IF in JCR CiteScore
Rank About IJO Current
Issue Featured Articles Articles In Press Recent Accepted
 International Journal
of Ophthalmology
International Journal
of Ophthalmology
2017; 10(9): 1337-1343
·Basic Research·
Suppression of fibrosis in human pterygium
fibroblasts by butyrate and phenylbutyrate
Yuka Koga1, Noriaki Maeshige1,2,
Hiroto Tabuchi1, Mikiko Uemura1, Michiko Aoyama-Ishikawa1,
Makoto Miyoshi1, Chikako Katakami3, Makoto Usami1,4
1Division of
Nutrition and Metabolism, Department of Biophysics, Kobe University Graduate
School of Health Sciences, Tomogaoka 7-10-2, Suma-ku, Kobe, Japan
2Department
of Rehabilitation Science, Kobe University Graduate School of Health Sciences,
Tomogaoka 7-10-2, Suma-ku, Kobe, Japan
3Department
of Ophthalmology, Saneikai Tsukazaki Hospital, Waku 68-1, Aboshi-ku, Himeji,
Japan
4Department
of Nutrition, Kobe University Hospital, Kobe University School of Medicine,
Kusunoki-cho 7-5-2, Chuo-ku, Kobe, Japan
Correspondence to: Noriaki
Maeshige. Division of Nutrition and Metabolism, Department of Biophysics, and
Department of Rehabilitation Science, Graduate School of Health Sciences, Kobe
University, Tomogaoka 7-10-2, Suma-ku, Kobe, Japan. nmaeshige@pearl.kobe-u.ac.jp
Received: 2017-02-18
Accepted: 2017-04-25
Abstract
AIM: To
evaluate the antifibrogenic effects of butyrate or phenylbutyrate, a chemical
derivative of butyrate, in human pterygium fibroblasts.
METHODS: Human
pterygium fibroblasts obtained from patient pterygium tissue were treated with
butyrate or phenylbutyrate for 48h. Expression of α-smooth
muscle actin, collagen I, collagen III and matrix metalloproteinase-1 mRNA was
measured by quantitative real-time reverse transcription polymerase chain
reaction, and acetylated histone was evaluated by Western blotting.
RESULTS: Butyrate
inhibited α-smooth
muscle actin, type III collagen and matrix metalloproteinase-1 expressions, and
phenylbutyrate inhibited types I and III collagen and matrix
metalloproteinase-1 expressions without changing cell viability as well as both
of these increased histone acetylation. These results suggested that butyrate
and phenylbutyrate suppress fibrosis through a mechanism involving histone
deacetylase inhibitor.
CONCLUSION: This
indicates that butyrate or phenylbutyrate have antifibrogenic effects in human
pterygium fibroblasts and could be novel types of prophylactic and/or
therapeutic drugs for pterygium, especially phenylbutyrate, which does not have
the unpleasant smell associated with butyrate.
KEYWORDS: butyrate;
phenylbutyrate; pterygium; fibroblasts; antifibrogenic effect
DOI:10.18240/ijo.2017.09.01
Citation: Koga Y, Maeshige N, Tabuchi H, Uemura M, Aoyama-Ishikawa M, Miyoshi M, Katakami
C, Usami M. Suppression of fibrosis in human pterygium fibroblasts by butyrate
and phenylbutyrate. Int J Ophthalmol 2017;10(9):1337-1343
INTRODUCTION
Pterygium is a triangular-shaped overgrowth
of the fibrovascular conjunctiva onto the nasal or temporal cornea, caused
mainly by chronic exposure to ultraviolet rays. It may cause ocular irritation,
cosmetic problems, astigmatism and visual impairment. Although surgical removal
is performed for these symptomatic cases[1-2],
post-surgical recurrence rates after excision of the pterygium have been
reported to be very high. Several intra- and post-operative treatments,
including mitomycin C, 5-fluorouracil and corticosteroids, have been
recommended for the prevention of postoperative recurrence of pterygium[2]. However, despite these treatments, the recurrence of
pterygium and/or occasional severe complications may occur. Therefore, safer
and securer treatments for the prevention of the recurrence of pterygium are
strongly desirable.
