IF in JCR CiteScore
Rank About IJO Current
Issue Featured Articles Articles In Press Recent Accepted
 International Journal
of Ophthalmology
International Journal
of Ophthalmology
2017; 10(9): 1354-1360
·Basic Research·
Intraocular pressure control of a novel glaucoma
drainage device - in vitro and in vivo studies
Li-Jun Cui1, Di-Chen Li2,
Jian Liu3, Lei Zhang4, Yao Xing1
1Department of
Ophthalmology, the First Affiliated Hospital of Xi’an Jiaotong
University, Xi’an 710061, Shaanxi Province, China
2Institute of
Advanced Manufacturing Technology, School of Mechanical Engineering, Xi’an
Jiaotong University, Xi’an 710061, Shaanxi Province, China
3Department
of Physiology and Pathophysiology, College of Medicine, Xi’an Jiaotong
University, Xi’an 710061, Shaanxi Province, China
4Department
of Cardiovascular Medicine, the Armed Police Corps Hospital of
Shaanxi, Xi’an 710054, Shaanxi Province, China
Correspondence
to: Li-Jun Cui. Department of Ophthalmology, the First
Affiliated Hospital of Xi’an Jiaotong University. No.277, West Yanta Street,
Xi’an 710061, Shaanxi Province, China. cuilijun@mail.xjtu.edu.cn
Received:
2017-04-12
Accepted: 2017-06-26
Abstract
AIM: To
evaluate the intraocular pressure (IOP) control of an artificial trabeculum
drainage system (ATDS), a newly designed glaucoma drainage device, and
postoperative complications in normal rabbit eyes.
METHODS:
Pressure drops in air and fluid of 30 ATDS were measured after being connected
to a closed manometric system. Twenty of them were then chosen and implanted
randomly into the eyes of 20 rabbits. Postoperative slit-lamp, gonioscopic
examination and IOP measurements were recorded periodically. Ultrasound
biomicroscopy and B-scan ultrasonography were also used to observe the
complications. Eyes were enucleated on day 60.
RESULTS:
Pressure drops of 4.6-9.4 mm Hg were obtained at physiological aqueous
flow rates in the tests in vitro. The average postoperative IOP of the
experimental eyes (11.6-12.8 mm Hg) was lower than the controls significantly (P<0.05)
at each time point. Complications of hemorrhage (n=1), cellulosic
exudation (two cases) and local iris congestion (two cases) were observed. The
lumina of the devices were devoid of obstructions in all specimens examined and
a thin fibrous capsule was found around the endplate.
CONCLUSION: ATDS
reduce IOP effectively. However, further studies on the structure are needed to
reduce complications.
KEYWORDS: drainage device; aqueous humor; outflow; intraocular pressure;
rabbit
Citation: Cui LJ, Li DC, Liu J, Zhang L,
Xing Y. Intraocular pressure
control of a novel glaucoma drainage device - in vitro and in vivo
studies. Int J Ophthalmol 2017;10(9):1354-1360
INTRODUCTION
Glaucoma is
the second most common cause of blindness and the leading cause of irreversible
blindness[1]. Glaucoma filtration surgery is a
fistulizing procedure that provides an alternative drainage route allowing
aqueous to escape from the anterior chamber (AC) to the subconjunctiva in order
to lower intraocular pressure
(IOP), which includes trabeculectomy and drainage implant surgery. Implantation of glaucoma drainage devices (GDDs)
has become a standard procedure in
various forms of complicated and refractory glaucoma with comparable IOP control and duration
of benefit[2-3]. In certain
conditions, such as neovascular glaucoma, irido-corneal syndrome, penetrating
keratopathy with glaucoma, and glaucoma following retinal detachment surgery,
uveitis, or trauma and so on, it is becoming the primary operation[4]. Such
devices enable percolation of aqueous liquid through a tube to a filtering
plate in the subconjunctival space, to the Schlemm's canal, or to the
suprachoroidal space[5].
There have
been many contemporary GDDs commercially available[6] since
Molteno implant was invented in 1969[7], but
success rates of most clinical series were not satisfied[8-9].
There are a number of unsolved
clinical drawbacks of existing systems[10].
While partly attributable to the complicated manifestations and strong
wound-healing trend of cases typically selected for implantation, various
complications also lead to filtration failure[11-14]. The most
significant complication related to exposure is endophthalmitis. Others include
hypotony, shallow AC, choroidal effusion, suprachoroidal hemorrhage, tube
migration and tube obstruction. The origin of most complications can be traced
to design inadequacies, poor flow control, lack of set resistance and
suboptimal material biocompatibility[3].
