the therapy of DME from the angle of trials from China.
MATERIALS AND METHODS
Literature Search An extensive search was conducted to spot relevant studies about DME patients’ therapy comparing IVC and IVR. Twelve databases were accessed as follows: PubMed, Medline, EMBASE, Web of Science, Springer, ScienceDirect, OVID, Cochrane Library, ClinicalTrials.gov, cqVIP, WanFangdata and China National Knowledge Infrastructure (CNKI). The following key words: “Diabetic Macular Edema” or “Diabetic Macular Oedema” or “DME” or “DMO”, and “ranibizumab or Lucentis”, and “conbercept” or “Lumitin” or “KH902” were used to identify relevant papers up to December 28, 2018, and the language restrictions were limited within English and Chinese in the search. In addition, the “pertinent articles” option was chosen to extend the search, and all search results were enrolled for further selection.
Inclusion Criteria and Exclusion Criteria Papers were picked out if they up to the following standards: 1) retrospective studies or randomized controlled trials (RCTs); 2) papers that compared the DME therapy of IVR with IVC; 3) papers that contained at least one of the interesting outcomes. The outcomes of interest contained optical coherence tomography-based (OCT-based) central macular thickness (CMT), best-corrected visual acuity (BCVA), effectiveness rate (ER) and adverse event (AE), and related results were able to be calculated or presented in the paper. Papers were dropped if one of the following criteria were met: 1) patients suffered more than one ocular disease, such as wAMD; 2) none of the interesting outcomes were reported, or it was hardly possible for reviewers to extrapolate the outcomes for either conbercept or ranibizumab from the papers; 3) reports were duplicated.
Outcomes of Interest and Data Extraction A thorough search of papers and a detailed assessment of the quality of studies were performed by two independent reviewers. All the data extracted from the literature was sorted with a standardized form, including the characteristics of basic information such as first author name, date of publication, collection period, DME type, number of patients administered with ICV or IVR therapy, treatment regimen, study design, and other interesting outcomes. log minimum angle of resolution (logMAR), to which all these values were transformed if visual acuity reported by other forms, was adopted to describe BCVA. All disparities with regard to data extraction were settled by discussion until the two reviewers reached an agreement.
Level of Evidence and Study Quality The two reviewers rated the coefficients of evidence of the nine trials, in accordance with the principles adopted by the Centre for Evidence-Based Medicine in Oxford, UK[19]. The quality of the studies was also evaluated by reviewers independently. Meanwhile, any discrepancy was solved by consensus.
Statistical Analyses The current Meta-analysis was performed in accordance with the Quality of Reporting of Meta-analyses guidelines, as well as the principles of the Cochrane Collaboration[20]. All the data analyses were conducted with the support of RevMan 5.3 software (Cochrane Library Software, Oxford, UK). Odds ratio (OR) was applied for dichotomous variables; weighted mean difference (WMD) was applied for continuous variables. All pooled estimates depended on the results of the Z test; P<0.05 was regarded as statistically significant, and the confidence interval (CI) was set at 95%. I2 test and the Chi-squared-based Q test were applied to determine the extent of heterogeneity among the studies; and P<0.05 as well as I2>50% were considered as heterogeneity. A random-effects model has been applied if the result of the heterogeneity test suggested that interstudy heterogeneity existed; otherwise, a fixed-effects model. Sensitivity analysis was conducted by dropping of specific studies. Outcomes were put together and analyzed if three or more studies reported the same variable through the whole Meta-analysis. Funnel plots were applied to evaluate the publication bias.
RESULTS
Literature Review Process A total of 9 studies with the number of 609 subjects were enrolled in the current Meta-analysis after selection[21-29]. The studies included five RCTs and four retrospective studies; 309 and 300 patients received IVC and IVR separately. Figure 1 presents a flowchart of the procedure of selection; Table 1 presents the characteristics of basic information.
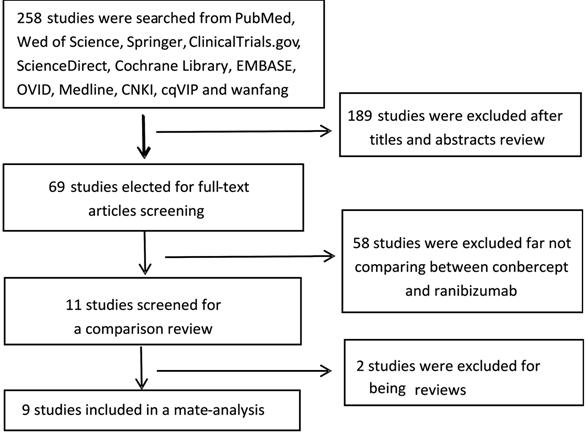
Figure 1 Flow diagram showing the procedure of selection for this study.