Aberrant extracellular matrix (ECM)
remodeling appears to be a major feature of pterygium, as evidenced by previous
studies indicating that ECM genes including fibronectin, collagen and versican
are upregulated in pterygium[3-4].
In particular, matrix metalloproteinases (MMPs), a family of structurally
related zinc-dependent ECM-degrading proteinases, are elevated in pterygium[5]. In the process of abnormal cell proliferation and
angiogenesis generation in primary and recurrent pterygium, modification or
degradation of ECM may be related to MMPs. It has been reported that altered
limbal basal epithelial cells may cause activation of fibroblasts at the head
of the pterygium, leading to degradation of Bowman’s layer as a result of the
production of MMP-1 derived from fibroblasts and further invasion of pterygium
into the cornea[6]. Regarding the proliferative
gain of function of fibroblasts, myofibroblasts express α-smooth muscle actin
(α-SMA), and its expression in pterygium has been reported in a previous study[7].
Butyrate, a predominant short-chain fatty
acid, is one of the end products of anaerobic bacterial fermentation of dietary
fibers in the colon, and has histone deacetylase (HDAC) inhibitor activity[8]. Butyrate or other HDAC inhibitors have antifibrogenic
effects that suppress collagen synthesis, α-SMA expression and increase of cell
number in various organs, such as pancreas or lung[9-10]. We have previously reported that the antifibrogenic
effects of butyrate are stronger than propionate in human dermal fibroblasts[11]. However, these effects of butyrate in human
pterygium fibroblasts (HPFs) have not been investigated. Because butyrate has a
characteristic unpleasant smell, it is considered that it might be unsuitable
for ocular administration in humans.
Phenylbutyrate (PB), a chemical derivative of
butyrate without the unpleasant smell, is a non-toxic pharmacological compound
that functions as a weaker HDAC inhibitor than butyrate. The Food and Drug
Administration has approved its clinical use in the United States in patients
with urea cycle disorders and hyperammonemia[12].
PB has also been used in clinics to treat β-thalassemia, sickle cell anemia and
cancer[13-14]. It has been
reported that PB decreases the expression of collagen I through histone
acetylation in fibroblast from human lung[10].
However, these effects of PB in HPFs have not been investigated. It has also
been reported that topical ocular PB administration shows beneficial effects in
improving glaucoma in a mouse model without causing eye abnormalities[15].
In the present study, we investigated the
antifibrogenic effects of butyrate and PB in HPFs by measuring profibrotic
factors and MMP expression, and the underlying mechanism involving histone
acetylation.
SUBJECTS AND METHODS
Cell Culture HPFs were
cultivated from pterygium obtained from two patients during surgical removal,
and the effects of butyrate and PB were evaluated in HPFs from each patient.
Patient 1 was a woman aged 68 with a primary pterygium, and patient 2 was a man
aged 78 with recurrent pterygium. The protocol for tissue collection and
analysis was approved by the ethics board of Kobe University Graduate School
and followed the Declaration of Helsinki. Written informed consents were
obtained from all of the participants. After surgical excision, subconjunctival
connective tissue of pterygium was propagated in Dulbecco’s modified Eagle
medium (DMEM; Wako, Osaka, Japan) supplemented with 10% fetal bovine serum
(FBS; Nichirei, Tokyo, Japan), 50 U/mL penicillin and 50 μg/mL streptomycin (MP
Biomedicals, Illkirch, France). The culture medium was changed every 2 or 3d
until approximately 80% confluence was reached. The cells were passaged by
incubation at 37℃ with 0.125%
trypsin-EDTA, and plated in culture dishes. Cells at passages 3 to 7 were used
for experiments, and we confirmed the fast growth of these cells. Four
independent experiments were performed for all analyses in HPFs from each
patient. The trial and additional data collection on the cause of visual loss
were approved by the relevant local research ethics committees.