In general, there are 2 types of GDDs[3-4,6,15], with or without set resistance mechanism or pressure sensible valve. The valved implants have a pressure-regulating mechanism to minimize overdrainage. The Ahmed glaucoma valve[16-17], in particular, is proved to function as a real valve that closely regulates pressure within a desired range by a variable resistance in response to changes in flow rate. But the potential site for obstruction by inflammatory debris and valve membrane adhesion[18], especially in Asian eyes[19], may cause surgery failure. On the other hand, hypotony caused by the bulk outflow of aqueous in the early postoperative period is much common in valveless implants. The inserted tube dimension is too large to produce resistance when aqueous humor flows through it at physiological rates. The Baerveldt GDD is available with a surface area of 350 mm² and requires temporary flow restriction to avoid early postoperative hypotony[20]. Either a two staged procedure or ligature technique[11,21-23], with or without fenestration of the tube, are therefore required to produce a temporary restriction of flow. However, these methods are sometimes cumbersome and time consuming, and many researchers[23-24] had proved that it was not possible to regulate pressure in a reliable and predictable way merely by constricting the tube lumen.
On the basis
of hydrodynamic principles, we developed a new restricted GDD without valve
membranes, which is named artificial trabeculum drainage system (ATDS). The
main purpose of this study is to evaluate flow characteristics of ATDS, and
observe IOP change and complications after implanted in rabbit eyes.
MATERIALS
AND METHODS
Artificial
Trabeculum Drainage System Following
the concept of tube and plate GDD, ATDS consists of a T-shaped silastic tube
(Medical Silicon Rubber Technical Institute of Rubber Goods Design Academy,
Beijing, China) and a pear-shaped plate (Institute
of Advanced Manufacturing Technology, School of Mechanical Engineering,
Xi’an Jiaotong University, Xi’an,
China) made of
medical-grade polyurethane (PUR) (Figure 1).
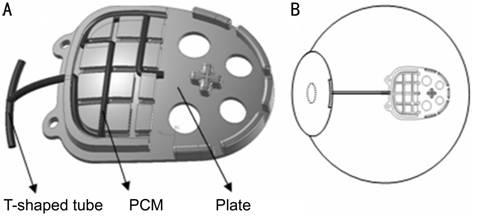
Figure 1
Simulated construction of ATDS A: The medical grade PUR plate, with a
spherical undersurface, has an area of 162.2 mm2. All the evections
lying on the posterior plate are designed after analyzing the contact surface
between Tenon’s capsule and plate using finite element analysis (FEA). Each one
has its optimal height, bulk and location to sustain Tenon’s capsule and keep
the largest drainage surface; B: Location of ATDS.
The
T-tube has the same dimension of 600 µm in outer diameter and 300 µm in inner
as the single round tube of GDD available in the market. Several micropores,
with diameter of 250 µm, distribute to the 6 mm-long horizontal tube to
decrease the blockage of the tube (Figure 2).
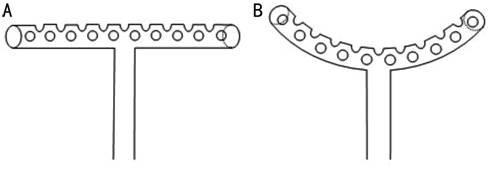
Figure 2
Structure of T-shaped tube The T-shaped
tube has an outer diameter of 600 µm and an inner diameter of 300 µm. Many
micropores distributing to horizontal tube except the surface connecting with
the perpendicular tube (A) are designed to increase the drainage surface and
decrease the opportunity of tube obstructed by fibrin clot or iris. When the
plate is pulled back, the 5 mm long horizontal tube will curve and match the AC
angle (B) to decrease tube movement, prevent extrusion and also block the
incision under scleral flap, which consequently, could avoid severe hypotony
caused by peritubular filtration.
