
·Basic Research·
The
expression of lacrimal androgen-binding proteins in mice Pseudomonas
aeruginosa keratitis
Le-Yu
Lyu, Qian Wang, Qiang Xu, Wen-Yi Zhao, Hua Yang, Cheng-Ye Che
Department of Ophthalmology, the
Affiliated Hospital of Qingdao University, Qingdao 266003, Shandong Province,
China
Correspondence to: Cheng-Ye Che. Department of
Ophthalmology, the Affiliated Hospital of Qingdao University, No.16 of Jiangsu
Road, Shinan District, Qingdao 266003, Shandong Province, China.
chechengye@126.com
Received:
Abstract
AIM: To investigate the expression
of lacrimal androgen-binding proteins (ABPs) in mice Pseudomonas aeruginosa
(P. aeruginosa) keratitis.
METHODS: P. aeruginosa mice model from
different gender was developed by intra-stromal injection. The expression of lacrimal
ABPs in lacrimal gland specimens from P. aeruginosa keratitis mice
was detected by the quantitative polymerase chain reaction (qRT-PCR). Corneal
virulence was evaluated based on clinical scores. To study the mechanism of
lacrimal ABPs’ expression, experimental subjects were pre-treated with 4E-BP1
inhibitor, and were used to evaluate the expression levels by qRT-PCR.
RESULTS: Compared with control
groups, the expression of ABPα, ABPη and ABPζ in lacrimal gland
from P. aeruginosa keratitis mice had no meaningful changes,
while ABPε and ABPδ were significantly
higher at 1d after infection. The expression of ABPδ in lacrimal gland of
male mice was higher than female mice, regardless of whether or not P.
aeruginosa keratitis occurred. After 4E-BP1 inhibitor subconjunctival
injection or lacrimal injection, the expression of ABPδ and ABPε has no significant
change compared with the control group.
CONCLUSION: ABPδ and ABPε secreted by mice
lacrimal gland may involve in the progress of alleviating the severity of
corneal damage in P. aeruginosa keratitis. The expression of ABPδ and ABPε upon P. aeruginosa
infection is independent of cap-dependent mRNA translation activated by 4E-BP1.
KEYWORDS: keratitis; Pseudomonas
aeruginosa; androgen-binding proteins; lacrimal gland
DOI:10.18240/ijo.2020.01.02
Citation:
Lyu LY, Wang Q, Xu Q, Zhao WY, Yang H, Che CY. The expression of lacrimal
androgen-binding proteins in mice Pseudomonas aeruginosa keratitis. Int
J Ophthalmol 2020;13(1):7-10
INTRODUCTION
Bacteria are the leading cause of
eye infections worldwide[1]. Ocular infections may
damage the structures and lead to blindness and visual impairment without
treatment[2]. Pseudomonas aeruginosa (P.
aeruginosa), which was found in 50% of keratitis diagnoses[3], is the most frequent isolate of Gram-negative ocular
infections[4]. P. aeruginosa was hard to
eradicate efficiently due to acquired antibiotic resistance and
pathoadaptation, making the urgent demands to seek for alternative therapeutic
methods[5-7].
The tear fluid plays the key role in
maintaining the stability of the intraocular environments by covering the
anterior corneal surface. The discharge of tears can flush pollutants and
irritants out, thereby playing the role as the first line of defense against
the invasion of pathogens for the anterior eye[8-9].
The androgen-binding proteins (ABPs)
containing a small family of secretory proteins were only found in mammalian
lineage. High concentrations of ABPs were found in many mammalian secretions,
such as fluids of the lacrimal gland, lung and salivary gland[10]. Five kinds of lacrimal ABPs are characteristic to
mice,including ABPα (Scgb1b27), ABPζ
(Scgb2b24), ABPη (Scgb1b2), ABPε (Scgb2b2) and ABPδ (Scgb2b20)[11]. Although the biological activities of ABPs in most
individuals have not been fully characterized, it has been found that this
family play an important role in the regulation of tissue repairment,
inflammation, and tumorigenesis[12]. There is a
slight self-healing tendency due to keratitis in mice, and lacrimal ABPs may
play a role against bacterial keratitis.