Trypan Blue Staining For
experimental treatments, the cells were seeded into six-well plates (Iwaki;
Tokyo, Japan) at a concentration of 2.8×105 cells/well and incubated
for 24h. The cells were then cultured in DMEM with 0 (control), 1, 4 or 16
mmol/L sodium butyrate (Sigma, St. Louis, MO, USA) or sodium PB (Sigma) for
48h. Trypan blue staining was used to calculate cell viability.
Quantitative Real-time Polymerase Chain
Reaction HPFs were
cultured in six-well plate using DMEM supplemented with 10% fetal bovine serum.
After 24h, the content of serum in the medium was reduced to 0.1% for 48h to
render the cells quiescent. Sodium butyrate and sodium PB were added and the
HPFs were incubated for 48h. Then, they were processed for total RNA isolation
using TRIzol (Invitrogen, Carlsbad, CA, USA) and reverse transcribed to yield a
single-stranded cDNA, with iScript cDNA synthesis kits (Bio-Rad, Hercules, CA,
USA). The expressions of collagen I, collagen III, α-SMA and MMP-1 were
detected by means of quantitative real-time polymerase chain reaction (PCR)
analysis, using SYBR Premix Ex Taq II (Takara Bio, Otsu, Japan) with each
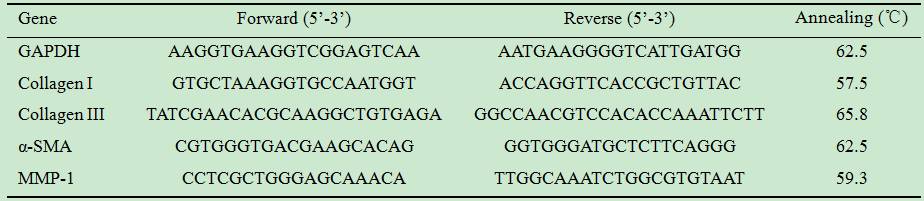
primer (Table 1). PCRs were run on iCycler IQ (Bio-Rad, Hercules, CA, USA) for
40 cycles at 95℃ for 30s, at
an annealing temperature (Table 1) for 30s, and at 72.0℃ for 30s. Post-PCR melting curves were confirmed by the
specificities of single-target amplification, and the relative expressions of
each gene were calculated based on glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) expression in duplicate.
Table 1 Primers used in real-time PCR
Western Blotting HPFs were
cultured in six-well plate using DMEM supplemented with 10% FBS. After 24h, the
content of serum in the medium was reduced to 0.1% for 48h to render the cells
quiescent. Sodium butyrate and sodium PB were added and the HPFs were incubated
for 48h. The HPFs were prepared in 1.5-mL tubes and then suspended in 100 μL of
ProPrep (iNtRON, Gyeonggi-do, Korea). Aliquots (5 µL) of the cell supernatants
were used to measure the protein concentration using Lowry’s method (RC DC
Protein Assay Kit: Bio-Rad, Hercules, CA, USA). Western blotting was performed
as described previously[16] using primary
antibodies against acetyl-histone H3 (1:1000; Cell Signaling Technology Inc.,
Danvers, MA, USA) and GAPDH (1:10000; Sigma), and appropriate horseradish
peroxidase-conjugated secondary antibody. Densitometric results were analyzed
using Image J software (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis The data are
expressed as the mean± standard error (SE). Differences were considered
statistically significant if the P value was <0.05, as determined
using the Tukey-Kramer post hoc test.
RESULTS
Cellular Toxicity The
viability of HPFs from patient 1 (Figure 1A) and patient 2 (Figure 1B) treated
with butyrate or PB was >96%, and there were no significant differences in
cell viability among all treatments.