The
pressure confined system (PCS) on the endplate, which is also a silastic tube,
with the inner diameter of 80 µm, circles as a certain mode. This mode is
selected from several designs in different tube lengths, calibers and circling
ways, by calculating and screening step by step using Poiseuille’s law,
Bernoulli’s formula and FLUNT hydrodynamic software. Pressure drop versus flow
rate from theoretical calculations is shown as Figure 3. The local
pressure impairments caused by 8 angles with different degrees and a diameter
change (from 300 µm to 80 µm) are calculated from Bernoulli formula: ![]()
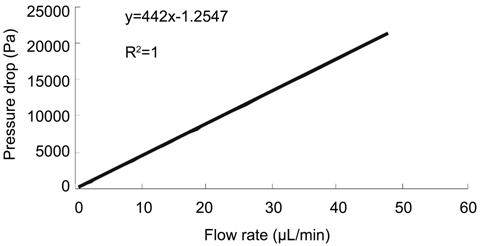
Figure
3 Pressure drops of PCS at different flow rates Pressure
drop produced by PCM had a linear correlation with flow rate at a range of
0.6-48.0 µL/min. The pressure drop across straight tube versus flow rate is
from Poiseuille’s formula[25]: pressure
drop=128nlQ/πd4, where n=aqueous viscosity=1.03×10-3 NS/m2;
l=length=25.7 mm in this study; Q=aqueous flow rate, d=diameter (metres).
But
the summation is too small to disturb the linear correlation because of the
slow flow.
Hydrodynamic
Test The
characteristic parameters were tested through a flow rig consisted of a tubing
compression pump (Model T-Y, TongYi Inc., Shanghai, China), a bridge amplifier
(Model ML110, AD Instruments, New South Wales, Australia), a
recorder (Model ML200, AD Instruments, New South Wales, Australia),
a pressure transducer (Powerlab, AFR Instruments, Tokyo, Japan) and
two three-way locks (Figure 4). Pressure changes were recorded and analyzed
using Chart 4 software.
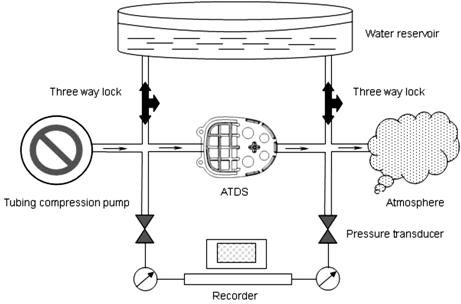
Figure 4
Flow rig for measuring pressure drop
Pressure drop were tested in air and water respectively. Repeated
flow measurements were taken (n=3), and each device was measured during
a 10min interval while a constant flow of fluid was pumped into the system.
Degassed
balanced salt solution (BSS) was infused by the pump with initial flow rates
preset to 0.6, 1.2, 2.4, 4.8, 9.0, 15.0, 24.0, 48.0 µL/min. All gas bubbles
were flushed out when BSS filled all the system. At this point, the two
three-way locks were turned to open the system to atmospheric pressure and the
pressure reading was zeroed on the recorder. The first three-way lock was then
turned to obtain a closed system and the infusion pump was started. The two
pressure readings were taken at the same time, and the difference between them
was recorded. Repeated flow measurements were taken (n=3). Pressure drops
in air and water of 30 ATDSs were tested respectively. Each device was measured
during a 10min interval while a constant flow of fluid was pumped into the
system.
Experimental
Animals A
prospective, randomized study was performed using 20 male and female New
Zealand White rabbits initially weighing 2.5 to 3.0 kg. The experiment was
performed in accordance with the ARVO Statement for the Use of Animals in
Ophthalmic and Vision Research. The project was also approved by the Local
Animal Research Review committee of the First Affiliated Hospital of Xi'an
Jiaotong University, Xi'an, China. All animals were maintained in a 12-hour day
and 12-hour night cycle. They were fed and had access to water ad libitum.
Twenty ATDSs
were implanted into the unilateral eyes of the rabbits. The horizontal part of
the T-shaped tube was inserted into the AC while the endplate was placed
subconjunctivally posterior to the equator of the eyeball (Figure 1B). The
fellow eyes were served as control.
Surgical
Procedure After adequate
general anesthesia [3% pentobarbital sodium (1.5-2.0 mL/kg) intravenous
injection], the eyes were prepared and draped with sterile towels. The lids
were secured with a lid speculum. Topical 0.5% tetracaine hydrochloride was
instilled to prevent any discomfort. A pair of eye scissors was used to perform
a superotemporal limbal peritomy from the 11- to 3-o’clock meridian. A 1-0 silk
retention suture was placed around the superior rectus muscle to hold the
eyeball and expose the surgical area. Conjunctiva and fascia were separated
from the globe and superficial bleeding vessels over the site of the intended
scleral flap were cauterized lightly. A rectangle scleral flap measuring 3 mm×5
mm and of one-half scleral thickness was dissected up to the limbal zone from
the 1- to 2-o’clock meridian (Figure 5A). A sterile ATDS was put into the
subconjunctival space in terms of sliding the plate along the scleral surface.