Interestingly, the secretion of some lacrimal ABPs is
sex-oriented. In the five lacrimal ABPs characteristic to mice, though ABPα and ABPζ are uncertain and ABPη and ABPε are unbiased, ABPδ shows obvious male bias[13]. Whether the gender response to P. aeruginosa keratitis
is different is also an interesting topic.
Based on these, present studies were designed to
investigate the expression levels and roles of lacrimal ABPs in P.
aeruginosa keratitis with different genders, as well as part of the
mechanism of ABPs’ functions.
MATERIALS AND METHODS
Ethical Approval All treatments on mice were complied
with the regulations of Statement on the Use of Animals in Ophthalmic and
Vision Research announced by Association for Research in Vision and
Ophthalmology (ARVO).
Anatomical Position of Lacrimal
Gland The main lacrimal gland of mice is
out of orbita, locating directly below the ear with the long axis perpendicular
to the zygomatic arch and connecting to the eye surface through a long
excretory duct[14].
The Establishment of Mouse Pseudomonas
aeruginosa Keratitis Eight-week-old specific
pathogen-free C57BL/6 mice (male and female) were purchased from the Changzhou
Cavens Laboratory (Jiangsu Province, China). The standard P. aeruginosa
strain was provided by the Affiliated Hospital of Qingdao University. Mice were
anesthetized by chloral hydrate (0.08 mL/mouse) through intraperitoneal
injection. One eye was randomly selected from each mouse. Next, a 33-gauge
Hamilton syringe was inserted through the tunnel, and 2.5 μL bacterial
suspension (2.5×10 bacteria/μL PBS) was injected into the corneal stroma[15]. The P. aeruginosa–infected mouse corneas
exhibited stromal infiltration 1d post-infection. To investigate the expression
of lacrimal ABPs in P. aeruginosa keratitis of the eye in mice, the mice
were divided into four groups, including normal control female, normal control
male, P. aeruginosa keratitis female and P. aeruginosa keratitis
male. To know the mechanism of ABPs, the experimental eyes were received a
subconjunctival injection (3 µL) containing 4E-BP1/eIF4E interaction inhibitor
4E1RCat (SelleckChem) or dimethyl sulfoxide (DMSO) as a control at 1d and 2h
before infection in group 1. Same as above, the experimental lacrimal glands
were received an injection (3 µL) containing 4E-BP1 inhibitor in group 2.
Clinical scores were used to
evaluate the degree of corneal infections: 0, transparent or slight opacity,
partly covering pupil; +1, slight opacity, completely covering cornea; +2,
dense opacity, partly or completely covering pupil; +3, dense opacity,
completely covering cornea; +4, corneal perforation or keratitis[16]. Lacrimal glands were collected one day after
establishing the mouse model for quantitative polymerase chain reaction
(qRT-PCR).
Quantitative Polymerase Chain
Reaction Under an operating microscope, whole
lacrimal gland of each mouse was then carefully cut off. RNA was extracted from
mice lacrimal gland using RNAiso plus reagent (Takara). To obtain cDNA, the
primescript RT Reagent Kit (Takara) was used to reverse transcript 2 µg total
RNA. Using Eppendorf Mastercycler and SYBR green, qRT-PCR was performed when
β-actin was used for internal control (Table 1).