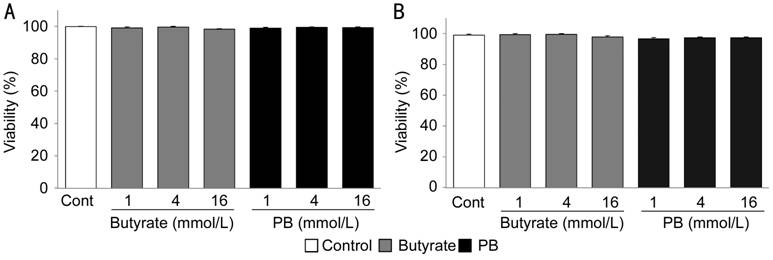
Figure 1
Effects of butyrate or PB on viability of HPFs HPFs of (A) patient 1 and (B) patient 2
were exposed to the indicated concentrations of PB or butyrate for 48h. Cell
viability were determined by trypan blue staining. Mean value and standard
error (SE) were calculated from data of four separate cultures.
Collagen I Expression in Human Pterygium
Fibroblasts Treated with Butyrate or Phenylbutyrate To
investigate the effective treatment time for butyrate or PB, we assessed
collagen I expression in HPFs of patient 1 treated with these compounds for 24
or 48h. Butyrate did not change collagen I expression at both 24 and 48h
(Figure 2A, 2B). PB at a concentration of 16 mmol/L significantly inhibited
collagen I expression at both 24 and 48h (P<0.05; Figure 2C, 2D);
however, PB at a concentration of 4 mmol/L significantly inhibited collagen I
expression only at 48h (P<0.05; Figure 2D). Therefore, the following
experiments were performed involving treatment with butyrate or PB for 48h.
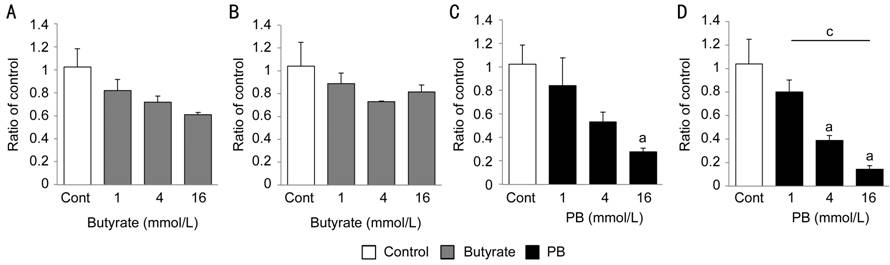
Figure 2
Collagen I expression in HPFs treated with butyrate or PB for 24 or 48h HPFs of patient 1 were exposed to the
indicated concentrations of PB or butyrate for 24 or 48h. mRNA expression of
collagen I at (A) 24h, (B) 48h treated with butyrate, (C) 24h and (D) 48h
treated with PB were analyzed using real-time PCR analysis. Mean value and SE
were calculated from data of four separate cultures. aP<0.05
vs control; cP<0.05 (Tukey-Kramer).
Effect of Butyrate on Profibrotic Factors and
Matrix Metalloproteinases Expressions
Regarding mRNA expression of profibrotic factors, butyrate at
concentrations of 4 and 16 mmol/L significantly inhibited α-SMA expression by
50% of the control level (P<0.01) and also collagen III mRNA
expression in HPFs of each patient (Figure 3A, 3C, 3E and 3G). In particular,
butyrate at a concentration of 16 mmol/L strongly suppressed α-SMA (patient 1,
22.7%; patient 2, 25.7%; P<0.01) and collagen III (patient 1, 7.7%;
patient 2, 3.6%; P<0.01) expressions; however, the expression of
collagen I was not suppressed (Figure 3B, 3F). Butyrate also significantly
inhibited MMP-1 mRNA expression in HPFs of patient 1 (P<0.01; Figure
3D), but not in HPFs of patient 2 (Figure 3H).