Adequate tube was kept for next step of insertion (Figure 5B). A 2-mm limbal
incision was made with a 45° blade. The horizontal tube was folded together by
a toothed forceps parallel to the iris plane, and sent into the AC through the
limbal incision. It stretched quickly and returned to the original shape under
its natural flexibility (Figure 5C). The scleral flap was closed with 4
interrupted 10-0 nylon sutures, 1 each on the sides and apexes. After that, the
T-shaped tube was pulled back along the surface of the eyeball to make sure the
horizontal tube was close to the anterior chamber angle. Fix the endplate to
sclera with 2 interrupted 8-0 nylon sutures through the two semicircle
protrusions on the head of the plate (Figure 5D). The conjunctival flap was
sutured to the limbus with 10-0 nylon suture. Tobradex eyedrop and 0.5%
erythromycin ointment were applied into conjunctival sac.
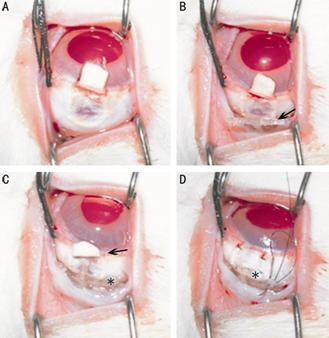
Figure 5
Main procedures of ATDS implanted surgery
Arrow shows the T-shaped tube, and asterisk indicates the plate of
ATDS.
All the
surgeries were aseptic and performed by the first author. Tobradex eyedrop was
instilled thrice daily for 3d.
Clinical
Observation Postoperative
evaluations were performed on postoperative days 1-3, 7, 14, 21, 30 and 60, or
more frequent when necessary, consisted of general health, Seidal test and red
reflex, IOP measurement by Perkins handheld applanation tonometer (HL-2, Kowa,
Japan), anterior segments observation by slit-lamp biomicroscopy (SL-8Z,
Topcon, Japan) and ultrasound biomicroscopy (UBM, 840, Humphrey, Germany) with
a 50 MHz transducer, vitreo-retinal complications by B-scan ultrasonography
(SW-2100, Suowei, China) with a 10 MHz probe onto the eyelid after performing
methylcellulose. IOP of bilateral eyes at each time point were evaluated using
a paired, 2-tailed, Student’s t-test.
Animals were
sacrificed at the end of research (sedation with a lethal dose of intravenous
pentobarbital sodium). The ATDS-implanted eyes were enucleated, with care taken
not to disturb the tissues around the implant. The appearance of fibrous
capsule and the tube lumen were observed.
RESULTS
Hydrodynamic
Tests The pressure
drops examined at different flow rates correlated closely with that predicted
by Poiseuille’s formula and Bernoulli’s equation (Figure 6). But
there was a tendency of larger variation with flow rate increasing.
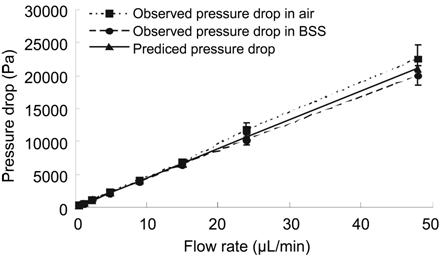
Figure 6
Observed pressure drop versus predicted
Fitting linear formula between 0.6 and 48.0 µL/min of the three plots:
predicted pressure drop, y=442x-1.2532, R2=1; observed pressure drop
in air, y=473.11x-52.539, R2=0.9992; observed pressure drop in BSS,
y=417.66x+72.411, R2=0.9998.
General
State and Intraocular Pressure There was no
discharge of all the participants, and red reflex remained normal. There was no
significant difference between bilateral baseline IOPs (Table 1), and IOPs of
the ATDS-implanted eyes were lower than the controls at each time point (P=0.000).
All the eyes were devoid of hypotony. Seidal tests were negative. The variation
of bilateral IOPs is shown as Figure 7.