Table 1 Nucleotide sequences of
mouse primers for qRT-PCR
|
Genes |
Primer sequence ( |
|
Scgb2b24-F |
GGAAGCAGGCTGTGGTTGTATC |
|
Scgb2b24-R |
GGAATAGTACTGCAGGCATTCTGG |
|
Scgb2b2-F |
TCTCTGGAAACAGGATTGGGTTA |
|
Scgb2b2-R |
CGACCTGCATTCTGAGCTGAAG |
|
Scgb2b20-F |
GGTGTGGTTGTATCAAGAACTCCAG |
|
Scgb2b20-R |
AGACCATAGTATGACAGGCATTCAG |
|
Scgb1b27-F |
TCTGATAGGACCTTGACCGAGGA |
|
Scgb1b27-R |
GCTGCATCTATGCTGGTGAGGA |
|
Scgb1b2-F |
TCGATAGGACGTTGACGAAGG |
|
Scgb1b2-R |
GTAGGGCTTGTTGCATCTATGTAGG |
|
β-actin-F |
GATTAC TGCTCTGGCTCCTAGC |
|
β-actin-R |
GACTCATCGTACTCCTGCTTGC |
Statistical Analysis Two-tailed, unpaired t-test
was used to determine the statistical significance of qRT-PCR data and clinical
score. Data were represented as mean±standard deviation and analyzed by
GraphPad 7.0 software. When P≤0.05, differences were considered
significant.
RESULTS
The Establishment of Pseudomonas
aeruginosa Keratitis Models in Female and Male Mice Images captured with a slit lamp
after infection at 1d illustrated the disease response to different genders
(Figure 1). Disease response was represented by a clinical score (n=8/group).
There was no statistical difference between the two groups (P>0.05).
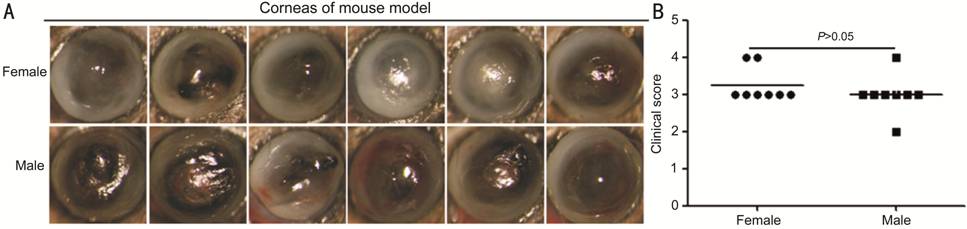
Figure 1 The establishment of a P.
aeruginosa keratitis model in female and male mice A: Images captured with a slit lamp
at 1d after infection; B: Disease response was showed by a clinical score (n=8/group),
which was no statistical difference between the two groups (P>0.05).
The Expression of Androgen-binding
Proteins in Pseudomonas aeruginosa Keratitis Compared with normal groups, the
expression of ABPα, ABPη and ABPζ in lacrimal gland had no meaningful changes (P>0.05),
while ABPε and ABPδ were significantly higher (P<0.05) at 1d after
infection (Figure 2). What’s more, the expression of ABPδ in lacrimal gland of
male mice was higher (P<0.05) than female mice, regardless of
whether or not P. aeruginosa keratitis occurred.
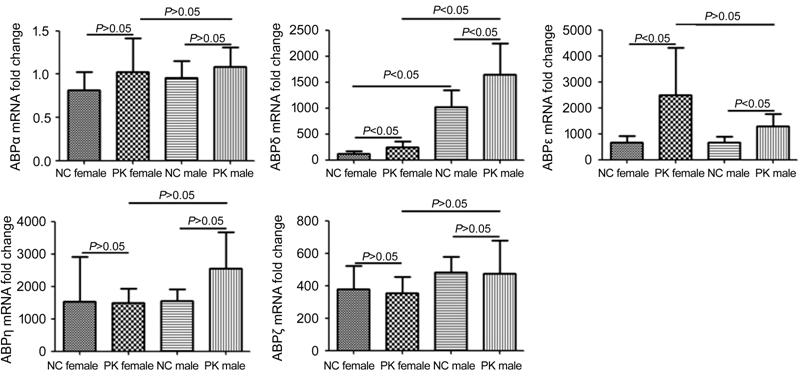
Figure 2 The expression of lacrimal
ABPs in mice P. aeruginosa keratitis The expression of ABPα, ABPη and
ABPζ in lacrimal gland had no meaningful changes (P>0.05), and the
expression of ABPε and ABPδ were significantly higher (P<0.05) at 1d
after infection. Meanwhile, the expression of ABPδ in lacrimal gland of male
mice was higher (P<0.05) than female mice, regardless of whether or
not P. aeruginosa keratitis occurred.