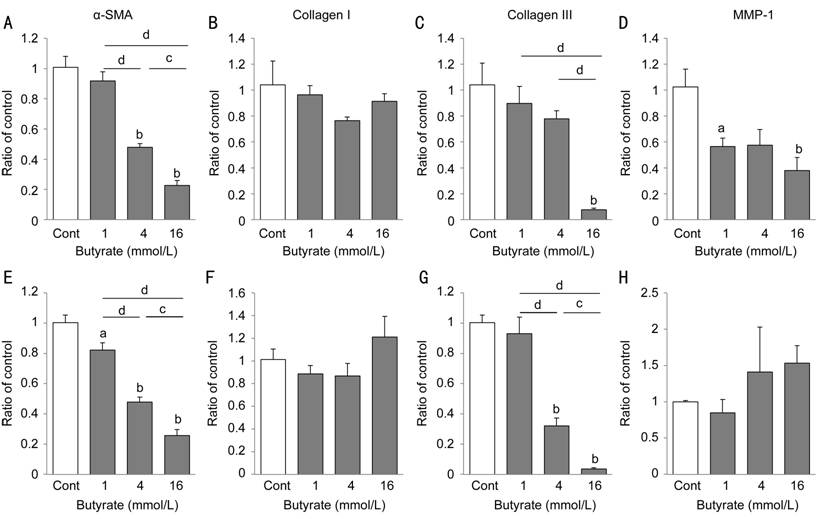
Figure 3 Effects of butyrate on the
expression of profibrotic factors and MMP in HPFs HPFs from (A-D) patient 1 and (E-H)
patient 2 were exposed to the indicated concentrations of butyrate for 48h.
mRNA expression of (A, E) α-SMA, (B, F) collagen I, (C, G) collagen III, (D, H)
MMP-1 were analyzed using real-time PCR analysis. Mean value and SE were
calculated from data of four separate cultures. aP<0.05; bP<0.01
vs control; cP<0.05; dP<0.01
(Tukey-Kramer).
Effect of Phenylbutyrate on Profibrotic
Factors and Matrix Metalloproteinase Expressions PB
significantly inhibited collagen I, collagen III and MMP-1 mRNA expressions in
HPFs of each patient (P<0.01; Figure 4B-4D, 4F-4H). In particular, PB
at a concentration of 16 mmol/L inhibited collagen I expression by 20% relative
to the control in both HPFs. In contrast, PB did not inhibit α-SMA expression
in both HPFs. These results suggest that butyrate and PB have antifibrogenic
effects.
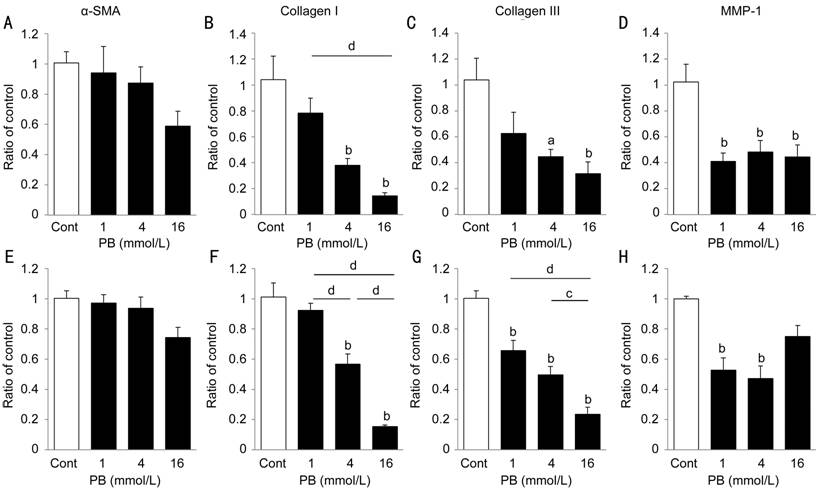
Figure 4 Effects of PB on the expression of
profibrotic factors and MMP in HPFs
HPFs from (A-D) patient 1 and (E-H) patient 2 were exposed to the
indicated concentrations of PB for 48h. mRNA expressions of (A, E) α-SMA, (B,
F) collagen I, (C, G) collagen III, (D, H) MMP-1 were analyzed using real-time
PCR analysis. Mean value and SE were calculated from data of four separate
cultures. aP<0.05; bP<0.01 vs
control; cP<0.05, dP<0.01
(Tukey-Kramer).