Table 1 IOPs
on each study point
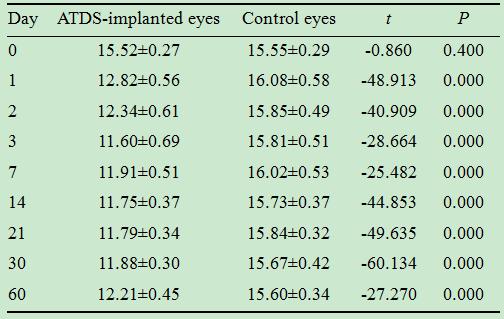
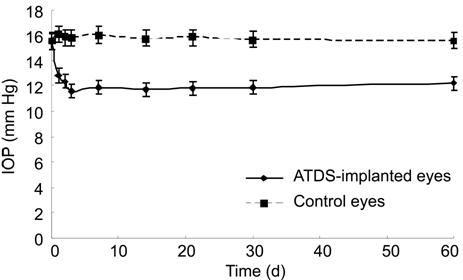
Figure 7 IOP
change of bilateral eyes A stable
reduction of mean IOP was obtained after it dropped slowly to the average of
11.6 mm Hg 3d after surgery in the ATDS-implanted eyes, and there was no
significant difference between consecutive study points.
Tissue
Responses Illustrated
as Figure 8, the thin, lucent and diffused bleb appeared on postoperative days
1 and 3, and it became localized with time. Conjunctival hyperemia triggered by
the surgery occurred on day 1 and lightened prominently on day 3 in most of the
ATDS-implanted eyes. Cellulosic exudation happened in 2 cases and resolved
spontaneously 5 and 7d after implantation. One case of AC hemorrhage occurred
on postoperative day 2 (Figure 9). It localized at the tube site, accompanied
with moderate corneal edema. The blood was absorbed 1wk after surgery, but
corneal edema still existed. Slight iris evection with actiniform vessels
injection was found at the tube site in several eyes (11/20). The local iris
congestion was slight and resolved in the end, but two of them aggravated at
first, and reached its apex as diffuse crimson red on postoperative days 14 and
25. Cornea edema (8/20) was localized at the site of surgery, and 6 of them
were accompanied with iris vessel injection. They were resolved 3-15d after
implantation. Tube erosion was found in 2 eyes at nearly the end of
observations, but the subconjunctival wound healed well and peritubular
filtration was not found. No tube migration or extrusion happened in the
experimental eyes.
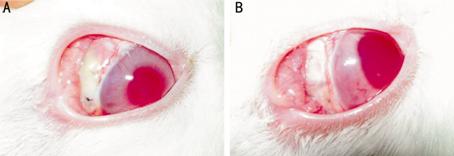
Figure 8
Bleb appearance of ATDS-implanted eyes
A: Bleb on postoperative day 3. It was thin, lucent and diffused
with slight hyperemia. Only the front part was available; B: Bleb on
postoperative day 14. Bleb was thick, transparent ivory white and localized
surround the plate.
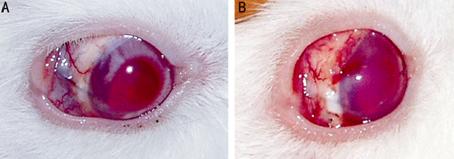
Figure 9 Hemorrhage of anterior chamber A: Slight hemorrhage on postoperative day 2. The T-shaped tube was
filled with blood; B: Major part of hemorrhage was absorbed on postoperative
day 7, but corneal edema still existed.
No focal
thickening or adherence of cornea and iris was found in the ATDS-implanted eyes
in UBM detection. The horizontal part of T-shaped tube was close to the angle
of AC (Figure 10A), while the perpendicular part lying straight on the surface
of the sclera (Figure 10B). On each time point, no obstruction was found in
tube lumen of all the samples. There was a low echogenic space between bleb
tissue and globe, with small punctiform echogenic distribution in the B-scan
ultrasonography image (Figure 10C). No ciliary body detachment, suprachoroidal
hemorrhage or retinal detachment was found according to the ultrasonography
detections.
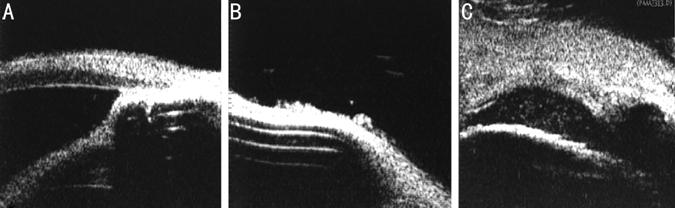
Figure 10
Ultrasound biomicroscopy images The T-shaped
tube was hyperechoic in UBM image, with band-shaped sound absorption beneath.
Enucleated
Eyes The local
tissue reaction typically consisted of a pink, thin and tenacious capsule. This
smooth fibrous layer covered the silastic tube and the endplate of ATDS
apparently (Figure 11). A small quantity of fluid flew out when the capsule was
opened along the edge of the plate. The lumen of the T-shaped tube and pressure
confined mechanism were filled with aqueous humor and devoid of obstructions in
all specimens examined, suggesting free flow of fluid.