Table 1 Characteristics of basic information
Study Year |
Collection period |
No. of eyes |
DME type |
Treatment regimen |
design |
||
IVC |
IVR |
||||||
Zhang et al[22] |
2018 |
2016.7-2017.7 |
25 |
25 |
dDME |
Monthly for 3mo |
RCT |
Guo et al[23] |
2018 |
2015.1-2016.1 |
55 |
55 |
DME |
Monthly for 3mo |
Retro |
Chen et al[21] |
2016 |
2012.1-2015.8 |
35 |
37 |
DME |
Monthly for 3mo |
RCT |
Ji[29] |
2018 |
2016.6-2017.11 |
42 |
43 |
DME |
Once |
Retro |
Hou and Hu[27] |
2018 |
2016.6-2017.4 |
29 |
29 |
DME |
Monthly for 3mo |
Retro |
Xiang[28] |
2018 |
2017.2-2017.12 |
30 |
30 |
dDME |
Monthly for 3mo |
RCT |
Lin[25] |
2016 |
2015.1-2015.6 |
36 |
24 |
DME |
Once |
RCT |
Yang and Chen[24] |
2017 |
2014.9-2015.9 |
33 |
33 |
DME |
Monthly for 3mo |
RCT |
Dong and Hou[26] |
2015 |
2014.6-2015.3 |
24 |
24 |
DME |
Once |
Retro |
Retro: Retrospective study; RCT: Randomized controlled trial; dDME: Diffuse diabetic macular edema; IVR: Intravitreal injections of ranibizumab; IVC: Intravitreal injections of conbercept.
Best-corrected Visual Acuity The BCVA of the follow-up in the 1st (n=220), 3rd (n=280) and 6th (n=160) month were reported. No statistically significant difference was found in the mean BCVA at beginning (WMD: -0.48; 95%CI: -1.06 to 0.10; P=0.1) and after therapy [the 1st month BCVA (WMD: 0.01; 95%CI: -0.26 to 0.27; P=0.96), the 3rd month BCVA (WMD: -0.04; 95%CI: -0.14 to 0.06; P=0.46); the 6th month BCVA (WMD: -0.24; 95%CI: -1.62 to 1.14; P=0.73)] between the IVC group and IVR group (Figure 2).
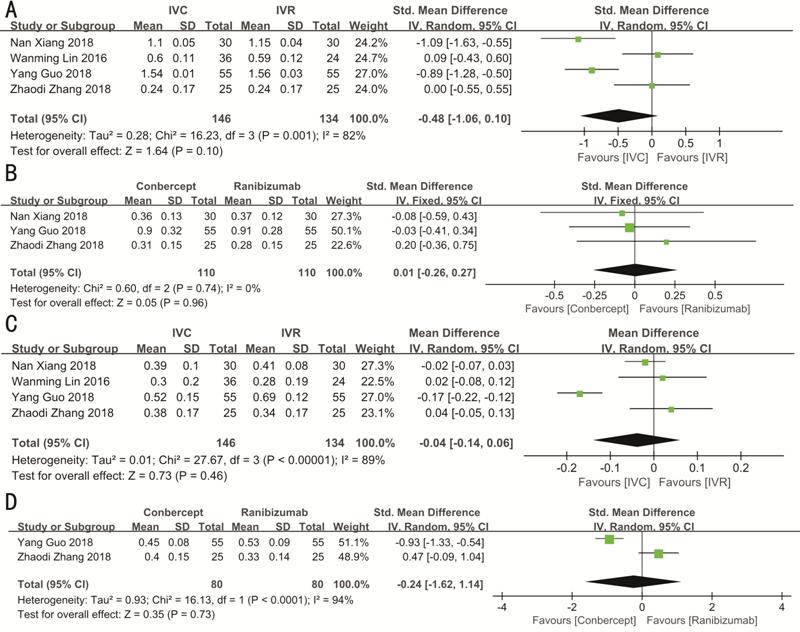
Figure 2 Forest plots of mean BCVA in DME patients before (A) and after 1 (B), 3 (C) and 6mo (D) of treatment with conbercept vs ranibizumab.