Alteration of Histone Acetylation To
investigate the mechanism of antifibrogenic effect by butyrate and PB,
acetyl-histone H3 protein was assessed. Butyrate induced acetylation of histone
H3 in HPFs of each patient (P<0.01; Figure 5A, 5B, P<0.05;
Figure 5D, 5E), indicating inhibition of HDAC activity. Although PB also
induced acetylation of histone H3 in HPFs of each patient (Figure 5A, 5C, 5D,
5F), only the change in HPFs of patient 2 was statistically significant (P<0.01).
The acetylation of histone H3 was more strongly induced by butyrate than PB in
both HPFs, indicating butyrate is a more effective HDAC inhibitor than PB.

Figure 5 Effects of butyrate or PB on histone
acetylation expression in HPFs HPFs of (A-C) patient 1 and (D-F) patient
2 were exposed to the indicated concentrations of PB or butyrate for 48h.
Acetylated histone was analyzed by Western blotting. A, D: Results of a
representative experiment are shown; B, C, E, F: Mean value and SE were
calculated from data of four separate cultures. aP<0.05, bP<0.01
vs control; cP<0.05, dP<0.01
(Tukey-Kramer).
DISCUSSION
This is the first report to demonstrate the
antifibrogenic effects of butyrate or PB in HPFs obtained from patients
undergoing pterygium surgery; there were three principle findings. First,
butyrate or PB did not affect the cell viability of HPFs from each patient.
Second, butyrate inhibited α-SMA, collagen III and MMP-1 expression. Third, PB
inhibited collagen I, collagen III and MMP-1 expression. PB and butyrate were
found to inhibit profibrotic factors and MMP; however, PB inhibited collagen
III expression at lower concentrations than butyrate. In particular, PB at a
concentration of 1 mmol/L, the lowest concentration in this study, inhibited
collagen III expression in HPFs of patient 2. These findings suggest that
butyrate or PB have therapeutic inhibitory effects on fibrogenesis and
progression of pterygium, and that PB might be more suitable for clinical use
than butyrate.
Expression of collagen I and collagen III was
significantly inhibited in HPFs treated with PB at concentrations of 4 and 16
mmol/L for 48h, suggesting that PB has antifibrogenic effects. The inhibition
of collagen I expression by PB is in agreement with a previous study involving
human lung fibroblasts[10]. Rishikof et al[10] suggested
that PB regulates collagen I expression by mechanisms that include stimulating
cAMP production and inhibiting HDAC activity. Although the well-known HDAC inhibitor
butyrate did not change collagen I expression, the weaker HDAC inhibitor PB
significantly inhibited collagen I expression. These results suggest that
histone acetylation may be only partially responsible for the effect of PB on
collagen I mRNA expression in HPFs, and that other mechanisms such as cAMP
production might exist.
Expressions of α-SMA and collagen III were
significantly inhibited in HPFs treated with butyrate at concentrations of 4
and 16 mmol/L for 48h. This inhibition of α-SMA and collagen III expressions by
butyrate is in agreement with previous reports involving several mesenchymal
cells[9]. Butyrate even at a concentration of 16
mmol/L did not inhibit collagen I expression; a similar response was observed
in our previous study involving human dermal fibroblasts[11].
However, high doses of butyrate may inhibit collagen I expression in HPFs,
because it has been reported that butyrate at a concentration of 20 mmol/L
decreases collagen I mRNA levels in human lung fibroblasts[10].
Our study show that the HDAC inhibitors,
butyrate and PB, suppress expression of profibrotic factors, indicate that the
histone acetylation may have role of transcriptional regulator. It has been
reported that HDAC inhibitors inhibit myofibroblastic differentiation and
migration by inducing cell senescence in corneal stromal cells[17]. In addition, senescent hepatic stellate cells have
been found to express reduced levels of ECM proteins, including collagens[18]. Therefore, the inhibition of profibrotic factor
expression by butyrate or PB might be associated with the induction of
senescence in HPFs. Krizhanovsky et al[19]
have demonstrated that senescence of activated hepatic stellate cells limits
liver fibrosis in a liver fibrosis model. Thus, senescence of HPFs might
suppress fibrosis in pterygium tissue.