The expression of ABPδ and ABPε upon
P. aeruginosa infection was independent of cap-dependent mRNA
translation activated by 4E-BP1. After 4E-BP1 inhibitor subconjunctival
injection or lacrimal injection, the expression of ABPδ and ABPε had no
significant change compared with the control group (P>0.05; Figure
3). There was also no significant difference between the two experimental
groups (P>0.05).
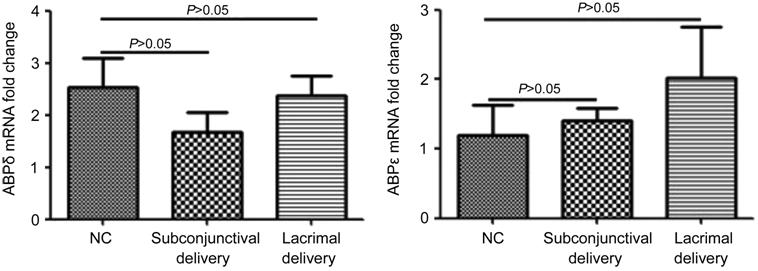
Figure 3 The expression of ABPδ and
ABPε upon P. aeruginosa infection was independent of cap-dependent mRNA
translation activated by 4E-BP1.
After 4E-BP1 inhibitor
subconjunctival injection or lacrimal injection, the expression of ABPδ and
ABPε has no significant change compared with the control group (P>0.05).
There was also no significant difference between the two experimental groups (P>0.05).
DISCUSSION
These results demonstrated that the
expressions of ABPδ and ABPε were increased in lacrimal gland of both female
and male mice against P. aeruginosa keratitis. As the members of
lacrimal ABPs, ABPδ and ABPε may be involved in P. aeruginosa
inflammation. Though the character of ABPs in P. aeruginosa keratitis
remains unclear, number of researches have shown their anti-inflammatory
effect. For instance, under the stimulation by the ABP dendrimer, interleukin
(IL)-10 could be produced by the same cellular subsets in vitro among
human immune cells[15,17]. And
the production of IL-10 was known as the paradigm of anti-inflammatory
cytokines[11]. Taken together, lacrimal ABPδ and
ABPε may have a protective effect in P. aeruginosa keratitis.
In addition, the expression of ABPδ
in lacrimal gland of male mice was higher than female mice, regardless of
whether or not P. aeruginosa keratitis occurred in our study. This
showed a clear gender bias. There was no significant difference between male
and female mice in P. aeruginosa keratitis. Besides, the expression of
ABP ε was slightly higher in female mice although without statistical difference.
The expression of ABP ε may be a kind of compensation for the lower expression
of ABP δ in female mice.
Eukaryotic translation initiation
factor (eIF4E), as the eukaryotic initiation factor, regulates the association
between eIF
After 4E-BP1 inhibitor
subconjunctival injection or lacrimal injection, the expression of ABPδ and
ABPε had no significant change compared with the control group. The expression
of ABPδ and ABPε upon P. aeruginosa infection was independent of
cap-dependent mRNA translation activated by 4E-BP1.
In summary, ABPδ and ABPε secreted
by mice lacrimal gland may be involved in the progress of alleviating the
severity of corneal damage in P. aeruginosa keratitis. The expression of
ABPδ and ABPε upon P. aeruginosa infection was independent of
cap-dependent mRNA translation activated by 4E-BP1. Unfortunately, the
researches on lacrimal ABPs are still rare. Moreover, there is no commercial
antibody to ABPs currently. So we bring the preliminary results about ABPs induced
by P. aeruginosa keratitis in this study. It’s promising that the veil
of ABPs will eventually be lifted in the further researches on ABPs.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural
Science Foundation of China (No.81300730); China Postdoctoral Science
Foundation (No
Conflicts of Interest: Lyu LY, None; Wang Q, None;
Xu Q, None; Zhao WY, None; Yang H, None; Che CY,
None.
REFERENCES