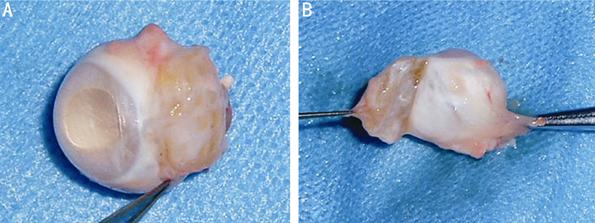
Figure 11
Fibrous capsule in an enucleated eye
A: The cornea was transparent, the wound of the limbus healed
well; B: The plate was encapsulated by a pink, thin and tenacious fibrous layer
including the undersurface connected with the eyeball, and it was easy to
separate.
DISCUSSION
ATDS was
developed in accordance with preventing hypotony in routine glaucoma filtration
surgery[26], and a new GDD must demonstrate
consistent control over internal flowed. The pressure difference
between the inlet and outlet of ATDS was mainly influenced by the aqueous flow
rate, and produced by frictions of tube wall, resistance of sinuosity, small
diameter and sudden dimension change.
According to
Energy Conservation Law, conversion of one type of matter into another are
always accompanied by the conversion of one form of energy into another. In
this study, the potential energy (in form of pressure drop), tube resistance
and kinetic energy of aqueous humor are conversed at any time if heat exchange
is not under consideration. Consequently, if fluid flows at a steady rate, the
pressure of inlet must be higher than that of the outlet to keep the potential
energy conversing to the kinetic energy. Pressure drop and flow rate influences
each other, and there should be 3 conditions as follows when applying to ATDS:
1) as is shown in the hydrodynamic tests, an 8.5 mm Hg pressure drop will be produced
by the pressure confined mechanism when aqueous humor is secreted and drained
out at a stable flow rate of 2.5 µL/min, or IOP will be 8.5 mm Hg higher than
the inner pressure of the filtration bleb. The success limit of 21 mm Hg needs
a steady flow rate of 6.2 µL/min, which is higher than the physiological range
of human aqueous secretion; 2) if the pressure difference between AC and
filtration bleb is higher than 8.5 mm Hg, the flow rate will grow bigger than
aqueous secretion, which will then cause pressure impairment to decrease the
pressure drop until the balance of 8.5 mm Hg is back; 3) aqueous humor could
flow outside under any pressure lower than 8.5 mm Hg but higher than its
hydrostatic pressure. However, the lowest flow rate in normal adult is thought
to be 1.4 µL/min during sleep[27], and the
resistance of ATDS at that speed is about 5 mm Hg in basis of the experimental
flow study. The kinetic energy of aqueous will accumulate gradually with
accrescent secretion rate, and conversed to the potential energy continuously.
The pressure difference between AC and filtration bleb will elevate in the end.
The good fit
between observed pressure drop across specimen ATDS and that predicted by Poiseuille’s
law and Bernoulli’s formula showed a good expected finding, but a
tendency of larger variation with increased flow rate was found simultaneously.
This may have been attributable to minor accretions of debris within
the tube lumens.
In the rabbit
experiment, ATDS-implanted eyes showed a significant IOP reduction of 20.9%,
20.5%, 25.3%, 23.3%, 24.3%, 24.0%, 23.5% and 21.3% of the baseline on each time
point, respectively. A stable pressure drop was obtained after day 3,
when the inflammatory reaction and surgical irritation had already lightened.
The reason why IOP fluctuated between 11.3-12.6 mm Hg and was higher than 8.5
mm Hg is that the physiological flow rates of aqueous humor in rabbit is bigger
than in human, and the hydrostatic pressure of filtration bleb should be
considered.
The
complications triggered by the surgery may have been attributable to the larger
incision to AC, reject reactions and irritation of the T-shaped tube. The local
evection of iris root demonstrated the pressing from the tube. Although the AC
angle in rabbit is longer and narrower than in human, the length and the outer
diameter of the T-shaped tube should be decreased to reduce the contact and
irritation, without hindering the blockage to the cornea incision. It is satisfactory
that all the ATDSs were filled with aqueous humor without any obstructions. The
micropores distributed to the horizontal tube are thought to be useful to
increase drainage surface and avoid tube obstruction. Blockage may happen in
some of the micropores, but others are still open to ensure the aqueous humor
outflow.