Inhibition of MMP-1 mRNA expression by
butyrate or PB suggests that they could suppress the degradation of Bowman’s
layer and the infiltration of HPFs. It has been reported that HDAC inhibitors,
including butyrate and trichostatin A, significantly reduce
interleukin-1β-induced MMP-1 and MMP-3 expressions in human colonic
subepithelial myofibroblasts[20]. Therefore, the
inhibition of MMP-1 expression by butyrate or PB might be associated with their
action regarding histone acetylation.
In the present study, we found the
antifibrogenic effects of butyrate or PB at the level of mRNA. Inhibition of
mRNA expression has been followed by decrease in protein expression in the
previous studies[9-10];
however, evaluation of butyrate and PB effects in a protein level are required
for sufficient assessment of their effects. In addition, the underlying
mechanisms of antifibrogenic effect of butyrate and PB are still unclear;
therefore, evaluation of the effects at further detailed studies including
in vivo study is required.
We showed that PB, a derivative of butyrate
without the unpleasant smell, has antifibrogenic effects at lower
concentrations than butyrate. In addition, PB eye drops have been tested in a
glaucoma mouse model, and did not cause eye abnormalities[15].
Thus, PB is considered to hold considerable promise in the treatment of
pterygium as an eye drop formulation.
In summary, we demonstrated that butyrate and
PB suppress fibrosis. These findings could contribute to the development of
novel types of prophylactic and/or therapeutic drugs for the treatment of
pterygium.
ACKNOWLEDGEMENTS
Foundation: Supported by
JSPS KAKENHI (No.23592648).
Conflicts of Interest: Koga Y, None; Maeshige
N, None; Tabuchi H, None; Uemura M, None; Aoyama-Ishikawa
M, None; Miyoshi M, None; Katakami C, None; Usami M, None.
References
1 Kheirkhah A, Hashemi H, Adelpour M, Nikdel
M, Rajabi MB, Behrouz MJ. Randomized trial of pterygium surgery with mitomycin
C application using conjunctival autograft versus conjunctival-limbal
autograft. <ii>Ophthalmology</ii> 2012;119(2):227-232. [CrossRef] [PubMed]
2 Hacıoğlu D, Erdöl H. Developments and
current approaches in the treatment of pterygium. <ii>Int Ophthalmol
</ii>2017;37(4):1073-1081. [CrossRef] [PubMed]
3 Cárdenas-Cantú E, Zavala J, Valenzuela J,
Valdez-García JE. Molecular basis of pterygium development. <ii>Semin
Ophthalmol </ii>2016;31(6):567-583. [PubMed]
4 Engelsvold DH, Utheim TP, Olstad OK,
Gonzalez P, Eidet JR, Lyberg T, Trøseid AM, Dartt DA, Raeder S. miRNA and mRNA
expression profiling identifies members of the miR-200 family as potential
regulators of epithelial-mesenchymal transition in pterygium. <ii>Exp Eye
Res</ii> 2013;115: 189-198. [CrossRef] [PMC free article]
[PubMed]
5 Di Girolamo N, Chui J, Coroneo MT,
Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and
matrix metalloproteinases. <ii>Prog Retinal Eye Res</ii>
2004;23(2):195-228. [CrossRef]
[PubMed]
6 Dushku N, John MK, Schultz GS, Reid TW.
Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing
altered limbal epithelial basal cells. <ii>Arch Ophthalmol </ii>
2001;119(5):695-706. [CrossRef]
[PubMed]
7 Touhami A, Di Pascuale MA, Kawatika T, Del
Valle M, Rosa RH Jr, Dubovy S, Tseng SC. Characterisation of myofibroblasts in
fibrovascular tissues of primary and recurrent pterygia. <ii>Br J
Ophthalmol</ii> 2005;89(3): 269-274. [CrossRef] [PMC free article]
[PubMed]