Central Macular Thickness All the CMTs were measured with the support of OCT at the beginning and duration of the follow-up period in the IVC group and IVR group. The CMT of the follow-up in the 1st (n=350), 3rd (n=410) and 6th (n=232) month were reported. No statistically significant difference was found in CMT at the baseline between the two cohorts (WMD: -0.83; 95%CI: -15.15 to 13.49; P=0.91). However, the IVC group significantly differed from the IVR group [1st month CMT (WMD: -19.88; 95%CI: -27.94 to -11.82; P<0.001), 3rd month CMT (WMD: -23.31; 95%CI: -43.30 to -3.33; P=0.02), 6th month CMT (WMD: -74.74; 95%CI: -106.22 to -43.26; P<0.001)] in mean CMT after administration with the conbercept or ranibizumab therapy. The study by Xu et al[30] was not enrolled since they only reported the CMT of the 12th month, rather than the 6th month’s CMT. DME patients administered with monthly injections of conbercept underwent a more remarkable reduction of mean CMT from the beginning compared with DME patients administered with ranibizumab. Figure 3 presents the forest plots reflecting mean CMT.
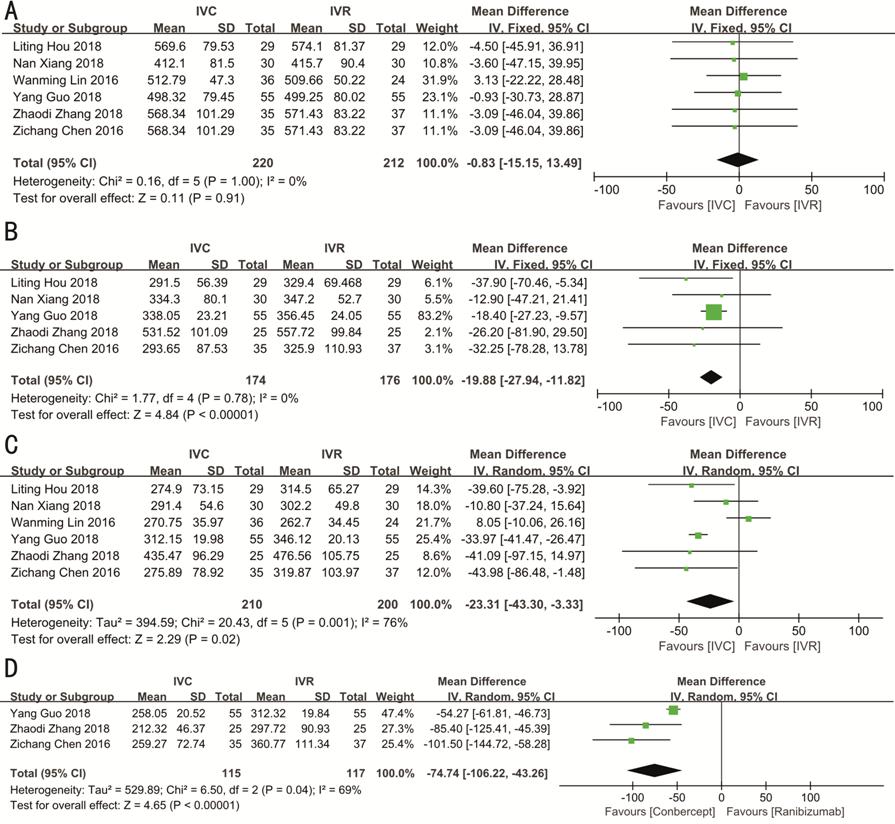
Figure 3 Forest plots of mean CMT in DME patients before (A) and at the 1st (B), 3rd (C) and 6th (D) month of treatment with conbercept vs ranibizumab.
Effectiveness Rate Treatment effectiveness is defined as an improvement in visual acuity ≥1 line in international standard vision chart or 5 letters in ETDRS chart after treatment or macular edema regression, complete or partial absorption of retinal hemorrhage. Seven studies (n=499) reported effectiveness. The fixed-effects model analysis was conducted, owing to the heterogeneity test results (P=0.84, I2=0). Meta-analysis results showed that there was a slightly statistical difference between the IVC group and IVR group in terms of ER (OR: 1.70; 95%CI: 0.97 to 2.96; P=0.06; Figure 4).