A thin
capsule was found around ATDS on day 60. The hygric granular inner surface with
aqueous filled, relating to the hypoechoic space in the B-scan ultrasonography
and the stable reduction of IOP, suggested a functional filtration bleb. Thin
capsules were also found after glaucoma implant inserted in human or rabbit
eyes, which consisted of lamellar collagen deposition surrounded by a granulomatous reaction
with multinucleate giant cells[28-29].
ATDS has an
exclusive surgical procedure and it is not difficult to implant. This study has
proved that ATDS could control IOP over internal flowed and provide consistent
protection from hypotony in the early postoperative period. With this basal
research, we are confident to improve the construction of ATDS and carry out
further long-term animal studies including histopathological research in the
future.
ACKNOWLEDGEMENTS
Li-Jun Cui
conceived and designed the study. Di-Chen Li designed and screened the pressure
confined system, and made ATDS to be available. Jian Liu and Lei Zhang contributed to the Hydrodynamic
tests. Li-Jun Cui, Lei Zhang
and Yao Xing performed the experiments and wrote the paper. All authors read
and approved the manuscript. Our team sincerely thanks to Mr. Jun-Tao Ning and
Ms. Li-Hua Liu, School of Mechanical
Engineering of Xi'an Jiaotong University, for assistance of
mode building and manufacture of ATDS.
Foundation: Supported
by National Natural Science Foundation of China (No.81300765).
Conflicts of
Interest: Cui LJ,
None; Li DC, None; Liu J, None; Zhang L, None; Xing Y, None.
REFERENCES
1 Bourne RR,
Taylor HR, Flaxman SR, Keeffe J, Leasher J, Naidoo K, Pesudovs K, White RA,
Wong TY, Resnikoff S, Jonas JB; Vision Loss Expert Group of the Global Burden
of Disease Study. Number of people blind or visually impaired by glaucoma
worldwide and in world regions 1990 - 2010: a meta-analysis. PLoS One 2016;11(10):e0162229. [CrossRef] [PMC free article]
[PubMed]
2 Yu DY,
Morgan WH, Sun X, Su EN, Cringle SJ, Yu PK, House P, Guo W, Yu X. The critical
role of the conjunctiva in glaucoma filtration surgery. Prog Retin Eye Res 2009;28(5):303-328. [CrossRef] [PubMed]
3 Levinson
JD, Giangiacomo AL, Beck AD, Pruett PB, Superak HM, Lynn MJ, Costarides AP.
Glaucoma drainage devices: risk of exposure and infection. Am J Ophthalmol 2015;160(3):516-521.e2. [CrossRef] [PMC free article]
[PubMed]
4 Chaudhry
M, Grover S, Baisakhiya S, Bajaj A, Bhatia MS. Artificial drainage devices for
glaucoma surgery: an overview. Nepal J
Ophthalmol 2012;4(2):295-302. [CrossRef]
5 Wischke C,
Neffe AT, Hanh BD, Kreiner CF, Sternberg K, Stachs O, Guthoff RF, Lendlein A. A
multifunctional bilayered microstent as glaucoma drainage devices. J Control Release 2013;172(3):1002-1010.
[CrossRef]
6 Gedde SJ,
Panarelli JF, Banitt MR, Lee RK. Evidenced-based comparison of aqueous shunts. Curr Opin Ophthalmol 2013;24(2):87-95. [CrossRef] [PubMed]
7
Razeghinejad MR, Spaeth GL. A history of the surgical management of glaucoma. Optom Vis Sci 2011;88(1):E39-E47. [CrossRef] [PubMed]
8 Molteno
AC, Bevin TH, Herbison P, Husni MA. Long-term results of primary
trabeculectomies and Molteno implants for primary open-angle glaucoma. Arch Ophthalmol 2011;129(11):1444-1450.
[CrossRef] [PubMed]
9 Hamanaka
T, Otora K, Ono K, Ishida N. Long-term results of non-valved glaucoma drainage
implant surgery and glaucoma drainage implant combined with trabeculectomy. Indian J Ophthalmol 2014;62(9):911-916.
[CrossRef] [PMC free article]
[PubMed]
10 Minckler
DS, Francis BA, Hodapp EA, Jampel HD, Lin SC, Samples JR, Smith SD, Singh K.
Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology 2008;115(6): 1089-1098. [CrossRef] [PubMed]
11 Riva I,
Roberti G, Oddone F, Konstas AG, Quaranta L. Ahmed glaucoma valve implant:
surgical technique and complications. Clin
Ophthalmol 2017;11:357-367. [CrossRef]
[PMC free
article] [PubMed]
12 Giovingo
M. Complications of glaucoma drainage device surgery: a review. Semin Ophthalmol 2014;29(5-6):397-402. [CrossRef] [PubMed]
13 Lee NY,
Hwang HB, Oh SH, Park CK. Efficacy of additional glaucoma drainage device
insertion in refractory glaucoma: case series with a systematic literature
review and Meta-analysis. Semin
Ophthalmol 2015;30(5-6):345-351. [CrossRef] [PubMed]
14 Bailey
AK, Sarkisian SR Jr. Complications of tube implants and their management. Curr Opin Ophthalmol 2014;25(2):148-153.
[CrossRef] [PubMed]
15 Aref AA,
Gedde SJ, Budenz DL. Glaucoma drainage implant surgery. Dev Ophthalmol 2012;50:37-47. [CrossRef] [PubMed]
16 Francis
BA, Cortes A, Chen J, Alvarado JA. Characteristics of glaucoma drainage
implants during dynamic and steady-state flow conditions. Ophthalmology 1998;105(9):1708-1714. [CrossRef]
17 Strubbe
DT, Gelatt KN, MacKay EO. In vitro flow characteristics of the Ahmed and
self-constructed anterior chamber shunts. Am
J Vet Res 1997;58:1332-1337. [PubMed]
18
Christakis PG, Kalenak JW, Tsai JC, Zurakowski D, Kammer JA, Harasymowycz PJ,
Mura JJ, Cantor LB, Ahmed II. The Ahmed versus Baerveldt study: five-year
treatment outcomes. Ophthalmology
2016;123(10):2093-2102. [CrossRef]
[PubMed]
19 Wang JC,
See JL, Chew PT. Experience with the use of Baerveldt and Ahmed glaucoma
drainage implants in an Asian population.
Ophthalmology 2004;111(7):1383-1388. [CrossRef] [PubMed]
20 Momont
AC, Stein JD, Lee PP, Weizer JS. Simultaneous placement of 2 glaucoma drainage
devices for uncontrolled glaucoma. Can J
Ophthalmol 2014;49(2):205-209. [CrossRef] [PubMed]
21 Kee C.
Prevention of early postoperative hypotony by partial ligation of silicone tube
in Ahmed glaucoma valve implantation. J
Glaucoma 2001;10(6):466-469. [CrossRef]
22 Tong L,
Frazao K,Labree L,
Varma R. Intraocular pressure control and complications with two-stage
insertion of the Baerveldt implant. Ophthalmology
2003;110(2):353-358. [CrossRef]
23 Marchini
G, Ceruti P, Vizzari G, Toscani M, Amantea C, Tosi R, Marchetti P. Long-term
outcomes of a modified technique using the Baerveldt glaucoma implant for the
treatment of refractory glaucoma. J
Glaucoma 2016;25(12):952-958. [CrossRef] [PubMed]
24 Kansal S,
Moster MR, Kim D, Schmidt CM Jr, Wilson RP, Katz LJ. Effectiveness of
nonocclusive ligature and fenestration used in Baerveldt aqueous shunts for
early postoperative intraocular pressure control. J Glaucoma 2002;11(1):65-70. [CrossRef]
25 Saleh JM.
Fluid flow handbook. New York:
McGram-Hill; 2002.
26 Freedman
J. What is new after 40y of glaucoma implants. J Glaucoma 2010;19(8):504-508. [CrossRef] [PubMed]
27 Fitt AD,
Gonzalez G. Fluid mechanics of the human eye: aqueous humour flow in the
anterior chamber. Bull Math Biol
2006;68(1):53-71. [CrossRef]
[PubMed]
28
Schoenberg ED, Blake DA, Swann FB, Parlin AW, Zurakowski D, Margo CE, Ponnusamy
T, John VT, Ayyala RS. Effect of two novel sustained-release drug delivery
systems on bleb fibrosis: an in vivo glaucoma drainage device study in a rabbit
model. Transl Vis Sci Technol
2015;4(3):4. [CrossRef] [PMC free article]
[PubMed]
29 McCluskey
P, Molteno A, Wakefield D, Di Girolamo N. Otago Glaucoma Surgery Outcome Study:
the pattern of expression of MMPs and TIMPs in bleb capsules surrounding
Molteno implants. Invest Ophthalmol Vis
Sci 2009;50(5):2161-2164. [CrossRef]
[PubMed]
--------------------------------------------------------------------------------------------------------------------------------
All rights reserved by Press of International Journal of Ophthalmology (IJO
PRESS)