8 Bourassa MW, Alim I, Bultman SJ, Ratan RR.
Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet
improve brain health? <ii>Neurosci Lett</ii> 2016;625:56-63. [CrossRef] [PMC free article]
[PubMed]
9 Bülow R, Fitzner B, Sparmann G, Emmrich J,
Liebe S, Jaster R. Antifibrogenic effects of histone deacetylase inhibitors on
pancreatic stellate cells. <ii>Biochem Pharmacol</ii>
2007;74(12):1747-1757. [CrossRef]
[PubMed]
10 Rishikof DC, Ricupero DA, Liu H, Goldstein
RH. Phenylbutyrate decreases type I collagen production in human lung
fibroblasts. <ii>J Cell Biochem</ii> 2004;91(4):740-748. [CrossRef] [PubMed]
11 Torii K, Maeshige N, Aoyama M, Imai M,
Tabuchi H, Miyoshi M, Hamada Y, Terashi H, Usami M. Antifibrogenic effects of
short chain fatty acids on human dermal fibroblasts. <ii>Wound Rep
Reg</ii> 2013;21:A1-A7.
12 Kusaczuk M, Bartoszewicz M,
Cechowska-Pasko M. Phenylbutyric Acid: simple structure-multiple effects.
<ii>Curr Pharm Des</ii> 2015;21(16): 2147-2166. [CrossRef]
13 Collins AF, Pearson HA, Giardina P,
McDonagh KT, Brusilow SW, Dover GJ. Oral sodium phenylbutyrate therapy in
homozygous beta thalassemia: a clinical trial. <ii>Blood</ii>
1995;85(1):43-49. [PubMed]
14 Zhang X, Wei L, Yang Y, Yu Q. Sodium
4-phenylbutyrate induces apoptosis of human lung carcinoma cells through
activating JNK pathway. <ii>J Cell Biochem</ii> 2004;93(4):819-829.
[CrossRef] [PubMed]
15 Zode GS, Bugge KE, Mohan K, Grozdanic SD,
Peters JC, Koehn DR, Anderson MG, Kardon RH, Stone EM, Sheffield VC. Topical
ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of
primary open-angle glaucoma. <ii>Invest Ophthalmol Vis Sci</ii>
2012;53(3): 1557-1565. [CrossRef]
[PMC free
article] [PubMed]
16 Fujiwara M, Miyoshi M, Sakai S, Nishiokada
A, Aoyama-Ishikawa M, Maeshige N, Usami Y, Hamada Y, Takahashi M, Usami M.
Lard-based high-fat diet increases secretory leukocyte protease inhibitor
expression and attenuates the inflammatory response of acute lung injury in
endotoxemic rats. <ii>Clin Nutr</ii> 2015;34(5):997-1009. [CrossRef] [PubMed]
17 Zhou Q, Wang Y, Yang L, Wang Y, Chen P,
Wang Y, Dong X, Xie L. Histone deacetylase inhibitors blocked activation and
caused senescence of corneal stromal cells. <ii>Mol Vis</ii>
2008;14:2556-2565. [PMC free article]
[PubMed]
18 Schnabl B, Purbeck CA, Choi YH, Hagedorn
CH, Brenner D. Replicative senescence of activated human hepatic stellate cells
is accompanied by a pronounced inflammatory but less fibrogenic phenotype.
<ii>Hepatology</ii> 2003;37(3):653-664. [CrossRef] [PubMed]
19 Krizhanovsky V, Yon M, Dickins RA, Hearn
S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated
stellate cells limits liver fibrosis. <ii>Cell</ii>
2008;134(4):657-667. [CrossRef]
[PMC free
article] [PubMed]
20 Kawamura T, Andoh A, Nishida A, Shioya M,
Yagi Y, Nishimura T, Hashimoto T, Tsujikawa T, Yasui H, Fujiyama Y. Inhibitory
effects of short-chain fatty acids on matrix metalloproteinase secretion from
human colonic subepithelial myofibroblasts. <ii>Dig Dis Sci</ii>
2009;54(2):238-245. [CrossRef]
[PubMed]
--------------------------------------------------------------------------------------------------------------------------------
All rights reserved by Press of International Journal of Ophthalmology (IJO
PRESS)