Figure 4 Conbercept versus ranibizumab for the effective rate in patients with DME.
Adverse Event Four studies (n=321) reported AEs (including anterior chamber inflammation, subconjunctival hemorrhage, vitreous hemorrhage, increased intraocular pressure, corneal edema, retinal proliferation, retinal tears, pain at the injection site, floaters in front of the eye, etc. Due to the heterogeneity test results (P=0.87, I2=0), fixed effects model analysis was performed. No statistically significant difference was found in the AEs between the IVC group and IVR group (OR: 0.84; 95%CI: 0.38 to 1.84; P=0.66) (Figure 5).

Figure 5 Conbercept versus ranibizumab for the AE in patients with DME.
Publication Bias and Sensitivity Analysis Three funnel plots (AEs, initial CMT, ER) were applied to evaluate the publication bias of the nine studies, and there was no significant publication bias observed in any of the comparison (Figure 6). Heterogeneities were obvious in initial BCVA (P=0.001, I2=82%), the 3rd month BCVA (P<0.001, I2=89%), the 3rd month CMT (P=0.001, I2=76%), and the 6th month CMT (P=0.04, I2=69%). Hence, a sensitivity analysis was performed. Generally, a specific study was dropped, and the remaining studies were reanalyzed to observe if the omitted study could have obviously affected the overall estimate. Sensitivity analyses indicated that no specific trial markedly affected the results of the BCVA and CMT. We inferred that the causes for heterogeneity could lie in small sample size which was insufficient to precisely predict heterogeneity, rather than clinical differences, DME type and treatment regimen, for instance. The remaining six analyses were also performed, and no significant heterogeneity has been found.
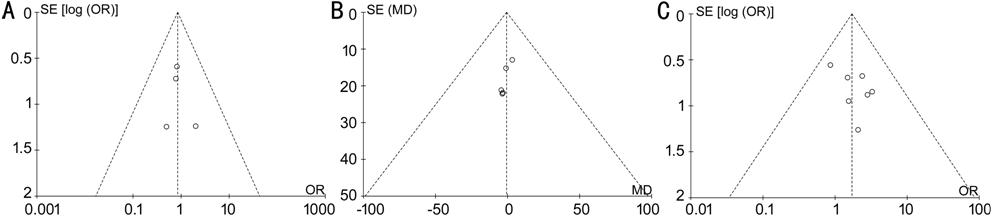
Figure 6 Funnel plots showing the publication bias A: AE; B: Initial CMT; C: ER.
DISCUSSION
In China, ranibizumab is deemed the first-line drug for the administration of wAMD, which also has been widely employed in retinal vein occlusion (RVO)[31-32]. It was learned recently that ranibizumab was authorized by the China State Drug and Food Administration for the therapy of DME, which is the third indication approved in China, providing a new treatment choice for DME patients. The efficacies and complications of IVC and IVR injection for DME have been reported by various researchers; both anti-angiogenesis drugs have been revealed obviously improving the visual acuity in DME patients. However, the results of these papers have not been systematically collected, sorted, and evaluated. This Meta-analysis provided further information with regard to the clinical efficacy and ocular complications of IVC and IVR from the point of clinical practice.
In the present Meta-analysis, no statistically significant difference was found in CMT and BCVA at the beginning of therapy, which suggested that IVC and IVR groups presented no statistically significant difference in baseline parameters. Surprisingly, CMT of the conbercept cohort decreased more obviously than that of the ranibizumab group during the short follow-up period whereas the BCVA has no statistically significant difference in the two cohorts. We speculated that conbercept has the greater binding affinity, higher concentration, and peculiar isoelectric point maybe account for this. First, the blocking ability of conbercept is 38 to 48 times more powerful than that of bevacizumab and ranibizumab, comparable with that of aflibercept[33]. Conbercept, a soluble decoy receptor that has a high binding affinity for VEGF[16], blocks PIGF as well as all isoforms of VEGF mentioned above. Second, conbercept, with a half-life of 4.2d for 0.5 mg, has a longer half-life than that of ranibizumab, with a half-life of 2.88d for 0.5 mg[34]. Therefore, conbercept might lasts longer from the angle of logical inference[35]. More specifically, there is evidence that the conbercept in choroid and retina still plays a role over 34d, and its concentration remained higher than 7 ng/g, the 50% inhibitory concentration, suggesting that an injection dose of 0.5 mg conbercept may last as long as 81d to compete with VEGF[33]. The additional domain 4 enhances the binding ability with VEGF, which assists conbercept in overmatching any of other anti-angiogenesis drugs in the half-life terms. The extended half-life of conbercept signifies the treatment effect could be extended, which would helpful to reduce the frequency of injections[33]. In addition, the introduction of domain 4 of VEGFR-2 results in a lower isoelectric point (PI). A previous study revealed that the poor pharmacokinetic peculiarities of fusion proteins might result from the high positive charge[36]. The special domain 4 of conbercept can decrease adhesion to the extracellular matrix as well as the positive charge, and the PI of conbercept (6.13-6.81) is obviously lower than that of aflibercept (8.82)[36]. As a consequence, this may be the reason why the CMT in conbercept group decreased more significantly than that in the ranibizumab group in a short follow-up period. No statistically significant difference, however, in mean BCVA between the two groups was found during the followed 6mo. The improvement of BCVA seemed to be unparallel to the reduction of CMT. The reason for this out of sync could be that BCVA was influenced by both the extent of macular edema and morphology, especially the dropout of photoreceptors[37]. The precursor to hard exudates was reported to have the erosion effect to the external limiting membrane and photoreceptors, which deposits in the outer nuclear layer, eventually leading to degeneration and apoptosis of photoreceptor[38]. Meanwhile, intracapsular pressure of the cysts also plays a part in the disruption of photoreceptors and external limiting membrane[39]. Therefore, we inferred that visual improvement is more relevant to the renewal and reconstruction of damaged cells and photoreceptors, rather than the VEGF level. Besides, Huang et al’s[40] report revealed no statistically significant difference in the visual gain of polypoidal choroidal vasculopathy (PCV) patients between the IVC group and IVR group at the 6th month. However, conbercept exhibited some advantages over ranibizumab treatment in the regression of polyps, which may partially substantiate this conjecture: conbercept is more likely to reduce the level of VEGF within the six months, not the improvement of the anatomic outcomes. In another Meta-analysis, the administration of macular edema secondary to wAMD, RVO or DME manifested surprisingly similar results: no significant difference in BCVA, but a significant difference in CMT in the short period[41]. However, we observed that Xu et al’s[30] study compared a 12-month efficacy of the two drugs in a relative long-term follow-up period, and concluded that conbercept and ranibizumab presented similar therapeutic effects as regard to the reduction of central retinal thickness and visual improvement at one year when injected in accordance a 3+PRN protocol. But still, the number of injections administered in the IVR group was significantly higher than that injected in the IVC group. We inferred that the renewal of the dysfunctional cells in the retina and the increased number of injections administered by ranibizumab, which compensated its disadvantages mentioned above, both lead to relatively similar outcomes.
Finally, as VEGF is also a necessary growth factor for maintaining normal retinal and choroidal microenvironment, anti-VEGF therapy can induce the apoptosis of retinal ganglion cells (RGCs) and amacrine cells as well as bipolar cells[42-43]. Studies suggest that multiple intravitreal injections may result in retinal atrophy in the macula, leading to ultimate visual impairment[44], which may partially account for the inconsistency between the BCVA and CMT. Therefore, whether repeated intravitreal-injection of anti-VEGF drugs may lead to functional damage in the macular area requires further clinical observation and verification.
There are several limitations to the current study. First, conbercept has only recently been employed clinically for DME. Hence, the available data are limited, which was our reason for including both retrospective studies and RCTs. More RCTs with longer-term follow-up periods and trials with therapeutic effects are needed to verify if the reduction in CMT at different time points as well as improvement in visual acuity remain stable over time. Second, additional clinical trials are necessary to compare the treatment efficacy of conbercept with other similar anti-angiogenesis drugs, for instance, aflibercept, which has recently been available in China. We are looking forward to seeing efficacy between conbercept with aflibercept in a short or long follow-up period, as both are more likely superior to ranibizumab, and have a similar structure.
In conclusion, pooled evidence suggested that both IVC and IVR are effective in the therapy of DME and affirmed that IVC presented superiority over IVR therapy in regard of CMT in patients with DME, but no statistically significant difference with regard to visual improvement. Relevant RCTs with longer-term follow-up are necessary to back up our conclusion.
ACKNOWLEDGEMENTS
Conflicts of Interest: Liu WS, None; Li YJ, None.
REFERENCES